Abstract
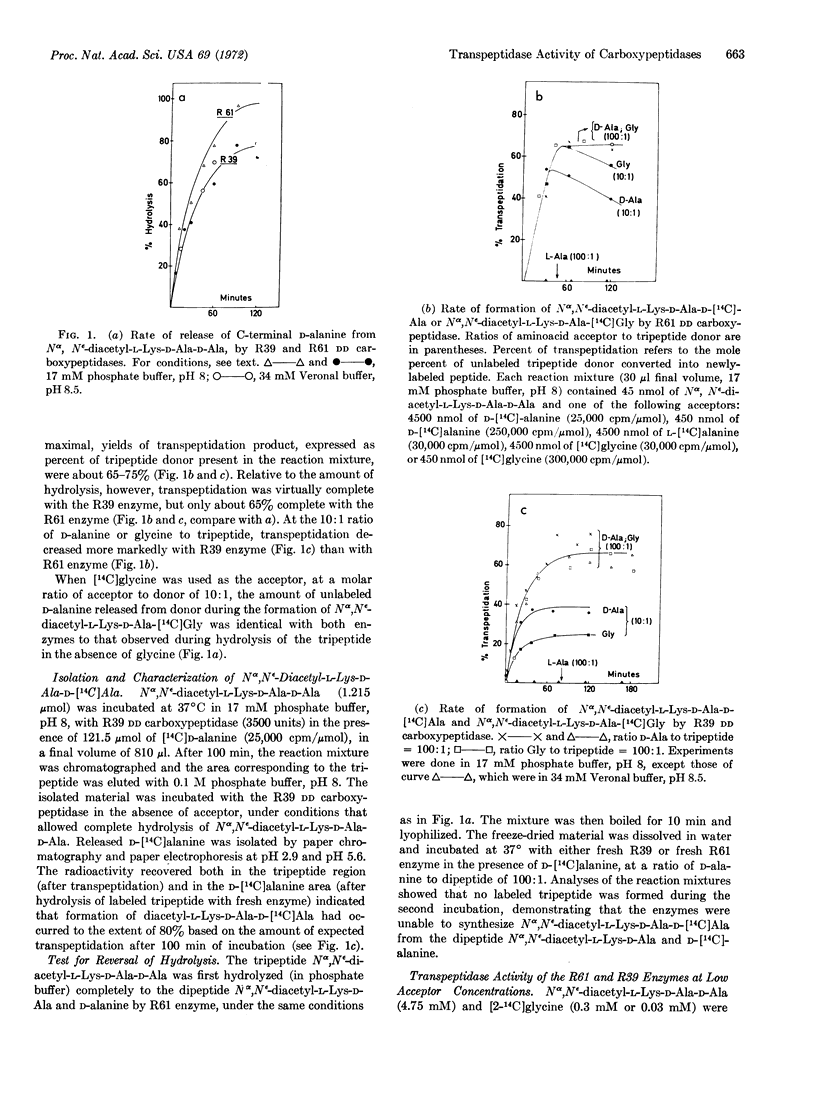
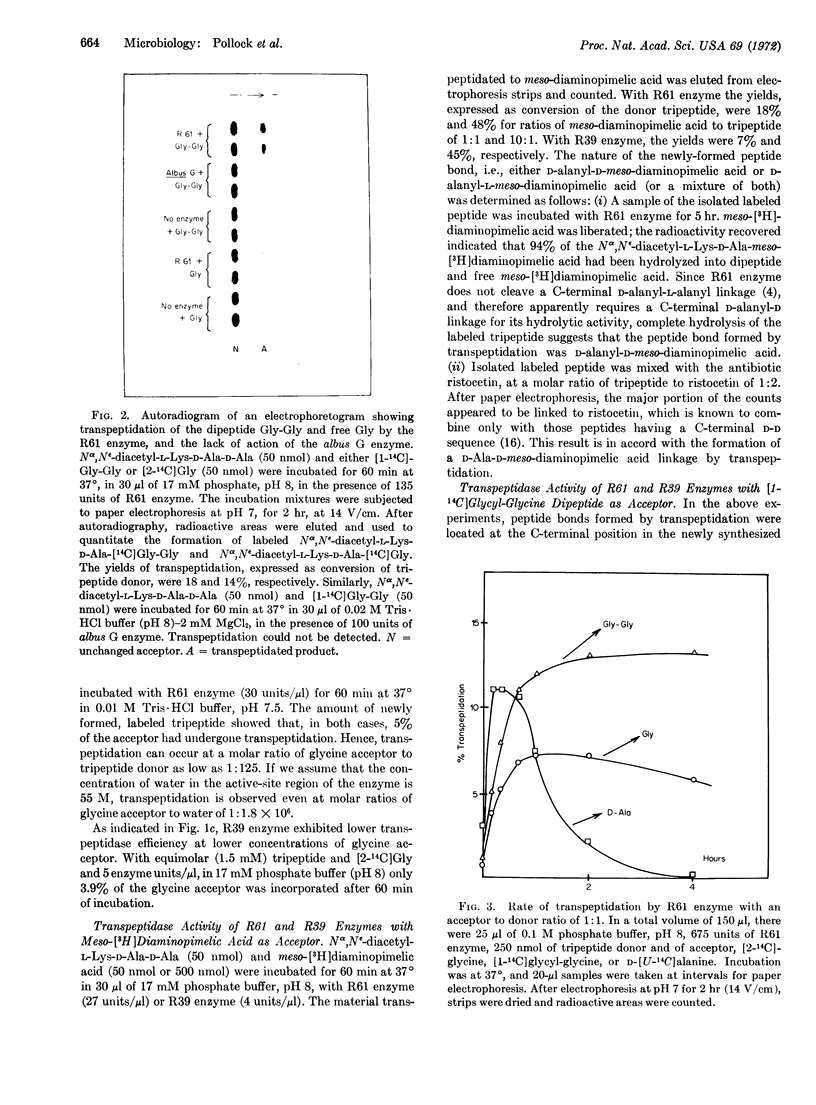
In the presence of Nα,Nε-diacetyl-L-Lys-D-Ala-D-Ala as donor, and either D-[14C]alanine, [14C]-glycine, or meso-[3H]diaminopimelic acid as acceptor, the DD carboxypeptidases from Streptomyces R61 and R39 catalyze a transpeptidation reaction with the release of terminal D-alanine from the donor and the formation of either Nα,Nε-diacetyl-L-Lys-D-Ala-D-[14C]Ala, Nα,Nε-diacetyl-L-Lys-D-Ala-[14C] Gly, or Nα,Nε-diacetyl-L-Lys-D-Ala-D-meso- [3H]diaminopimelic acid. The reaction appears to be a true transpeptidation, and is not simply a “reversal of hydrolysis”. Transpeptidation is inhibited by pencillin at concentrations that inhibit hydrolysis (carboxypeptidase action) of the donor peptide. There are differences in the specificity profiles of the Streptomyces enzymes for acceptor molecules:only the R61 enzyme used [14C]Gly-Gly as acceptor; transfer of Nα,Nε-diacetyl-L-Lys-D-Ala to this acceptor resulted in the formation of Nα,Nε-diacetyl-Lys-D-Ala-[14C] Gly-Gly, with the synthesis of a (D-Ala-Gly) peptide bond in an endoposition.
Keywords: penicillin, bacterial cell-wall synthesis, ristocetin donor and acceptor configuration
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREITENBACH J. W., DERKOSCH J., WESSELY F. Energetics of peptide formation. Nature. 1952 May 31;169(4309):922–922. doi: 10.1038/169922a0. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M., Bricas E., Lache M., Leyh-Bouille M. Structure of the cell walls of Micrococcus lysodeikticus. 3. Isolation of a new peptide dimer, N-alpha-[L-alanyl-gamma-(alpha-D-glutamylglycine)]-L-lysyl-D-alanyl-N-alpha-[L-alanyl-gamma-(alpha-D-glutamylglycine)]-L-lysyl-D-alanine. Biochemistry. 1968 Apr;7(4):1450–1460. doi: 10.1021/bi00844a030. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M., Leyh-Bouille M., Bonaly R., Nieto M., Perkins H. R., Schleifer K. H., Kandler O. Isolation of DD carboxypeptidase from Streptomyces albus G culture filtrates. Biochemistry. 1970 Jul 21;9(15):2955–2961. doi: 10.1021/bi00817a004. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Leyh-Bouille M., Bonaly R., Ghuysen J. M., Tinelli R., Tipper D. LL-diaminopimelic acid containing peptidoglycans in walls of Streptomyces sp. and of Clostridium perfringens (type A). Biochemistry. 1970 Jul 21;9(15):2944–2952. doi: 10.1021/bi00817a002. [DOI] [PubMed] [Google Scholar]
- Leyh-Bouille M., Coyette J., Ghuysen J. M., Idczak J., Perkins H. R., Nieto M. Penicillin-sensitive DD-carboxypeptidase from Streptomyces strain R 61. Biochemistry. 1971 May 25;10(11):2163–2170. doi: 10.1021/bi00787a032. [DOI] [PubMed] [Google Scholar]
- Leyh-Bouille M., Ghuysen J. M., Bonaly R., Nieto M., Perkins H. R., Schleifer K. H., Kandler O. Substrate requirements of the Streptomyces albus G DD carboxypeptidase. Biochemistry. 1970 Jul 21;9(15):2961–2970. doi: 10.1021/bi00817a005. [DOI] [PubMed] [Google Scholar]
- Leyh-Bouille M., Ghuysen J. M., Nieto M., Perkins H. R., Schleifer K. H., Kandler O. On the Streptomyces albus G DD carboxypeptidase mechanism of action of penicillin, vancomycin, and ristocetin. Biochemistry. 1970 Jul 21;9(15):2971–2975. doi: 10.1021/bi00817a006. [DOI] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R. The specificity of combination between ristocetins and peptides related to bacterial cell wall mucopeptide precursors. Biochem J. 1971 Oct;124(5):845–852. doi: 10.1042/bj1240845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J. J., Sharon N. Studies on the acceptor specificity of the lysozyme-catalyzed transglycosylation reaction. Biochemistry. 1970 Sep 29;9(20):3913–3925. doi: 10.1021/bi00822a009. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E. Peptidoglycan synthesis in bacilli. I. Effect of temperature on the in vitro system from Bacillus megaterium and Bacillus stearothermophilus. Biochim Biophys Acta. 1971 May 18;237(2):239–254. [PubMed] [Google Scholar]
- Reynolds P. E. Peptidoglycan synthesis in bacilli. II. Characteristics of protoplast membrane preparations. Biochim Biophys Acta. 1971 May 18;237(2):255–272. doi: 10.1016/0304-4165(71)90316-3. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heijenoort J., Elbaz L., Dezélée P., Petit J. F., Bricas E., Ghuysen J. M. Structure of the meso-diaminopimelic acid containing peptidoglycans in Escherichia coli B and Bacillus megaterium KM. Biochemistry. 1969 Jan;8(1):207–213. doi: 10.1021/bi00829a030. [DOI] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]




