Abstract
Objectives
We evaluated changes in oral DM medication adherence and persistence, as well as glycemic control for the year prior to breast cancer (BC) diagnosis (Year −1), during BC treatment, and in subsequent years.
Methods
Cohort study of 4,216 women diagnosed with incident early stage (I,II) invasive BC from 1990-2008, enrolled in Group Health Cooperative. Adherence was measured in prevalent users at baseline (N=509), during treatment, and 1-3 years post-diagnosis using medication possession ratio (MPR), %-adherent (MPR≥0.80) and discontinuation rates (DR). Laboratory data on glycosylated hemoglobin (HbA1C) was obtained for the corresponding periods.
Results
Compared to Year −1, mean MPR for metformin/sulfonylureas (0.86 versus 0.49, P<0.001) and %-adherent (75.3% versus 24.6%, P<0.001) declined during BC treatment. MPR and %-adherent rose slightly during years 1-3 post diagnosis but never returned to baseline. DR increased from treatment to Year +1 (59.3% versus 75.6%, P<0.001) and remained elevated during subsequent observation periods. Compared to baseline, increased HbA1C (7.0% versus 7.4%, P=0.001) and % women with high HbA1C >7.0% (34.9% versus 51.1%, P<0.001) coincided with decreased adherence.
Conclusion
DM medication adherence declined following BC diagnosis while discontinuation rates were relatively stable but poor overall. The proportion of adherent users increased only marginally following treatment, while the proportion of women meeting goals for HbA1C decreased considerably. These data support the hypothesis that adherence and subsequent glycemic control are sensitive to BC diagnosis and treatment. Confirmatory studies in other settings, on reasons for reduced adherence post-cancer diagnosis, and on subsequent indicators of glycemic control are warranted.
Keywords: Breast cancer, diabetes mellitus, medication adherence, glycemic control
Introduction
The incidence of breast cancer (BC) increases with age,1 as does the incidence and prevalence of comorbid conditions such as diabetes mellitus (DM)2 that are managed by multiple medications. Breast cancer patients with DM are part of a growing population of aging individuals with multi-morbidity, and oncologists can expect more than half of the patients they see ages 65 years and older to have ≥1 other meaningful chronic condition that may affect their treatment.3 However, overall adherence to DM medications in the general population is low, between 50-75% on average,4-6 and attainment of DM treatment goals with oral medications is strongly tied to adherence.6 Further, nonadherence to DM medications is associated with increased risk of glycometabolic disturbance and all-cause mortality,7 as well as increased costs and all-cause hospitalization.8 Adherence to DM therapy is known to decrease following major life events and with psychological stressors,9 although little is known about DM management following cancer diagnosis.
Numerous studies including meta-analyses support an association between diabetes and increased risk of breast cancer.10-12 Diabetes is also hypothesized to be an indicator of poor prognosis13-16 and possibly a risk factor for second contralateral breast cancer.17 DM may promote carcinogenesis through increased insulin-like growth factors and sex-steroid bioavailability, hyperglycemia, and chronic inflammation.18, 19 Other factors may influence the association between diabetes and breast cancer, including extent of glycemic control and impacts of certain drugs such as metformin used to manage DM.20, 21 As such, adherence to DM medications has the potential to not only alter DM outcomes but also breast cancer outcomes. The need for high quality management of comorbid conditions will continue to increase as improvements in diagnosis and treatment lead to longer lives for cancer survivors. For cancers such as early stage breast cancer with 5-year survival rates of >90%,1 increasing numbers of survivors are burdened with the challenges of polypharmacy and chronic condition care, and are more likely to die from causes other than cancer.3 While there are considerable data documenting the decline in medication adherence for adjuvant hormone therapies,22 there is relatively little evidence regarding adherence to medications used to control important comorbid conditions post-breast cancer.
The estimated 2.8 million breast cancer survivors living in the U.S.1 and the increasingly high prevalence of DM2 warrants a better understanding of adherence to medications for DM and goals for glycemic control. The objective of our study was to estimate adherence to commonly used oral DM medications, biguanides (i.e., metformin) and sulfonylureas, in the year before breast cancer diagnosis, during cancer treatment, and in subsequent years among a retrospective cohort of women diagnosed with early stage breast cancer. Further, we evaluated glycemic control, measured by glycosylated hemoglobin (HbA1C), among women taking oral DM medications in the corresponding periods.
Materials & Methods
We sampled women from the previously established Commonly Used Medications and Breast Cancer Outcomes (COMBO) cohort of 4,216 women diagnosed with incident early stage (I, II), invasive breast cancer between 1990 and 2008 at Group Health Cooperative (GH).23, 24 Women without at least 1 year of GH enrollment prior and after breast cancer diagnosis (unless they died) and women with bilateral breast cancer were excluded. GH is a large integrated delivery system that provides comprehensive medical care to approximately 620,000 enrollees in Washington State and parts of Idaho. Incident breast cancers and tumor characteristics were identified through linkage to the Surveillance, Epidemiology and End Results Seattle-Puget Sound registry.25 In this study, we included all women diagnosed through August 2007 so each woman had the potential for 3 years of follow-up. Follow-up was then through the earliest of second breast cancer event (SBCE), death, disenrollment, or end of the study period (August 2010). SBCE is defined as the first of a ductal carcinoma in situ or invasive cancer of the ipsilateral (recurrence) or contralateral (second primary) breast. Patient characteristics were obtained through GH automated data files,26 which include laboratory results, inpatient and outpatient diagnoses, procedures, enrollment, pharmacy dispensings, and death (internal records and Washington state death tapes).27 Information on breast cancer treatment and outcomes (e.g., recurrence) were obtained through review of medical records. For the current study, we selected only women with ≥1 dispensings of GH's first-line DM medications, metformin and/or sulfonylureas (N=509) alone or in combination, out of the 516 women treated with oral DM medications in the year before breast cancer diagnosis. Since the majority of oral DM medication users were taking metformin or sulfonylureas, we refer to these women as users of oral DM medication. Insulin use was also identified for women using oral DM medications.
Measures of medication adherence
Medication adherence and persistence were measured using medication possession ratio (MPR) and discontinuation rate (DR), respectively. Shorter days' supply associated with repeated DM medication dispensings prompted calculation of measures to incorporate both information on oversupply and medication gaps, a more recently validated method using automated pharmacy/claims data.28 Recent reviews in the scientific literature identify MPR and DR among the most commonly used and reproducible measures of medication adherence.29 We defined MPR as the proportion of days' supply of medication dispensed over the number of days for which the patient had been prescribed oral DM medication, or the intended period of treatment. For example, in a period of 180 days, five dispensings of 30 days' supply (150 days) of glyburide would result in an estimated MPR of 0.83 (150/180). MPR ≥0.80 was considered the threshold for which women were adherent to DM pharmacotherapy.29 DR was calculated using the observed number of discontinuation episodes, defined as a gap of ≥90 days between the end of a previous days' supply and the subsequent dispensing of DM medication.29 DR is equal to the proportion of users with ≥1 discontinuation episode within an observation period. Thus, for periods of one year, DR is the one-year cumulative incidence of discontinuation and persistence among users (i.e., continuous treatment with no gaps ≥90 days) in that period is represented as 1–DR.
Observation periods
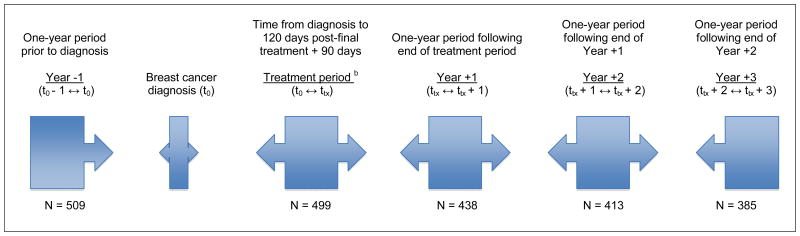
Using dispensing data from the GH automated pharmacy database, MPR and DR were calculated for the 1-year period before breast cancer diagnosis (Year −1, t0 − 1 year ↔ t0), treatment period (t0 ↔ ttx = trx + 90 days), 1-year period following end of treatment (Year +1, tx ↔ tx + 1 year), and two subsequent 1-year periods (Years +2 and +3) following end of treatment (Figure 1). The treatment period was defined as time from diagnosis to 120 days post-final treatment (last of surgery, radiation, or chemotherapy) plus 90 days. Among women on oral DM medication at any point during the year before breast cancer diagnosis (n=509), mean time to end of primary treatment (last of surgery, radiation or chemotherapy) was 133.5 days (SD 112.9) (Table 1). Sensitivity analyses were conducted on the definition of the treatment period. Specifically, we examined differences in varying definitions of treatment length (range: 180 to 365 days). No substantial differences were observed, and thus, we present results only on the treatment period defined as 120 days post-final treatment plus 90 days. Women contributed to the four post-diagnosis observation periods only if they were using DM medication (i.e., no discontinuation) in the prior observation period.
Figure 1. Timeline of observation periods for adherence and persistence of DM medication users a relative to breast cancer diagnosis date.
a. ≥1 Dispensing of metformin and/or sulfonylureas in the year prior to breast cancer diagnosis
b. Treatment period: SEER diagnosis date to 120 days post-final breast cancer treatment noted in the medical chart (surgery, radiation, or chemotherapy) plus 90 days
Table 1. Characteristics of oral DM medication users at breast cancer diagnosis.
| During breast cancer treatment period | ||||||
|---|---|---|---|---|---|---|
| All DM medication users a (n=516) | Adherent DM medication users b (n=123) | Non-adherent DM medication users b (n=376) | ||||
| Year of breast cancer diagnosis | ||||||
| 1990-2000 | 302 | (58.5) | 66 | (53.7) | 232 | (61.7) |
| 2001-2004 | 120 | (23.3) | 39 | (31.7) | 77 | (20.5) |
| 2005-2008 | 94 | (18.2) | 18 | (14.6) | 67 | (17.8) |
| Length of cancer treatment period (days) c | ||||||
| Median (IQR) | 110 | (59-185) | 113 | (76-191) | 109 | (57-193) |
| Age (years) | ||||||
| Median (IQR) | 64.3 | (11.4) | 68 | (55-71) | 63 | (56-76) |
| 18-39 | 9 | (1.8) | 1 | (0.8) | 8 | (2.1) |
| 40-49 | 57 | (11.4) | 13 | (10.6) | 44 | (11.7) |
| 50-59 | 116 | (23.2) | 22 | (17.9) | 89 | (23.7) |
| 60-69 | 150 | (30.1) | 32 | (26.0) | 115 | (30.6) |
| 70-79 | 132 | (26.5) | 38 | (30.9) | 91 | (24.2) |
| 80+ | 51 | (10.2) | 17 | (13.8) | 29 | (7.7) |
| Menopausal status | ||||||
| Premenopausal | 105 | (20.3) | 23 | (18.7) | 80 | (21.3) |
| Postmenopausal | 411 | (79.7) | 100 | (81.3) | 296 | (78.7) |
| Race | ||||||
| White | 423 | (82.0) | 107 | (87.0) | 293 | (77.9) |
| African American | 26 | (5.0) | 3 | (2.4) | 23 | (6.1) |
| American Indian / Alaska Native | 20 | (3.9) | 3 | (2.4) | 20 | (5.3) |
| Asian / Pacific Islander | 44 | (8.5) | 9 | (7.3) | 39 | (10.4) |
| Unknown | 3 | 1 | 2 | |||
| Ethnicity | ||||||
| Not Hispanic | 480 | (93.0) | 116 | (94.3) | 347 | (92.3) |
| Hispanic | 36 | (7.0) | 7 | (5.7) | 29 | (7.7) |
| Education | ||||||
| High school or less | 82 | (31.5) | 16 | (13.0) | 46 | (12.2) |
| Some college | 106 | (40.8) | 18 | (14.6) | 65 | (17.3) |
| College or post graduate | 72 | (27.7) | 16 | (13.0) | 50 | (13.3) |
| Unknown | 256 | 73 | 215 | |||
| Body mass index (kg/m2) | ||||||
| Mean (SD) | 32.3 | (6.3) | 33.2 | (7.4) | 31.4 | (6.5) |
| <18.5 | 4 | (0.8) | 2 | (1.6) | 2 | (0.5) |
| 18.5-24.9 | 69 | (13.4) | 12 | (9.8) | 56 | (14.9) |
| 25.0-29.9 | 139 | (26.9) | 33 | (26.8) | 106 | (28.2) |
| 30.0-34.9 | 139 | (26.9) | 31 | (25.2) | 108 | (28.7) |
| 35.0+ | 165 | (32.0) | 50 | (40.7) | 103 | (27.4) |
| Smoking status | ||||||
| Ever | 79 | (15.3) | 17 | (13.8) | 55 | (14.6) |
| Never | 433 | (84.7) | 106 | (86.2) | 321 | (85.4) |
| AJCC stage | ||||||
| I | 318 | (61.6) | 84 | (68.3) | 222 | (59.0) |
| IIA | 138 | (26.7) | 25 | (20.3) | 108 | (28.7) |
| IIB | 60 | (11.6) | 14 | (11.4) | 46 | (12.2) |
| Lymph node status d | ||||||
| Negative | 383 | (74.4) | 82 | (66.7) | 253 | (67.3) |
| Positive | 132 | (25.6) | 22 | (17.9) | 91 | (24.2) |
| Unknown | 1 | 19 | 32 | |||
| Comorbidities | ||||||
| Hypertension | 440 | (85.3) | 104 | (84.5) | 320 | (85.1) |
| Ischemic heart disease | 175 | (35.1) | 53 | (43.1) | 122 | (32.4) |
| Charlson comorbidity index e,h | ||||||
| 0 | 177 | (34.2) | 28 | (22.8) | 175 | (46.5) |
| 1 | 197 | (38.2) | 51 | (41.5) | 118 | (31.4) |
| 2+ | 122 | (23.6) | 37 | (30.1) | 72 | (19.1) |
| Missing (pre-1993) | 20 | 7 | 11 | |||
| Surgical procedure | ||||||
| Mastectomy ± radiation | 195 | (39.7) | 57 | (46.3) | 138 | (36.7) |
| Breast conserving, radiation (+) | 258 | (51.5) | 60 | (48.8) | 191 | (50.8) |
| Breast conserving, radiation (-) | 63 | (12.2) | 13 | (10.6) | 40 | (10.6) |
| Other breast cancer treatments | ||||||
| Chemotherapy h | 153 | (29.7) | 27 | (22.0) | 120 | (31.9) |
| Endocrine therapy | 301 | (58.3) | 65 | (52.8) | 226 | (60.1) |
| Completed 5 years endocrine therapy | 130 | (43.2) | 26 | (40.0) | 104 | (46.0) |
| Chemotherapy + Endocrine therapy | 109 | (21.1) | 16 | (13.0) | 78 | (20.7) |
| # Primary care physician visits within one year post-diagnosis | ||||||
| Mean (SD) | 4.0 | (3.9) | 4.3 | (3.6) | 3.9 | (4.0) |
| 0-1 visit only h | 147 | (28.5) | 28 | (22.8) | 113 | (30.1) |
| ≥2 visits | 369 | (71.5) | 95 | (77.2) | 263 | (69.9) |
| DM medication use | ||||||
| Metformin only | 149 | (28.9) | 38 | (30.9) | 111 | (29.5) |
| Sulfonylureas only | 195 | (37.8) | 48 | (39.0) | 147 | (39.1) |
| Metformin plus sulfonylureas | 165 | (32.0) | 37 | (30.1) | 128 | (34.0) |
| Any DM medication plus insulin | 220 | (42.6) | 39 | (31.7) | 148 | (39.4) |
| Other DM medications only f | 7 | (1.4) | ||||
| # CVD prescriptions used concurrently throughout study period g | ||||||
| 1 medication only | 102 | (19.8) | 23 | (18.7) | 67 | (17.8) |
| ≥2 medications | 414 | (80.2) | 100 | (81.3) | 309 | (82.2) |
| ≥3 medications | 248 | (48.1) | 52 | (42.3) | 195 | (51.9) |
| ≥4 medications h | 150 | (29.1) | 33 | (26.8) | 140 | (37.2) |
Abbreviations: IQR interquartile range, SD standard deviation, AJCC American Joint Committee on Cancer
Note: Values are presented as n (%) unless otherwise noted
≥1 dispensing of oral DM medication in the year prior to breast cancer diagnosis
Post-diagnosis adherence defined as MPR ≥0.80 to metformin/sulfonylureas, breast cancer diagnosis through treatment period
Last date of primary breast cancer treatments (surgery, radiation or chemotherapy)
From SEER registry or chart when missing from SEER
Deyo RA, Cherkin DC, Ciol MA. J Clin Epidemiol. 1992;45: 613-619.
Other DM medications: meglitinides, thiazolidinediones or DPP-4 inhibitors
CVD prescriptions used: highest number through Year +3 of concurrent oral medications to treat DM, dyslipidemias or hypertension
Indicates that differences between adherent and non-adherent users were significant at P<0.05 using χ2 test for categorical variables and Fisher's exact test for continuous variables
Glycemic control
We obtained laboratory data on HbA1C for DM medication users within corresponding time periods in which medication adherence was calculated. Approximately 85% of DM medication users received ≥1 laboratory measurement of HbA1C in the year prior to breast cancer diagnosis. Similar proportions (80-85%) of users had HbA1C data in subsequent observation periods. The highest of HbA1C in a given period of interest was used to determine glycemic control and standard goals for management of DM30 (defined as HbA1C ≤7.0%) as well as a less rigid measure of glycemic control (HbA1C ≤8.0%). We performed sensitivity analyses using the lowest HbA1C and mean value of multiple measures. We also limited our analysis of medication adherence to only women with complete HbA1C data in all periods. Results from these sensitivity analyses were not appreciably different from our first approach, and thus, we report on only our main analyses. All analyses of medication adherence and glycemic control were also stratified by concurrent insulin use.
Statistical analysis
We evaluated differences in the characteristics between adherent and non-adherent users during the treatment period using χ2 test for categorical variables and Fisher's exact test for continuous variables. We considered P-values <0.05 to be of statistical significance.
Statistical tests for within-subjects' comparisons of measures of adherence and glycemic control were performed. Statistical methods for the analysis of paired data were used to test the hypothesis of no difference between the year prior to diagnosis and each subsequent year. Paired t-tests were used for the continuous measures of mean MPR and HbA1C. McNemar exact tests were used to test the hypothesis of no difference for dichotomous measures of persistence and adherence to DM therapy and glycemic control goals met. Our analyses tested differences between Year −1 and subsequent years' mean MPR, % adherent, % persistent, mean HbA1C and % at goal overall and by adherence status yielding a total of 48 comparisons. To account for these multiple comparisons, we set an alpha level of 0.001 for determining statistical significance, following the approach of Bonferroni.31 This alpha level allows us to conduct up to 50 hypothesis tests without exceeding a family-wise type I error rate of 0.05. Analyses were performed using Stata 13 (College Station, TX: StataCorp LP).
Results
Of the 509 women using metformin and/or sulfonylureas in the year prior to BC diagnosis (Year−1), the median age at BC diagnosis was 65 years, the majority presented with AJCC Stage I tumors (61.6%), and 23.6% of women scored ≥2 on the Charlson comorbidity index (Table 1).32 Prevalence of other comorbidities was high with 121 (23.4%) women having a history of ischemic heart disease and 440 (85.3%) having a history of hypertension. Compared with adherent users during the diagnosis through treatment period, non-adherent users of oral DM medications were more likely to be diagnosed with Stage II tumors (40.9% versus 31.7%, P=0.149), and more likely treated with adjuvant chemotherapy (31.9% versus 22.0%, P=0.035) and endocrine therapy (60.1% versus 52.8%, P=0.156) but only chemotherapy was significantly different. Non-adherent users also had a marginally higher proportion of women with 0-1 visit only to a primary care provider (30.1% versus 22.8%, P=0.042) within the year following diagnosis. Per pharmacy dispensings, non-adherent oral DM medication users were more likely to be also concurrently using ≥4 CVD medications compared with adherent users (37.2% versus 26.8%, P=0.035). Between the year before diagnosis and Year +3, 124 women were censored from analyses due to discontinuation of oral DM therapy (n=64), death (n=23), disenrollment (n=17), or SBCE (n=20) (Table 2).
Table 2. Adherence and persistence of metformin and sulfonylureas users prior to and following breast cancer diagnosis and treatment.
| Adherence | Persistence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MPR | Adherent users (MPR ≥0.80) | Discontinuation episodes | DR | Persistent users (1–DR) | |||||||||
| N | Mean | SD | IQR | n | (%) | Mean | SD | Median | IQR | (%) | n | (%) | |
| Year −1 | 509 | 0.86 | 0.26 | 0.67-0.99 | 383 | 75.3% | 1.23 | 1.41 | 1 | 0-2 | 74.7% | 129 | 25.3% |
| Treatment period | 499 | 0.49* | 0.31 | 0.25-0.67 | 123 | 24.6%* | 1.06 | 1.25 | 1 | 0-1 | 59.3% | 186 | 40.7%* |
| Year +1 | 438 | 0.48* | 0.32 | 0.25-0.82 | 118 | 27.1%* | 1.16 | 1.50 | 1 | 0-2 | 75.6% | 107 | 24.4% |
| Year +2 | 413 | 0.48* | 0.30 | 0.25-0.74 | 100 | 24.2%* | 1.22 | 1.56 | 1 | 0-2 | 71.5% | 118 | 28.5% |
| Year +3 | 385 | 0.52* | 0.32 | 0.25-0.87 | 122 | 31.8%* | 1.97 | 2.57 | 2 | 0-2 | 70.5% | 113 | 29.5% |
Abbreviations: MPR, medication possession ratio; DR, discontinuation rate; SD, standard deviation; IQR, interquartile range
Note: Statistical hypothesis tests were performed comparing means and proportions to baseline, Year −1 values;
indicates difference of statistical significance at P<0.001
Medication adherence and persistence
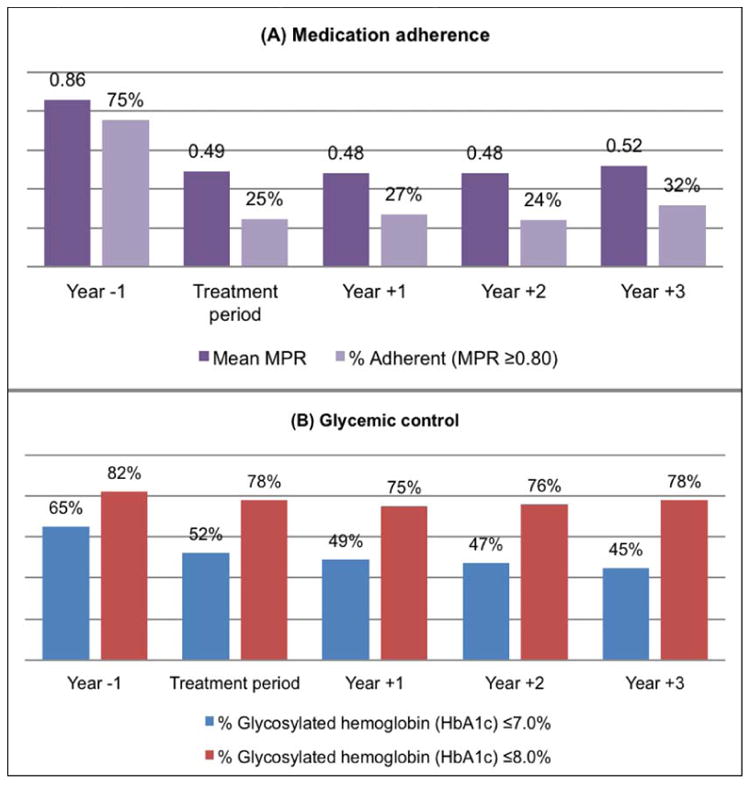
Estimated MPR and DR among oral DM medication users are reported in Table 2. Mean MPR for oral DM medication use in the year before diagnosis (Year −1) was highest overall, 0.85. In Year −1 there were 383 (75.3%) DM medication users adherent (MPR ≥0.80) to medication therapy. Mean MPR was lower in the treatment period, 0.49 (P <0.001) compared to Year−1. Accordingly, DM medication users considered adherent during treatment declined to only 24.6%. In the subsequent three years of observation, mean MPR and proportion adherent remained considerably low (Figure 2). Adherence was poorest in Year +2, MPR = 0.48 and proportion adherent of 24.2%, but overall similar to that observed during the treatment period. The proportion of persistent users, those that did not experience a discontinuation episode (1–DR), was 25.3% at Year−1 and greatest in the treatment period (40.7%, P<0.001), although in each of the 3 years following treatment persistence levels were similar to that of baseline. While adherence throughout the follow-up period was similar between oral DM medication users on insulin therapy and those on oral medications only, persistence (1–DR) was greater among insulin users in all observation periods (Online Supplementary Material – Table S1).
Figure 2. Medication adherence (A) and glycemic control (B) for users of metformin and sulfonylureas prior to and following breast cancer diagnosis and treatment.
Glycemic control
Results on measured HbA1C are reported in Table 3. Among DM medication users with laboratory values for HbA1C during periods of interest (n=433), mean HbA1C and proportion not at goal HbA1C were higher during the treatment period (HbA1C 7.32%, P=0.001 and 47.8% not at goal HbA1C, P<0.001) in comparison to Year−1 (HbA1C 6.96% and 34.9% not at goal HbA1C). Achievement of treatment goal HbA1C continued to decline slightly through Year +3 (Figure 3). Despite the trend in increasing mean HbA1C over time, the majority of women maintained relatively good control with fewer women having HbA1C >8.0% (24.7% in Year +1, 24.2% in Year +2, and 21.8% in Year +3).
Table 3. Glycosylated hemoglobin (HbA1C) of metformin and sulfonylureas users prior to and following breast cancer diagnosis and treatment.
| Glycemic control | ||||||||
|---|---|---|---|---|---|---|---|---|
| HbA1C | HbA1C >7.0% | HbA1C >8.0% | ||||||
| N | Mean | SE | 95% CI | n | (%) | n | (%) | |
| All users | ||||||||
|
| ||||||||
| Year −1 | 433 | 6.96 | 0.080 | 6.80-7.12 | 151 | 34.9% | 77 | 17.8% |
| Treatment period | 399 | 7.32* | 0.072 | 7.18-7.46 | 191 | 47.9% | 87 | 21.8% |
| Year +1 | 372 | 7.41* | 0.072 | 7.27-7.55 | 190 | 51.1%* | 92 | 24.7% |
| Year +2 | 351 | 7.42* | 0.074 | 7.28-7.56 | 185 | 52.7%* | 85 | 24.2% |
| Year +3 | 327 | 7.30 | 0.064 | 7.17-7.43 | 181 | 55.4%* | 73 | 22.3% |
|
| ||||||||
| Adherent users (MPR ≥0.80) | ||||||||
|
| ||||||||
| Year −1 | 326 | 6.45 | 0.049 | 6.35-6.55 | 113 | 34.7% | 52 | 16.0% |
| Treatment period | 105 | 6.83* | 0.081 | 6.67-6.99 | 42 | 40.0% | 19 | 18.1% |
| Year +1 | 101 | 6.90* | 0.078 | 6.75-7.05 | 47 | 46.5% | 21 | 20.8% |
| Year +2 | 84 | 6.96* | 0.087 | 6.79-7.13 | 31 | 36.9% | 27 | 32.1%* |
| Year +3 | 103 | 6.96* | 0.081 | 6.80-7.12 | 36 | 35.0% | 22 | 21.4% |
|
| ||||||||
| Non-adherent users (MPR <0.80) | ||||||||
|
| ||||||||
| Year −1 | 107 | 7.32 | 0.157 | 7.01-7.63 | 38 | 35.5% | 19 | 17.8% |
| Treatment period | 294 | 7.46 | 0.080 | 7.30-7.62 | 148 | 50.3% | 67 | 22.8% |
| Year +1 | 271 | 7.52 | 0.081 | 7.36-7.68 | 143 | 52.8% | 70 | 25.8% |
| Year +2 | 267 | 7.53 | 0.081 | 7.37-7.69 | 154 | 57.7%* | 60 | 22.5% |
| Year +3 | 224 | 7.42 | 0.073 | 7.28-7.56 | 145 | 64.7%* | 51 | 22.8% |
Abbreviations: SE, standard error; CI, confidence interval of the mean
Note: Statistical hypothesis tests were performed comparing means and proportions to baseline, Year −1 values;
indicates difference of statistical significance at P<0.001
Glycemic control also varied by adherence status (Table 3). Among adherent oral DM medication users mean HbA1C increased from Year−1 to the treatment period (6.45% to 6.83%, P<0.001) and remained elevated throughout subsequent years of follow-up. Adherent users had a slightly higher proportion with high HbA1C (>7.0%) during treatment and Year +1 (40.0%, P=0.343 and 46.5%, P=0.032) compared with Year−1 (34.7% high HbA1C). Nonadherent DM medication users (MPR <0.80) also had a marginally increased mean HbA1C from baseline to treatment (7.32% to 7.46%, P=0.390) that remained similarly elevated and consistently higher compared to adherent users. The proportion of nonadherent users with high HbA1C at Year−1 (35.5%) was higher during treatment (50.3%, P=0.009) and was greatest in Year +3 (64.7%, P<0.001). Insulin users consistently had higher mean HbA1C throughout all observation periods (Online Supplementary Material – Table S2).
Discussion
A non-trivial number of women diagnosed with breast cancer will have ≥1 concurrent, comorbid conditions for which a need for evidence on quality of survivor care has been identified.33-35 Our results suggest that adherence to oral DM medications as measured by MPR and DR may be sensitive to timing of breast cancer diagnosis and treatment, and that these effects continue in the years that follow. Medication adherence decreased in the treatment period and remained low in the years following breast cancer diagnosis. Achieving goals for glycemic control in DM treatment also appeared to vary in the years following diagnosis with increased mean HbA1C compared to baseline. While many factors influence glycemic control among women with DM, these results signal a possible opportunity for improved management of DM among breast cancer survivors particularly with respect to medication adherence.
There is evidence from some but not all epidemiologic studies that diabetes and abnormal glucose tolerance are associated with cancer-related death,36, 37 and several reports link pre-existing diabetes to increased risk of all-cause mortality in breast cancer.14-16 In a meta-analysis comparing overall survival in cancer patients with and without pre-existing diabetes,14 there was a 61% increased risk (95% CI, 1.46-1.78) of long-term, all-cause mortality in breast cancer patients with diabetes. It is hypothesized that less aggressive primary breast cancer treatment or diabetes care, both of which could compromise survival, are responsible for such observed associations.15 Here we consider the latter scenario, in which management of DM through adherence to medications and glycemic control may be compromised during breast cancer treatment and the following years of recovery. Also, certain DM medications, such as metformin, are hypothesized to improve breast cancer prognosis and survival.21, 38-40 Such a protective effect is potentially mediated through metformin's role in reducing hyperglycemia, decreasing circulating insulin levels and suppressing several metabolic processes that contribute to tumorigenesis.41 Therefore, adherence to DM medications may become important for improving cancer outcomes in addition to diabetes management and glycemic control.
Relevant epidemiological studies for direct comparison are limited. In a large, independent practice model health maintenance organization (HMO), a cross-sectional study of 6,000 patients in a DM management program6 described correlations between HbA1C and MPR for use of sulfonylureas (r=-0.295, P<0.001) and metformin (r=-0.285, P<0.001). As such, mean MPR of patients at goal HbA1C ≤7.0% compared with those that did not meet glycemic goals was higher for users of sulfonylureas (0.82 versus 0.72, P<0.001) and metformin users (0.77 versus 0.62, P<0.001) over two years. Using data from an integrated health system, Rolnick et al5 described medication adherence among a sample of 4,631 patients taking a single oral DM medication and having no other major chronic disease diagnoses. In this select group of patients, median MPR over a 12-month period was 0.81, and only 50% of female and 55% of male DM medication users were considered adherent (MPR ≥0.80). These estimates are similar to women in this study with regard to adherence in the year before diagnosis (MPR=0.86, 75% adherent) and differ from our MPR observed during treatment (MPR=0.49, 25% adherent). However, while observed adherence declined post-diagnosis and remained low in subsequent years, glycemic control among DM medication users in our cohort was only marginally clinically worse and seems to improve or stabilize by Year +3, particularly for insulin users.
The Institute of Medicine report, From Cancer Patient to Cancer Survivor: Lost in Transition,34 describes the lack of guidelines for and possible inconsistencies on the transfer from cancer-directed care back to primary care providers. Illustrating this possibly complex transition, Snyder et al35 compared 23,731 breast cancer survivors in the 366 to 730 days post-cancer diagnosis. Women seeing both a primary care provider and oncology specialist versus only a single provider were the most likely to receive recommended cancer screenings (i.e., colorectal cancer and mammography) and other preventive care (i.e., influenza vaccination, cholesterol screening, bone densitometry). Our results add to this limited body of work on chronic comorbid condition care in cancer patients because, to our knowledge, this analysis is the first to report on longitudinal measures of medication adherence and glycemic control among women diagnosed with breast cancer.
Limitations and strengths
Some important limitations to our study should be noted. Although use of automated pharmacy records provides objective and reproducible adherence measures, this methodology has its drawbacks. First, a dispensed medication does not guarantee patients ingested medication as directed, potentially overestimating adherence. Similarly, patients may receive medications from other sources not captured by health plan data and therefore DR may be overestimated. However, this is unlikely given that approximately 97 % of GH enrollees fill their medications at GH-owned or contracted pharmacies.26, 42, 43 We used two of the most commonly reported and reproducible measures of medication adherence (MPR and DR), but results using other methods to measure adherence and discontinuation may yield different results, particularly with longer periods of observation.29 We accounted for therapeutic interchange in DM management by considering all days' supply from metformin and sulfonylureas together when calculating MPR and DR. Therefore, this approach was conservative in that changes in therapy would tend toward medication oversupply in MPR and not inflate DR. We note the possible limitations in our study's generalizability. GH enrollees represent a predominantly White, insured population in the United States, thereby excluding a proportion of breast cancer survivors. This is noteworthy given that minority, uninsured, and/or low-income women may have worse adherence due to financial constraints or problems with access to services. Although we can make broad comparisons to studies of DM medication adherence in the general population, adherence among women in our population without a history of breast cancer would be informative, but beyond the scope of this analysis.
Data from the parent study only went back one year before breast cancer diagnosis, limiting our ability to evaluate the influence of duration of DM medication use before breast cancer diagnosis on post-diagnosis adherence. Analysis of prevalent users, women followed from Year −1 versus incident “new users,” may introduce selection bias because prevalent users have, by definition, survived under treatment. The mix of incident and prevalent users stands to dilute differences in adherence behavior between those recently starting DM medications and those on long-term treatment. Also, HbA1C estimates average plasma glucose in the prior 4-12 weeks. Therefore, this measure may not reflect glycemic control entirely throughout each observation period. We lacked information on other factors that can alter glycemic control such as health behaviors (e.g., diet and exercise), short-term corticosteroids co-administered with adjuvant chemotherapy, and nausea/anorexia side effects of chemotherapy. Long-term changes in health behaviors post-breast cancer diagnosis (e.g., adopting healthier eating habits or increasing exercise) could improve glycemic control and lead to medication dose reductions or even warranted discontinuation of medication. We were unable to measure dose reductions but the drop in glycemic control and relatively constant discontinuation rate (except for the treatment period) does not support this argument in our data. Short-term changes in diet coinciding with chemotherapy such as nausea and anorexia may preclude use of oral DM medications and potentially lower MPR. If chemotherapy-related nausea or loss of appetite alone accounted for our observed decreases in adherence then we would perhaps expect adherence to promptly return to pre-diagnosis levels. Although the role of chemotherapy side effects as a cause for glycometabolic disturbance warrants further investigation, the observed sustained decline in MPR suggests that these short-term changes are not the sole explanation for poor adherence. It is also possible that corticosteroids altered glycemic control in the short term, which could actually result in improved adherence and/or addition of therapies. To that end, goal HbA1C and glycemic control are intermediate therapeutic outcomes, not end-point clinical outcomes such as hospitalizations or emergency department visits. Rather, we answer a specific question regarding how clinical management (often driven by HbA1C values) varies from prior to and in the years following breast cancer diagnosis and treatment. Loss to follow-up due to disenrollment is a potential limitation since these women may differ from women who stay with a health plan longer. However, only 17 (3.3%) cohort members disenrolled during the study period so this did not substantively affect out results.
In the Cochrane review of interventions to improve medication adherence,44 confounding by severity of disease is noted to be particularly problematic in studies of DM management. For example, intensive insulin therapy is indicated to be added to oral DM therapy when oral therapies alone have failed and glycemic control has worsened. Poorly controlled diabetes also often triggers closer management by nutritionists and diabetes educators to monitor therapy and titrate insulin dosing, which in turn influences both measured adherence and glycemic control.30 Reporting one of these measures not in the context of other data (e.g., DR with no information on MPR or HbA1C) may limit interpretation of continuance/discontinuation of therapy.45 Thus, by design, we chose to use multiple measures to examine DM management (i.e., adherence, discontinuation, and glycemic control) and stratify glycemic control and adherence to oral DM medications by insulin use. Understanding adherence to intensive insulin therapy would also be informative but is less reliably measured using automated pharmacy dispensing data.
Our study adds to the current literature and has many strengths including a large population-based cohort of women with (1) automated pharmacy records considered to be valid, complete, and used in other epidemiologic studies; (2) longitudinal, long-term follow-up; (3) complete capture of cancer and recurrences through the SEER registry and medical charts; (4) cancer and treatment characteristics; and (5) information on diagnoses, laboratory values, and demographics. Also, our approach uses multiple measures of adherence and glycemic control such that comparison to future studies and potential interventions to improve outcomes modifiable by drug therapy are possible.46 Further studies allowing for comparison of medication adherence in both incident and prevalent users among breast cancer survivors and the general population will be important for understanding any differences in the reasons for nonadherence and the role providers may have in managing comorbidities among cancer survivors.
Conclusion
Efforts to understand multiple-comorbidity following cancer diagnosis and improve self-management are important to the growing population of breast cancer survivors. We believe our results lend further evidence to and raise awareness of the importance of DM management following breast cancer diagnosis and subsequent years following treatment. Population-level measures to improve diabetes care have been identified and applied to integrated primary care models at GH,47, 48 and multidisciplinary, tailored approaches such as these may be important tools for addressing adherence and glycemic control among these women. We hope that our results further motivate efforts to address the complex needs for comorbidity care in breast cancer survivorship. While not the focus of this study, patient characteristics (e.g., treatment with adjuvant chemotherapy, frequency of visits to primary care providers) identified to be more prevalent among the non-adherent group versus adherent group during the breast cancer treatment period may provide clues for further research and potential interventions.
Supplementary Material
Key Points.
Many women diagnosed with breast cancer will have ≥1 concurrent, chronic comorbid conditions for which a need for evidence on quality of survivor care has been identified. Our results suggest that adherence to oral DM medications as measured by MPR and DR may be sensitive to timing of breast cancer diagnosis, treatment, and recovery.
Medication adherence decreased greatly in the treatment period compared to the year prior and remained low in the years following breast cancer diagnosis. Women meeting treatment goals for DM management also decreased in the subsequent years after breast cancer diagnosis compared to the year prior to diagnosis.
While many factors influence glycemic control among women with DM, these results signal a possible opportunity for improved management of DM among breast cancer survivors particularly with respect to medication adherence.
Acknowledgments
The National Cancer Institute (R01CA120562 to D.M.B) provided funding for this study at the Group Health Research Institute. The National Institutes of Health Cancer Prevention Training Grant in Nutrition, Exercise, and Genetics (R25CA094880) at the University of Washington and Fred Hutchinson Cancer Research Center supported G.S.C. Authors of this manuscript are affiliated with the Departments of Epidemiology (G.S.C., A.S., K.E.M., and D.M.B.) and Biostatistics (R.A.H.) at the University of Washington, Group Health Research Institute (R.A.H. and D.M.B.), Seattle Cancer Care Alliance (J.R.G.) and Division of Public Health Sciences at the Fred Hutchinson Cancer Research Center (G.S.C. and K.E.M.).
Funding: The National Cancer Institute (R01 CA120562 to D.M.B) provided funding for this study at the Group Health Research Institute. The National Institutes of Health Cancer Prevention Training Grant in Nutrition, Exercise, and Genetics (R25 CA094880) at the University of Washington and Fred Hutchinson Cancer Research Center supported G.S.C.
Footnotes
Prior Presentation: Results from this analysis were presented, in part, in an oral presentation at the International Conference on Pharmacoepidemiology and Therapeutic Risk Management in August 2013. Pharmacoepidemiology and Drug Safety, 2013; 22: (Suppl. 1): 26. DOI: 10.1002/pds.3512
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2013-2014. Atlanta: American Cancer Society, Inc.; 2013. [Google Scholar]
- 2.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchie CS, Kvale E, Fisch MJ. Multimorbidity: an issue of growing importance for oncologists. Journal of oncology practice / American Society of Clinical Oncology. 2011;7:371–374. doi: 10.1200/JOP.2011.000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. The American journal of medicine. 2005;118(Suppl 5A):27S–34S. doi: 10.1016/j.amjmed.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Rolnick SJ, Pawloski PA, Hedblom BD, Asche SE, Bruzek RJ. Patient characteristics associated with medication adherence. Clin Med Res. 2013;11:54–65. doi: 10.3121/cmr.2013.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence DB, Ragucci KR, Long LB, Parris BS, Helfer LA. Relationship of oral antihyperglycemic (sulfonylurea or metformin) medication adherence and hemoglobin A1c goal attainment for HMO patients enrolled in a diabetes disease management program. J Manag Care Pharm. 2006;12:466–471. doi: 10.18553/jmcp.2006.12.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hepke KL, Martus MT, Share DA. Costs and utilization associated with pharmaceutical adherence in a diabetic population. Am J Manag Care. 2004;10:144–151. [PubMed] [Google Scholar]
- 8.Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care. 2004;27:2149–2153. doi: 10.2337/diacare.27.9.2149. [DOI] [PubMed] [Google Scholar]
- 9.Peyrot M, McMurry JF, Jr, Kruger DF. A biopsychosocial model of glycemic control in diabetes: stress, coping and regimen adherence. J Health Soc Behav. 1999;40:141–158. [PubMed] [Google Scholar]
- 10.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 11.Liao S, Li J, Wei W, et al. Association between diabetes mellitus and breast cancer risk: a meta-analysis of the literature. Asian Pac J Cancer Prev. 2011;12:1061–1065. [PubMed] [Google Scholar]
- 12.Boyle P, Boniol M, Koechlin A, et al. Diabetes and breast cancer risk: a meta-analysis. Br J Cancer. 2012;107:1608–1617. doi: 10.1038/bjc.2012.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peairs KS, Barone BB, Snyder CF, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011;29:40–46. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. 2007;120:1986–1992. doi: 10.1002/ijc.22532. [DOI] [PubMed] [Google Scholar]
- 16.Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013 doi: 10.1093/annonc/mdt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li CI, Daling JR, Tang MT, Malone KE. Relationship between diabetes and risk of second primary contralateral breast cancer. Breast Cancer Res Treat. 2011;125:545–551. doi: 10.1007/s10549-010-1035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 19.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soranna D, Scotti L, Zambon A, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. The oncologist. 2012;17:813–822. doi: 10.1634/theoncologist.2011-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chlebowski RT, McTiernan A, Wactawski-Wende J, et al. Diabetes, metformin, and breast cancer in postmenopausal women. J Clin Oncol. 2012;30:2844–2852. doi: 10.1200/JCO.2011.39.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aiello Bowles EJ, Boudreau DM, Chubak J, et al. Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. Journal of oncology practice / American Society of Clinical Oncology. 2012;8:e149–157. doi: 10.1200/JOP.2012.000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wirtz HS, Buist DS, Gralow JR, et al. Frequent antibiotic use and second breast cancer events. Cancer Epidemiol Biomarkers Prev. 2013;22:1588–1599. doi: 10.1158/1055-9965.EPI-13-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boudreau DM, Yu O, Chubak J, et al. Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res Treat. 2014;144:405–416. doi: 10.1007/s10549-014-2870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Group Health Breast Cancer Surveillance Registry. [Accessed June 15, 2011]; http://www.grouphealthresearch.org/surveillanceproject/
- 26.Saunders KW, Davis RL, Stergachis A Group Health Cooperative. In: Pharmacoepidemiology. Strom BL, editor. Chichester ; Hoboken, NJ: J. Wiley; 2005. pp. 223–239. [Google Scholar]
- 27.Washington State Department of Health, Center for Health Statistics. Death Data. [Accessed June 15, 2011]; http://www.doh.wa.gov/ehsphl/CHS/chs-data/death/deatmain.htm.
- 28.Bryson CL, Au DH, Young B, McDonell MB, Fihn SD. A refill adherence algorithm for multiple short intervals to estimate refill compliance (ReComp) Med Care. 2007;45:497–504. doi: 10.1097/MLR.0b013e3180329368. [DOI] [PubMed] [Google Scholar]
- 29.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574. doi: 10.1002/pds.1230. discussion 575-567. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes A. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 33.Richardson LC, Pollack LA. Therapy insight: Influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nature clinical practice Oncology. 2005;2:48–53. doi: 10.1038/ncponc0062. [DOI] [PubMed] [Google Scholar]
- 34.Hewitt ME, Ganz PA Institute of Medicine (U.S.), American Society of Clinical Oncology (U.S.) From cancer patient to cancer survivor : lost in transition : an American Society of Clinical Oncology and Institute of Medicine Symposium. Washington, D.C.: National Academies Press; 2006. [Google Scholar]
- 35.Snyder CF, Frick KD, Kantsiper ME, et al. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009;27:1054–1061. doi: 10.1200/JCO.2008.18.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 37.Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidemiol. 2003;157:1092–1100. doi: 10.1093/aje/kwg100. [DOI] [PubMed] [Google Scholar]
- 38.Lega IC, Austin PC, Gruneir A, Goodwin PJ, Rochon PA, Lipscombe LL. Association Between Metformin Therapy and Mortality After Breast Cancer: A Population-Based Study. Diabetes Care. 2013 doi: 10.2337/dc12-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peeters PJ, Bazelier MT, Vestergaard P, et al. Use of metformin and survival of diabetic women with breast cancer. Curr Drug Saf. 2013;8:357–363. doi: 10.2174/15680266113136660069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang ZJ, Li S. The prognostic value of metformin for cancer patients with concurrent diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2014 doi: 10.1111/dom.12267. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: a therapeutic opportunity in breast cancer. Clin Cancer Res. 2010;16:1695–1700. doi: 10.1158/1078-0432.CCR-09-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buist DS, LaCroix AZ, Brenneman SK, Abbott T., 3rd A population-based osteoporosis screening program: who does not participate, and what are the consequences? J Am Geriatr Soc. 2004;52:1130–1137. doi: 10.1111/j.1532-5415.2004.52311.x. [DOI] [PubMed] [Google Scholar]
- 43.Boudreau DM, Doescher MP, Jackson JE, Fishman PA, Saver BG. Impact of healthcare delivery system on where HMO-enrolled seniors purchase medications. Ann Pharmacother. 2004;38:1317–1318. doi: 10.1345/aph.1D569. [DOI] [PubMed] [Google Scholar]
- 44.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 45.Weiss NS. Estimating the impact of the discontinuation of medical interventions on health outcomes. Am J Epidemiol. 2009;169:653–656. doi: 10.1093/aje/kwn386. [DOI] [PubMed] [Google Scholar]
- 46.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 47.Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695–700. doi: 10.2337/diacare.24.4.695. [DOI] [PubMed] [Google Scholar]
- 48.McCulloch DK, Price MJ, Hindmarsh M, Wagner EH. A population-based approach to diabetes management in a primary care setting: early results and lessons learned. Eff Clin Pract. 1998;1:12–22. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.