Abstract
The orphan nuclear receptor TLX, also known as NR2E1, is an essential regulator of neural stem cell (NSC) self-renewal, maintenance, and neurogenesis. In vertebrates, TLX is specifically localized to the neurogenic regions of the forebrain and retina throughout development and adulthood. TLX regulates the expression of genes involved in multiple pathways, such as the cell cycle, DNA replication, and cell adhesion. These roles are primarily performed through the transcriptional repression or activation of downstream target genes. Emerging evidence suggests the misregulation of TLX might play a role in the onset and progression of human neurological disorders making this factor an ideal therapeutic target. Here, we review the current understanding of TLX function, expression, regulation, and activity significant to NSC maintenance, adult neurogenesis, and brain plasticity.
Keywords: TLX, Neurogenesis, Neural Stem Cell, NR2E1, Nuclear Receptor
INTRODUCTION
The orphan nuclear receptor subfamily 2 group E member 1 (NR2E1), commonly known as TLX, is an evolutionarily conserved member of the nuclear receptor superfamily of transcription factors found in both vertebrates and invertebrates [1]. An alignment of frog, mouse, chick, zebrafish and human TLX proteins reveals remarkable interspecies conservation with at least 89%–97% homology between the five species [2–6]. The vertebrate Tlx gene was first cloned nearly 20 years ago from a chick cDNA library screen using an RXRβ probe [3]. The following year, the mouse Tlx gene was cloned using the Drosophila tailless (tll) as the probe [4]. With a mouse model in hand, TLX was extensively characterized and implicated in the regulation of neurogenesis and the maintenance of neural stem cell (NSC) populations. TLX expression is specific to the neurogenic regions of the forebrain and retina during mouse development and adulthood [4]. The regulation of progenitor cell proliferation and timing of neurogenesis by TLX is necessary for the correct establishment of the pallio-subpallial boundary, ventral pallial identity, and superficial cortical layers during mouse forebrain development. Further, TLX regulates retinal stem cell proliferation and cell cycle re-entry during retinogenesis by directly controlling the expression of the tumor suppressor Pten.
Spatiotemporal expression of Tlx
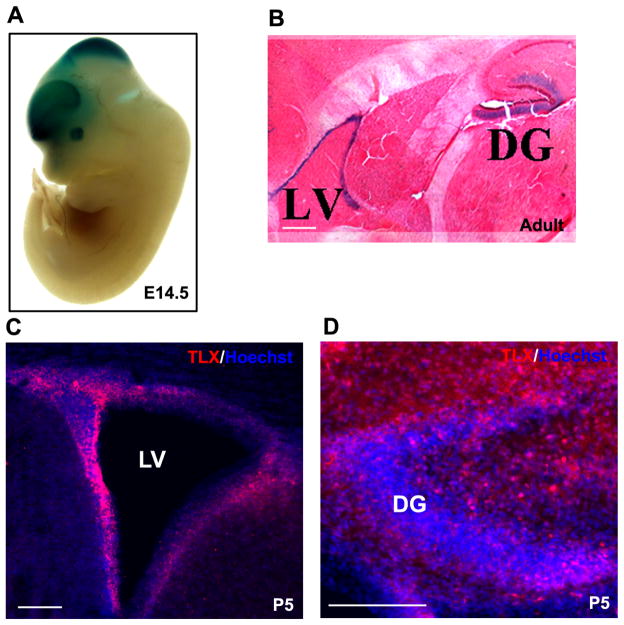
Expression of Tlx is specific to the developing forebrain and retina in multiple species, including the frog [2], zebrafish [6], and mouse [4]. In the developing embryo and adult mouse, Tlx is specifically localized to the neurogenic regions of the telencephalon, diencephalon, nasal placode, and retina (Figure 1A, B, C, D) [3, 4]. This Tlx expression becomes detectable at embryonic day 8 (E8), peaks at E13.5, and declines from E13.5 until birth. Tlx expression then gradually increases with high levels detectable in the adult brain [4]. Immunohistochemical staining with a TLX-specific antibody reveals a sparse distribution of TLX throughout the cortex, strong yet dispersed expression in the subgranular zone (SGZ) of the dentate gyrus (DG), and clustered expression in the subventricular zone (SVZ) of lateral ventricle (LV; Figure 1C, D) [7]. The NSCs found in the SVZ are a rare population of relatively quiescent cells [8]. Recently, TLX expression has been shown in the rapidly dividing neural progenitor cells of the adult SVZ, although most TLX-positive cells in the SVZ are quiescent [9].
Figure 1. Spatiotemporal TLX expression.
A, B. Expression of the lacZ marker targeted to the endogenous locus of Tlx in the embryonic (A) and adult (B) mouse. C, D. TLX-specific immunostaining of postnatal day 5 (P5) mouse brain sections reveals TLX expression in the subventricular zone (SVZ) of the lateral ventricle (LV) and the subgranular zone (SGZ) of the dentate gyrus (DG). Scale bars: 20 μm (B,C,) and 10 μm (D).
Biological roles for TLX
Brain formation and behavior
The brains of Tlx-null mice exhibit no obvious defects during early development; however, mature mice suffer retinopathies, limbic defects, reduced copulation, and progressively violent behavior [10, 11]. Adult Tlx-mutant brains also show severe deficits in active neurogenic regions such as reduced hippocampal DG size, significantly expanded lateral ventricles, and smaller olfactory bulbs [12, 13]. The mutation of mouse Tlx results in hyperactivity and extremely aggressive behavior suggesting a potential role for TLX in human neurological disorders [14]. In support of this hypothesis, mutations in the regulatory regions of the human Tlx gene have been positively correlated with bipolar disorder. Similarly, several mutations in the transcriptional regulatory elements of Tlx are strongly associated with microcephaly.
Eye development
Tlx can be detected in the optic processes of the developing mouse eye as early as E9 [4]. By E11.5, expression is restricted to the innermost retinal surface corresponding to the end feet of retinal progenitor cells found in the neuroblast layer of the developing embryo [15]. Peak Tlx expression in this layer is observed at E15.5 supporting its role in early retinogenesis [15, 16]. Tlx-null mice exhibit retinal and optic nerve dystrophy resulting in blindness [11, 16, 17]. A developing Tlx-null mouse exhibits deregulated retinal progenitor cell proliferation and increased apoptosis in the ganglion cell layer. This results in a marked reduction of thickness for each distinct layer of the adult retina [15, 17]. Additionally, Tlx-null animals suffer from retinal vasculature defects illustrating the critical role for this gene in the assembly of fibronectin matrices secreted by proangiogenic astrocytes [18].
NSC self-renewal
The primary function of TLX in the developing and adult brain is to prevent the precocious differentiation of NSCs [7, 12, 19]. This key transcriptional regulator controls the expression of a broad network of genes to maintain NSC pools in an undifferentiated, self-renewing state [12, 20, 21]. These undifferentiated precursor cells, with the capacity to give rise to both neuronal and glial lineages, are the driving force behind the formation of a complete and functional central nervous system (CNS) [22–25].
Tlx-null neural cells isolated from adult Tlx-mutant mice are non-proliferative and fail to self-renew. Importantly, the ectopic expression of Tlx in these cells rescues their ability to proliferate and self-renew [12]. Inducible lineage tracing and genetic markers for adult NSCs have demonstrated that Tlx-expressing cells can generate both quiescent and activated postnatal NSCs [20]. TLX plays an essential role in NSC activation and governs the localization of NSCs to the neurogenic niche [20]. Furthermore, whole-genome RNA-sequencing revealed that TLX coordinates multiple signaling pathways to regulate NSC behavior [20]. Specifically, TLX modulates signaling in the p53 pathway to control NSC activation.
Adult Neurogenesis
NSCs are found in at least two discrete regions of the adult CNS, the SGZ and SVZ. It is here that multipotent neural stem/progenitor cells generate new neurons, a fundamental process known as neurogenesis. This process occurs throughout the developing embryonic brain and localizes to these distinct regions during adulthood. In most mammals, new neurons are continually generated throughout life. As a central regulator of adult hippocampal neurogenesis, TLX balances the maintenance of NSC populations with terminal neuronal differentiation [12, 26].
Recent studies show that activated NSCs and transit-amplifying progenitors (TAPs) in the neonatal subependymal zone of the LV express TLX [27]. TLX-positive cells in the adult SVZ are relatively quiescent stem cells and the inactivation of TLX in these cells leads to a complete loss of neurogenesis in the SVZ [9, 28, 29]. TLX positively and negatively modulates gene expression in both cell types. The absence of Tlx in activated NSCs, but not TAPs, leads to the upregulation of negative regulators of the cell cycle and proliferation arrest. Moreover, the homeobox transcription factor DLX2, which promotes neurogenesis, is downregulated in both cell types in the absence of Tlx. This is paralleled by increased OLIG2 expression in activated NSCs and heightened GFAP expression in TAPs, indicating that TLX decreases gliogenesis in both populations [27].
Unlike the TLX-positive NSCs of the SVZ, NSCs in the DG produce neurons with unique roles in hippocampal-dependent learning and memory [30]. The conditional disruption of Tlx in the mouse has been shown to reduce cognitive aptitude and incite abnormal behavior [30]. This might suggest that the status of adult neural progenitors in the SGZ and aberrations in neurogenesis are forerunners to neuropsychiatric diseases such as dementia, mood disorders, and cognitive deficits [31]. TLX-positive NSCs of the adult SGZ (type 1 NSCs) have long radial glia-like processes spanning the entire granule cell layer. These cells express nestin, GFAP, SOX2, and brain lipid-binding protein (BLBP). Although the majority of type 1 NSCs remain in an inactive state, a subset divides slowly to yield transiently amplifying type 2 cells. These type 2 cells are tangentially oriented with short processes and rapidly proliferate to generate type 3 cells. These cells resemble immature DCX+ neuroblasts and ultimately mature into granule neurons that functionally integrate into the existing neural networks. Deletion of Tlx in the DG results in complete loss of transiently amplifying cells and neuroblasts.
Mechanism of action
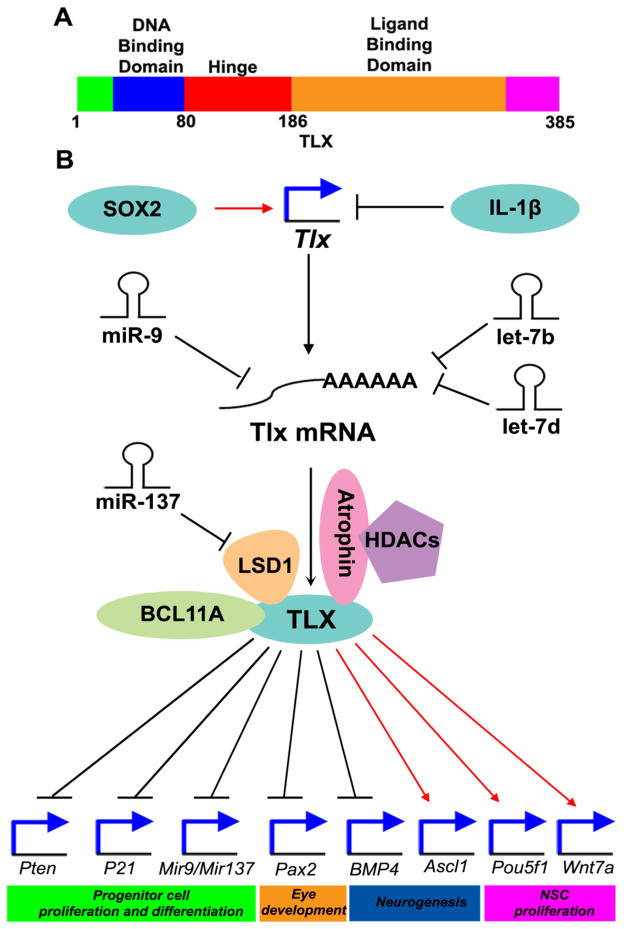
Like most members of the nuclear receptor superfamily, TLX has at least two structural domains. The first is a highly conserved DNA-binding domain (DBD) required for targeting a consensus DNA motif - AAGTCA. The second is a moderately conserved ligand-binding domain (LBD) critical to cofactor interactions (Figure 2A) [3, 32]. Despite its conservation, no ligand has yet been identified for TLX, hence this receptor is classified as an orphan nuclear receptor.
Figure 2. The TLX-associated regulatory pathway.
A. A schematic diagram depicting the basic structure of TLX. DBD, DNA-binding domain; LBD, ligand-binding domain. B. A TLX-centric overview of gene expression, protein interaction, target regulation, and biological functions.
TLX has been shown to function primarily through the transcriptional repression of downstream target genes by complexing with transcriptional corepressors like the epigenetic modifier lysine-specific histone demethylase 1 (LSD1) [33–36]. TLX also recruits histone deacetylases (HDACs) to target genes, which repress transcription and, in turn, regulate NSC proliferation [33]. TLX was shown to recruit both HDAC3 and HDAC5 to target gene promoters in cultured NSCs (Figure 2B) [33].
Recently, yeast two-hybrid screens of cDNA libraries prepared from adult human brain tissues were used to identify and characterize novel proteins that interact with TLX [37]. The physical interaction of TLX and atrophin-1 (ATN1), a member of a newly identified class of nuclear receptor corepressors, earlier reported by Zhang et al. and Wang et al. was confirmed in this study (Figure 2B) [16, 38–42]. The direct association of TLX and ATN1 has been shown to prevent retinal dystrophy, a condition of visual impairment typically observed in developing Tlx-null mice [16]. In addition to ATN1, the screen by Estruch et al. identified B-cell lymphoma/leukemia 11A/CTIP1 (BCL11A), an oncoprotein and transcription factor, as a novel TLX regulator [37]. This selective interaction with TLX relies on two copies of the BCL11A signature motif F/YSXXLXXL/Y [43].
With respect to intercellular signaling, bone morphogenetic protein 7 (BMP7) and Sonic Hedgehog (SHH) have been shown to relieve TLX-mediated PAX2 repression [44]. SMAD1 of the BMP7 pathway and GLI2 of the SHH pathway are suspected of binding TLX to modulate PAX2 expression.
TLX downstream targets
TLX has diverse roles in gene regulation that affect a broad range of cellular processes from the cell cycle and DNA replication to mitogen-activated protein kinase signaling and even cell adhesion [20, 29, 30, 33]. TLX binds a highly conserved 5′-AAGTCA-3′ motif to promote or repress target gene expression [16]. The TLX targets Ascl1, Pou5f1, Pax2, Mir9, Mir137, Pten, Cdkn1a, Sirt1, and Wnt7a were each identified through DNA binding assays or conserved motif analyses [11, 12, 16, 26, 27, 33, 35, 45–49].
Transcription factors: ASCL1, POU5F1, and PAX2
Proneural basic helix-loop-helix (bHLH) transcription factors are integral to the initiation of neurogenesis and terminal neuronal specification. ASCL1 (MASH1), an early proneural bHLH transcription factor broadly expressed in brain and spinal cord progenitors, drives the specification of neurons and oligodendrocytes in multiple neurogenic brain regions like the hippocampus [50–52]. Recently, TLX was shown to target and activate Ascl1 thereby promoting neuronal induction in adult hippocampal neuroprogenitors [26].
Interestingly, TLX balances dichotomous roles under hypoxic conditions, the regulation of NSC commitment to the neuronal lineage by Ascl1 and the maintenance of proliferating neural progenitor pools through the fine-tuning of Pou5f1 (Oct3/4) expression [26, 27, 45]. In this second role, TLX serves as a critical mediator of hypoxia-induced NSC proliferation and neural progenitor pluripotency. Early hypoxia-induced Tlx expression potentiates neural progenitor proliferation and imparts a stem cell-like phenotype exhibiting NSC markers under differentiation conditions [45]. TLX is recruited to the Pou5f1 proximal promoter under hypoxic conditions to augment transcription and, in turn, promote proliferation and progenitor pluripotency. Pou5f1 knockdown significantly reduces TLX-mediated NSC proliferation. This highlights the TLX-POU5F1 interdependent relationship required for maintenance of the progenitor cell reservoir [45].
Another downstream target of TLX, Pax2, was shown to transiently overlap with Tlx expression in the developing chick optic vesicle. TLX represses Pax2 transcription by binding a conserved 5′-AAGTCA-3′ motif approximately 80 nucleotides upstream of the TATA box [11, 15].
microRNAs: miR-137 and miR-9
Recent studies have uncovered multiple brain-specific microRNAs (miRNAs) with roles in neuronal differentiation and maturation [53, 54]. The brain-specific miR-137 has been shown to promote the neuronal differentiation of adult subventricular NSCs and to inhibit the maturation of adult hippocampal neurons [55, 56]. Mir137 is a bonafide TLX target and a novel upstream regulator of LSD1. TLX represses Mir137 in NSCs by recruiting LSD1 to the genomic regions of Mir137 [34]. Mir9, another direct TLX target, forms a negative-feedback loop regulating TLX expression in NSCs to affect the status of progenitor proliferation and differentiation [57].
PTEN
The tumor suppressor gene Pten was identified as a TLX target during a global gene expression-profiling study [16]. The tight regulation of stem cell proliferation by TLX was demonstrated in mice with the individual deletion of Cdkn1a (P21, Cip1), Tp53 (P53), and Pten [58]. PTEN has a fundamental role in brain development and mutations can result in multiple forms of human cancer [59–62]. Through negative regulation of the G0 to G1 cell cycle transition, TLX precisely balances progenitor cell proliferation and differentiation via the PTEN-cyclin D1 pathway [16, 63]. As a negative regulator of NSC proliferation, Pten is repressed by TLX in both the developing retina and the adult brain [16, 28, 33, 60, 63]. TLX is thought to regulate NSC proliferation by governing expression of the Cip/Kip family cyclin-dependent kinase inhibitors such as Cdkn1a (P21), Cdkn1c (P57, Kip2) and several genes downstream of Tp53 (P53) [12, 16, 20, 28–30]. The observation that p21 and p57 are expressed in differentiating neural precursor cells (NPCs) supports this molecular paradigm [64].
SIRT1
SIRT1, a NAD-dependent protein deacetylase, has been shown to co-localize with TLX in neural precursor cells [49]. In HEK293 cells, TLX increases Sirt1 expression by binding to the TLX-activating element in the Sirt1 promoter. Moreover, Tlx knockdown with small interfering RNAs diminishes SIRT1 protein expression in NPCs [49].
WNT7A
Wnt/β-catenin signaling influences the self-renewal of multiple stem cell types, including hematopoietic stem cells, epidermal progenitors, and adult brain NSCs [65–69]. TLX activates this pathway in the adult mouse to stimulate NSC proliferation [48]. A gene profiling analysis of RNAs isolated from the brains of adult wild-type and Tlx mutant mice identified Wnt7a as a direct target of TLX [48]. The promoter region of Wnt7a contains multiple consensus TLX binding sites and cell-based reporter assays indicate that TLX interacts with this regulatory region [48].
BMP-SMAD signaling pathway
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor beta (TGFβ) superfamily that signals through the phosphorylation of SMAD family transcription factors. BMPs play a vital role in NSC neurogenesis and astrogliogenesis [70]. It was recently shown that TLX controls the timing of postnatal astrogenesis by modulating the BMP-SMAD signaling pathway [71]. TLX directly binds to the enhancer region of Bmp4 to suppress its expression in the NSCs and Bmp4 is markedly upregulated in nestin-positive cells from Tlx−/− mice [71].
TLX regulation
While the fundamental roles of TLX in NSC self-renewal and neurogenesis are well established, relatively little is known about the molecular mechanisms that regulate the spatiotemporal expression and activity of TLX (Figure 2B). Mounting evidence points to both transcriptional and post-transcriptional mechanisms of regulation. miRNAs post-transcriptionally regulate a diverse number of neurogenic genes, generally negatively, by binding the 3′ untranslated region of target mRNAs [72–76]. The overexpression of miR-9, a highly expressed miRNA in the vertebrate CNS, such as zebrafish embryo, mouse embryonic cortex, and chick spinal cord, reduces the proliferation of neural progenitors [57, 77, 78]. Interestingly, this reduction can be rescued by TLX overexpression suggesting that TLX and miR-9 might participate in a negative feedback loop [57]. Additional molecular evidence for this claim is found in the Mir9 locus where consensus TLX binding sites have been identified. Moreover, cells derived from adult Tlx-null mice exhibit increased expression of pre-miR-9 transcripts [57].
In an attempt to shed light on the dynamic multidimensional regulation of TLX expression and activity, Zhao et al. recently demonstrated that the miRNA let-7d modulates TLX expression through a conserved binding site in the 3′ untranslated region of Tlx mRNA transcripts [79]. The overexpression of let-7d inhibits NSC proliferation, promotes neuronal differentiation, and induces neuronal migration in embryonic mouse brains, a phenotype similar to TLX knockdown models. This phenotype can be rescued by the in vivo co-electroporation of a truncated Tlx transcript lacking a 3′ untranslated region [79]. Prior to this work on let-7d, let-7b and miR-137 were also highlighted as potential regulators of NSC proliferation [34, 80]. Remarkably, miR-137 targets the TLX transcriptional corepressor LSD1, which itself is recruited by TLX to the regulatory regions of Mir137 to downregulate its expression.
Interleukin-1 beta (IL-1β), a negative regulator of embryonic and adult hippocampal neurogenesis, represses TLX expression in NPCs. IL-1β also acts to repress TLX in differentiating new-born neurons, mature neurons, and astrocytes [81–83]. Recently, it has been demonstrated that this repression of TLX and hippocampal NPC proliferation is mediated through the interleukin-1 receptor type I [84].
Sex determining region Y-box 2 (SOX2), another transcription factor with prominent roles in the regulation of adult NSC proliferation, was recently shown to bind the upstream regulatory region of Tlx and activate its promoter activity in adult mouse NSCs [85–88]. SOX2 knockdown in cultured adult mouse NSCs moderately reduces TLX expression indicating multiple independent factors are involved in TLX regulation [88].
TLX as a therapeutic target
Bipolar disorder is a highly heritable multifactorial psychiatric disorder linked to abnormalities in the 6q21–22 chromosomal locus [89–95]. Interestingly, Tlx is located within this region and has been genetically linked to bipolar I disorder. Several strains of Tlx-null mice exhibit neuroanatomical abnormalities similar to those observed in human bipolar patients. These include diminished neurogenesis, dysfunction of GABAergic interneuron, olfactory dysfunction, enlarged lateral ventricles, and reductions in the size of the hippocampus, cerebral cortex, corpus callosum, amygdala, and cortical layers II/III [10, 12, 17, 19, 30, 96–103].
As a potent regulator of stem cell proliferation, TLX has been implicated in gliomagenesis. Gene expression profiling has shown that various types of human brain tumors, such as astrocytoma and ependymomas, exhibit elevated Tlx expression [104–109]. Similarly, a fraction of primary glioblastoma patients exhibit increased Tlx copy number [29]. The ectopic expression of Tlx, in combination with p53 or Ink4a/arf inactivation, is sufficient to induce gliomagenesis in the mouse [29, 110]. Genetic analyses further revealed that Tlx deletion significantly impedes this glioma formation within the adult neurogenic niches, while the potential for gliomagenesis in other brain regions remains unaffected [58]. Interestingly, the direct TLX target and tumor suppressor Pten is often mutated in cases of malignant glioma [16, 28]. These findings suggest that the misregulation of TLX expression or activity can promote the initiation of gliomagenesis in the SVZ.
Conclusion
TLX is a master regulator of adult NSC behavior. A detailed, mechanistic understanding of TLX activity and regulation will provide significant insights into NSC maintenance, adult neurogenesis, and brain plasticity. Its essential role in postnatal neurogenesis makes TLX an attractive candidate for further studies into the relationship of adult neurogenesis and tumorigenesis. With fundamental roles in NSC maintenance and neurogenesis timing, TLX holds promise as an ideal therapeutic target for glioblastoma, neurological injury, and neurodegenerative diseases.
Highlights for Review.
A thorough review of current literatures on TLX in the central nervous system.
TLX is an orphan nuclear receptor controlling gene expression.
TLX plays a critical role in neural stem cells and neurogenesis.
TLX is regulated at both the transcriptional and posttranscriptional level.
Dysregulation of TLX might lead to neurological diseases.
Acknowledgments
We would like to thank Derek K. Smith for critical reading and helpful comments on the manuscript. This work was supported by the Whitehall Foundation (2009-12-05), the Welch Foundation (I-1724), the Ellison Medical Foundation (AG-NS-0753-11), the American Heart Association (09SDG2260602), and the NIH grants (1DP2OD006484 and R01NS070981; to C.-L.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollemann T, Bellefroid E, Pieler T. The Xenopus homologue of the Drosophila gene tailless has a function in early eye development. Development. 1998;125:2425–2432. doi: 10.1242/dev.125.13.2425. [DOI] [PubMed] [Google Scholar]
- 3.Yu RT, McKeown M, Evans RM, Umesono K. Relationship between Drosophila gap gene tailless and a vertebrate nuclear receptor Tlx. Nature. 1994;370:375–379. doi: 10.1038/370375a0. [DOI] [PubMed] [Google Scholar]
- 4.Monaghan AP, Grau E, Bock D, Schutz G. The mouse homolog of the orphan nuclear receptor tailless is expressed in the developing forebrain. Development. 1995;121:839–853. doi: 10.1242/dev.121.3.839. [DOI] [PubMed] [Google Scholar]
- 5.Jackson A, Panayiotidis P, Foroni L. The human homologue of the Drosophila tailless gene (TLX): characterization and mapping to a region of common deletion in human lymphoid leukemia on chromosome 6q21. Genomics. 1998;50:34–43. doi: 10.1006/geno.1998.5270. [DOI] [PubMed] [Google Scholar]
- 6.Kitambi SS, Hauptmann G. The zebrafish orphan nuclear receptor genes nr2e1 and nr2e3 are expressed in developing eye and forebrain. Gene Expr Patterns. 2007;7:521–528. doi: 10.1016/j.modgep.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Sun G, Yang S, Qu Q, Nakashima K, Shi Y. Nuclear receptor TLX regulates cell cycle progression in neural stem cells of the developing brain. Mol Endocrinol. 2008;22:56–64. doi: 10.1210/me.2007-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Sun G, Murai K, Ye P, Shi Y. Characterization of TLX expression in neural stem cells and progenitor cells in adult brains. PLoS One. 2012;7:e43324. doi: 10.1371/journal.pone.0043324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monaghan AP, Bock D, Gass P, Schwager A, Wolfer DP, Lipp HP, Schutz G. Defective limbic system in mice lacking the tailless gene. Nature. 1997;390:515–517. doi: 10.1038/37364. [DOI] [PubMed] [Google Scholar]
- 11.Yu RT, Chiang MY, Tanabe T, Kobayashi M, Yasuda K, Evans RM, Umesono K. The orphan nuclear receptor Tlx regulates Pax2 and is essential for vision. Proc Natl Acad Sci U S A. 2000;97:2621–2625. doi: 10.1073/pnas.050566897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 13.Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juarez P, Valdovinos MG, May ME, Lloyd BP, Couppis MH, Kennedy CH. Serotonin(2)A/C receptors mediate the aggressive phenotype of TLX gene knockout mice. Behav Brain Res. 2013;256:354–361. doi: 10.1016/j.bbr.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 15.Miyawaki T, Uemura A, Dezawa M, Yu RT, Ide C, Nishikawa S, Honda Y, Tanabe Y, Tanabe T. Tlx, an orphan nuclear receptor, regulates cell numbers and astrocyte development in the developing retina. J Neurosci. 2004;24:8124–8134. doi: 10.1523/JNEUROSCI.2235-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang CL, Zou Y, Yu RT, Gage FH, Evans RM. Nuclear receptor TLX prevents retinal dystrophy and recruits the corepressor atrophin1. Genes Dev. 2006;20:1308–1320. doi: 10.1101/gad.1413606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young KA, Berry ML, Mahaffey CL, Saionz JR, Hawes NL, Chang B, Zheng QY, Smith RS, Bronson RT, Nelson RJ, Simpson EM. Fierce: a new mouse deletion of Nr2e1; violent behaviour and ocular abnormalities are background-dependent. Behav Brain Res. 2002;132:145–158. doi: 10.1016/s0166-4328(01)00413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uemura A, Kusuhara S, Wiegand SJ, Yu RT, Nishikawa S. Tlx acts as a proangiogenic switch by regulating extracellular assembly of fibronectin matrices in retinal astrocytes. J Clin Invest. 2006;116:369–377. doi: 10.1172/JCI25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy K, Kuznicki K, Wu Q, Sun Z, Bock D, Schutz G, Vranich N, Monaghan AP. The Tlx gene regulates the timing of neurogenesis in the cortex. J Neurosci. 2004;24:8333–8345. doi: 10.1523/JNEUROSCI.1148-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niu W, Zou Y, Shen C, Zhang CL. Activation of postnatal neural stem cells requires nuclear receptor TLX. J Neurosci. 2011;31:13816–13828. doi: 10.1523/JNEUROSCI.1038-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y, Sun G, Zhao C, Stewart R. Neural stem cell self-renewal. Critical reviews in oncology/hematology. 2008;65:43–53. doi: 10.1016/j.critrevonc.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Buylla A, Temple S. Stem cells in the developing and adult nervous system. J Neurobiol. 1998;36:105–110. [PubMed] [Google Scholar]
- 24.Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Weiss S, van der Kooy D. CNS stem cells: where’s the biology (a.k.a. beef)? J Neurobiol. 1998;36:307–314. doi: 10.1002/(sici)1097-4695(199808)36:2<307::aid-neu14>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 26.Elmi M, Matsumoto Y, Zeng ZJ, Lakshminarasimhan P, Yang W, Uemura A, Nishikawa S, Moshiri A, Tajima N, Agren H, Funa K. TLX activates MASH1 for induction of neuronal lineage commitment of adult hippocampal neuroprogenitors. Mol Cell Neurosci. 2010;45:121–131. doi: 10.1016/j.mcn.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Obernier K, Simeonova I, Fila T, Mandl C, Holzl-Wenig G, Monaghan-Nichols P, Ciccolini F. Expression of Tlx in both stem cells and transit amplifying progenitors regulates stem cell activation and differentiation in the neonatal lateral subependymal zone. Stem Cells. 2011;29:1415–1426. doi: 10.1002/stem.682. [DOI] [PubMed] [Google Scholar]
- 28.Liu HK, Belz T, Bock D, Takacs A, Wu H, Lichter P, Chai M, Schutz G. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 2008;22:2473–2478. doi: 10.1101/gad.479308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu HK, Wang Y, Belz T, Bock D, Takacs A, Radlwimmer B, Barbus S, Reifenberger G, Lichter P, Schutz G. The nuclear receptor tailless induces long-term neural stem cell expansion and brain tumor initiation. Genes Dev. 2010;24:683–695. doi: 10.1101/gad.560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 31.Kempermann G, Krebs J, Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Current opinion in psychiatry. 2008;21:290–295. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- 32.Bain DL, Heneghan AF, Connaghan-Jones KD, Miura MT. Nuclear receptor structure: implications for function. Annual review of physiology. 2007;69:201–220. doi: 10.1146/annurev.physiol.69.031905.160308. [DOI] [PubMed] [Google Scholar]
- 33.Sun G, Yu RT, Evans RM, Shi Y. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc Natl Acad Sci U S A. 2007;104:15282–15287. doi: 10.1073/pnas.0704089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun G, Ye P, Murai K, Lang MF, Li S, Zhang H, Li W, Fu C, Yin J, Wang A, Ma X, Shi Y. miR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat Commun. 2011;2:529. doi: 10.1038/ncomms1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun G, Alzayady K, Stewart R, Ye P, Yang S, Li W, Shi Y. Histone demethylase LSD1 regulates neural stem cell proliferation. Mol Cell Biol. 2010;30:1997–2005. doi: 10.1128/MCB.01116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama A, Takezawa S, Schule R, Kitagawa H, Kato S. Transrepressive function of TLX requires the histone demethylase LSD1. Mol Cell Biol. 2008;28:3995–4003. doi: 10.1128/MCB.02030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estruch SB, Buzon V, Carbo LR, Schorova L, Luders J, Estebanez-Perpina E. The oncoprotein BCL11A binds to orphan nuclear receptor TLX and potentiates its transrepressive function. PLoS One. 2012;7:e37963. doi: 10.1371/journal.pone.0037963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Rajan H, Pitman JL, McKeown M, Tsai CC. Histone deacetylase-associating Atrophin proteins are nuclear receptor corepressors. Genes Dev. 2006;20:525–530. doi: 10.1101/gad.1393506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S, Xu L, Lee J, Xu T. Drosophila atrophin homolog functions as a transcriptional corepressor in multiple developmental processes. Cell. 2002;108:45–56. doi: 10.1016/s0092-8674(01)00630-4. [DOI] [PubMed] [Google Scholar]
- 40.Shen Y, Lee G, Choe Y, Zoltewicz JS, Peterson AS. Functional architecture of atrophins. J Biol Chem. 2007;282:5037–5044. doi: 10.1074/jbc.M610274200. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Tsai CC. Atrophin proteins: an overview of a new class of nuclear receptor corepressors. Nucl Recept Signal. 2008;6:e009. doi: 10.1621/nrs.06009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen Y, Peterson AS. Atrophins’ emerging roles in development and neurodegenerative disease. Cell Mol Life Sci. 2009;66:437–446. doi: 10.1007/s00018-008-8403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan CM, Fulton J, Montiel-Duarte C, Collins HM, Bharti N, Wadelin FR, Moran PM, Mongan NP, Heery DM. A signature motif mediating selective interactions of BCL11A with the NR2E/F subfamily of orphan nuclear receptors. Nucleic Acids Res. 2013;41:9663–9679. doi: 10.1093/nar/gkt761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sehgal R, Sheibani N, Rhodes SJ, Belecky Adams TL. BMP7 and SHH regulate Pax2 in mouse retinal astrocytes by relieving TLX repression. Dev Biol. 2009;332:429–443. doi: 10.1016/j.ydbio.2009.05.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chavali PL, Saini RK, Matsumoto Y, Agren H, Funa K. Nuclear orphan receptor TLX induces Oct-3/4 for the survival and maintenance of adult hippocampal progenitors upon hypoxia. J Biol Chem. 2011;286:9393–9404. doi: 10.1074/jbc.M110.167445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Liu HK, Schutz G. Role of the nuclear receptor Tailless in adult neural stem cells. Mech Dev. 2013;130:388–390. doi: 10.1016/j.mod.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Gui H, Li ML, Tsai CC. A tale of tailless. Dev Neurosci. 2011;33:1–13. doi: 10.1159/000321585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qu Q, Sun G, Li W, Yang S, Ye P, Zhao C, Yu RT, Gage FH, Evans RM, Shi Y. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol. 2010;12:31–40. doi: 10.1038/ncb2001. sup 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwahara N, Hisahara S, Hayashi T, Horio Y. Transcriptional activation of NAD+-dependent protein deacetylase SIRT1 by nuclear receptor TLX. Biochem Biophys Res Commun. 2009;386:671–675. doi: 10.1016/j.bbrc.2009.06.103. [DOI] [PubMed] [Google Scholar]
- 50.Parras CM, Galli R, Britz O, Soares S, Galichet C, Battiste J, Johnson JE, Nakafuku M, Vescovi A, Guillemot F. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guillemot F, Joyner AL. Dynamic expression of the murine Achaete-Scute homologue Mash-1 in the developing nervous system. Mech Dev. 1993;42:171–185. doi: 10.1016/0925-4773(93)90006-j. [DOI] [PubMed] [Google Scholar]
- 52.Kim EJ, Leung CT, Reed RR, Johnson JE. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci. 2007;27:12764–12774. doi: 10.1523/JNEUROSCI.3178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 54.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 55.Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello JF, Bergers G, Weiss WA, Alvarez-Buylla A, Hodgson JG. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smrt RD, Szulwach KE, Pfeiffer RL, Li X, Guo W, Pathania M, Teng ZQ, Luo Y, Peng J, Bordey A, Jin P, Zhao X. MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells. 2010;28:1060–1070. doi: 10.1002/stem.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nature structural & molecular biology. 2009;16:365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zou Y, Niu W, Qin S, Downes M, Burns DK, Zhang CL. The nuclear receptor TLX is required for gliomagenesis within the adult neurogenic niche. Mol Cell Biol. 2012;32:4811–4820. doi: 10.1128/MCB.01122-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO, Mak TW. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001;29:396–403. doi: 10.1038/ng782. [DOI] [PubMed] [Google Scholar]
- 60.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 61.Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet. 2001;29:404–411. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- 62.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 63.Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, Geschwind DH, Liu X, Kornblum HI, Wu H. PTEN negatively regulates neural stem cell self-renewal by modulating G0–G1 cell cycle entry. Proc Natl Acad Sci U S A. 2006;103:111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gui H, Li S, Matise MP. A cell-autonomous requirement for Cip/Kip cyclin-kinase inhibitors in regulating neuronal cell cycle exit but not differentiation in the developing spinal cord. Dev Biol. 2007;301:14–26. doi: 10.1016/j.ydbio.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, Okano H, Sawamoto K. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 66.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 67.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 68.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 69.Zhu AJ, Watt FM. beta-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development. 1999;126:2285–2298. doi: 10.1242/dev.126.10.2285. [DOI] [PubMed] [Google Scholar]
- 70.Bond AM, Bhalala OG, Kessler JA. The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Dev Neurobiol. 2012;72:1068–1084. doi: 10.1002/dneu.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin S, Niu W, Iqbal N, Smith DK, Zhang C-L. Orphan nuclear receptor TLX regulates astrogenesis by modulating BMP signaling. Front Neurosci. 2014;8:74. doi: 10.3389/fnins.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi Y, Zhao X, Hsieh J, Wichterle H, Impey S, Banerjee S, Neveu P, Kosik KS. MicroRNA regulation of neural stem cells and neurogenesis. J Neurosci. 2010;30:14931–14936. doi: 10.1523/JNEUROSCI.4280-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lang MF, Shi Y. Dynamic Roles of microRNAs in Neurogenesis. Frontiers in neuroscience. 2012;6:71. doi: 10.3389/fnins.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Asuelime GE, Shi Y. The little molecules that could: a story about microRNAs in neural stem cells and neurogenesis. Frontiers in neuroscience. 2012;6:176. doi: 10.3389/fnins.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shibata M, Nakao H, Kiyonari H, Abe T, Aizawa S. MicroRNA-9 regulates neurogenesis in mouse telencephalon by targeting multiple transcription factors. J Neurosci. 2011;31:3407–3422. doi: 10.1523/JNEUROSCI.5085-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leucht C, Stigloher C, Wizenmann A, Klafke R, Folchert A, Bally-Cuif L. MicroRNA-9 directs late organizer activity of the midbrain-hindbrain boundary. Nat Neurosci. 2008;11:641–648. doi: 10.1038/nn.2115. [DOI] [PubMed] [Google Scholar]
- 78.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao C, Sun G, Ye P, Li S, Shi Y. MicroRNA let-7d regulates the TLX/microRNA-9 cascade to control neural cell fate and neurogenesis. Scientific reports. 2013;3:1329. doi: 10.1038/srep01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao C, Sun G, Li S, Lang MF, Yang S, Li W, Shi Y. MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc Natl Acad Sci U S A. 2010;107:1876–1881. doi: 10.1073/pnas.0908750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Green HF, Treacy E, Keohane AK, Sullivan AM, O’Keeffe GW, Nolan YM. A role for interleukin-1beta in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci. 2012;49:311–321. doi: 10.1016/j.mcn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 82.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Green HF, Nolan YM. Unlocking mechanisms in interleukin-1beta-induced changes in hippocampal neurogenesis--a role for GSK-3beta and TLX. Translational psychiatry. 2012;2:e194. doi: 10.1038/tp.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryan SM, O’Keeffe GW, O’Connor C, Keeshan K, Nolan YM. Negative regulation of TLX by IL-1beta correlates with an inhibition of adult hippocampal neural precursor cell proliferation. Brain, behavior, and immunity. 2013;33:7–13. doi: 10.1016/j.bbi.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 85.Catena R, Tiveron C, Ronchi A, Porta S, Ferri A, Tatangelo L, Cavallaro M, Favaro R, Ottolenghi S, Reinbold R, Scholer H, Nicolis SK. Conserved POU binding DNA sites in the Sox2 upstream enhancer regulate gene expression in embryonic and neural stem cells. J Biol Chem. 2004;279:41846–41857. doi: 10.1074/jbc.M405514200. [DOI] [PubMed] [Google Scholar]
- 86.Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell stem cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pevny LH, Nicolis SK. Sox2 roles in neural stem cells. Int J Biochem Cell Biol. 2010;42:421–424. doi: 10.1016/j.biocel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 88.Shimozaki K, Zhang CL, Suh H, Denli AM, Evans RM, Gage FH. SRY-box-containing gene 2 regulation of nuclear receptor tailless (Tlx) transcription in adult neural stem cells. J Biol Chem. 2012;287:5969–5978. doi: 10.1074/jbc.M111.290403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dick DM, Foroud T, Flury L, Bowman ES, Miller MJ, Rau NL, Moe PR, Samavedy N, El-Mallakh R, Manji H, Glitz DA, Meyer ET, Smiley C, Hahn R, Widmark C, McKinney R, Sutton L, Ballas C, Grice D, Berrettini W, Byerley W, Coryell W, DePaulo R, MacKinnon DF, Gershon ES, Kelsoe JR, McMahon FJ, McInnis M, Murphy DL, Reich T, Scheftner W, Nurnberger JI., Jr Genomewide linkage analyses of bipolar disorder: a new sample of 250 pedigrees from the National Institute of Mental Health Genetics Initiative. American journal of human genetics. 2003;73:107–114. doi: 10.1086/376562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hayden EP, Nurnberger JI., Jr Molecular genetics of bipolar disorder. Genes, brain, and behavior. 2006;5:85–95. doi: 10.1111/j.1601-183X.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- 91.Kohn Y, Lerer B. Excitement and confusion on chromosome 6q: the challenges of neuropsychiatric genetics in microcosm. Molecular psychiatry. 2005;10:1062–1073. doi: 10.1038/sj.mp.4001738. [DOI] [PubMed] [Google Scholar]
- 92.McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW, Abou Jamra R, Albus M, Bacanu SA, Baron M, Barrett TB, Berrettini W, Blacker D, Byerley W, Cichon S, Coryell W, Craddock N, Daly MJ, Depaulo JR, Edenberg HJ, Foroud T, Gill M, Gilliam TC, Hamshere M, Jones I, Jones L, Juo SH, Kelsoe JR, Lambert D, Lange C, Lerer B, Liu J, Maier W, Mackinnon JD, McInnis MG, McMahon FJ, Murphy DL, Nothen MM, Nurnberger JI, Pato CN, Pato MT, Potash JB, Propping P, Pulver AE, Rice JP, Rietschel M, Scheftner W, Schumacher J, Segurado R, Van Steen K, Xie W, Zandi PP, Laird NM. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. American journal of human genetics. 2005;77:582–595. doi: 10.1086/491603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Middleton FA, Pato MT, Gentile KL, Morley CP, Zhao X, Eisener AF, Brown A, Petryshen TL, Kirby AN, Medeiros H, Carvalho C, Macedo A, Dourado A, Coelho I, Valente J, Soares MJ, Ferreira CP, Lei M, Azevedo MH, Kennedy JL, Daly MJ, Sklar P, Pato CN. Genomewide linkage analysis of bipolar disorder by use of a high-density single-nucleotide-polymorphism (SNP) genotyping assay: a comparison with microsatellite marker assays and finding of significant linkage to chromosome 6q22. American journal of human genetics. 2004;74:886–897. doi: 10.1086/420775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pato CN, Pato MT, Kirby A, Petryshen TL, Medeiros H, Carvalho C, Macedo A, Dourado A, Coelho I, Valente J, Soares MJ, Ferreira CP, Lei M, Verner A, Hudson TJ, Morley CP, Kennedy JL, Azevedo MH, Daly MJ, Sklar P. Genome-wide scan in Portuguese Island families implicates multiple loci in bipolar disorder: fine mapping adds support on chromosomes 6 and 11, American journal of medical genetics. Part B. Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2004;127B:30–34. doi: 10.1002/ajmg.b.30001. [DOI] [PubMed] [Google Scholar]
- 95.Schulze TG, Buervenich S, Badner JA, Steele CJ, Detera-Wadleigh SD, Dick D, Foroud T, Cox NJ, MacKinnon DF, Potash JB, Berrettini WH, Byerley W, Coryell W, DePaulo JR, Jr, Gershon ES, Kelsoe JR, McInnis MG, Murphy DL, Reich T, Scheftner W, Nurnberger JI, Jr, McMahon FJ. Loci on chromosomes 6q and 6p interact to increase susceptibility to bipolar affective disorder in the national institute of mental health genetics initiative pedigrees. Biological psychiatry. 2004;56:18–23. doi: 10.1016/j.biopsych.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 96.Anand A, Shekhar A. Brain imaging studies in mood and anxiety disorders: special emphasis on the amygdala. Ann N Y Acad Sci. 2003;985:370–388. doi: 10.1111/j.1749-6632.2003.tb07095.x. [DOI] [PubMed] [Google Scholar]
- 97.Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Molecular psychiatry. 2003;8:721–737. 715. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- 98.Goldberg JF, Chengappa KN. Identifying and treating cognitive impairment in bipolar disorder. Bipolar disorders. 2009;11(Suppl 2):123–137. doi: 10.1111/j.1399-5618.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 99.Kruger S, Frasnelli J, Braunig P, Hummel T. Increased olfactory sensitivity in euthymic patients with bipolar disorder with event-related episodes compared with patients with bipolar disorder without such episodes. Journal of psychiatry & neuroscience : JPN. 2006;31:263–270. [PMC free article] [PubMed] [Google Scholar]
- 100.Land PW, Monaghan AP. Expression of the transcription factor, tailless, is required for formation of superficial cortical layers. Cereb Cortex. 2003;13:921–931. doi: 10.1093/cercor/13.9.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McCurdy RD, Feron F, Perry C, Chant DC, McLean D, Matigian N, Hayward NK, McGrath JJ, Mackay-Sim A. Cell cycle alterations in biopsied olfactory neuroepithelium in schizophrenia and bipolar I disorder using cell culture and gene expression analyses. Schizophrenia research. 2006;82:163–173. doi: 10.1016/j.schres.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 102.Stenman JM, Wang B, Campbell K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J Neurosci. 2003;23:10568–10576. doi: 10.1523/JNEUROSCI.23-33-10568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Swayze VW, 2nd, Andreasen NC, Alliger RJ, Ehrhardt JC, Yuh WT. Structural brain abnormalities in bipolar affective disorder. Ventricular enlargement and focal signal hyperintensities. Archives of general psychiatry. 1990;47:1054–1059. doi: 10.1001/archpsyc.1990.01810230070011. [DOI] [PubMed] [Google Scholar]
- 104.Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, Macdonald T, Rutka J, Guha A, Gajjar A, Curran T, Gilbertson RJ. Radial glia cells are candidate stem cells of ependymoma. Cancer cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 105.Modena P, Lualdi E, Facchinetti F, Veltman J, Reid JF, Minardi S, Janssen I, Giangaspero F, Forni M, Finocchiaro G, Genitori L, Giordano F, Riccardi R, Schoenmakers EF, Massimino M, Sozzi G. Identification of tumor-specific molecular signatures in intracranial ependymoma and association with clinical characteristics. J Clin Oncol. 2006;24:5223–5233. doi: 10.1200/JCO.2006.06.3701. [DOI] [PubMed] [Google Scholar]
- 106.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 107.Sim FJ, Keyoung HM, Goldman JE, Kim DK, Jung HW, Roy NS, Goldman SA. Neurocytoma is a tumor of adult neuronal progenitor cells. J Neurosci. 2006;26:12544–12555. doi: 10.1523/JNEUROSCI.0829-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharma MK, Mansur DB, Reifenberger G, Perry A, Leonard JR, Aldape KD, Albin MG, Emnett RJ, Loeser S, Watson MA, Nagarajan R, Gutmann DH. Distinct genetic signatures among pilocytic astrocytomas relate to their brain region origin. Cancer Res. 2007;67:890–900. doi: 10.1158/0008-5472.CAN-06-0973. [DOI] [PubMed] [Google Scholar]
- 109.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park HJ, Kim JK, Jeon HM, Oh SY, Kim SH, Nam DH, Kim H. The neural stem cell fate determinant TLX promotes tumorigenesis and genesis of cells resembling glioma stem cells. Molecules and cells. 2010;30:403–408. doi: 10.1007/s10059-010-0122-z. [DOI] [PubMed] [Google Scholar]