Abstract
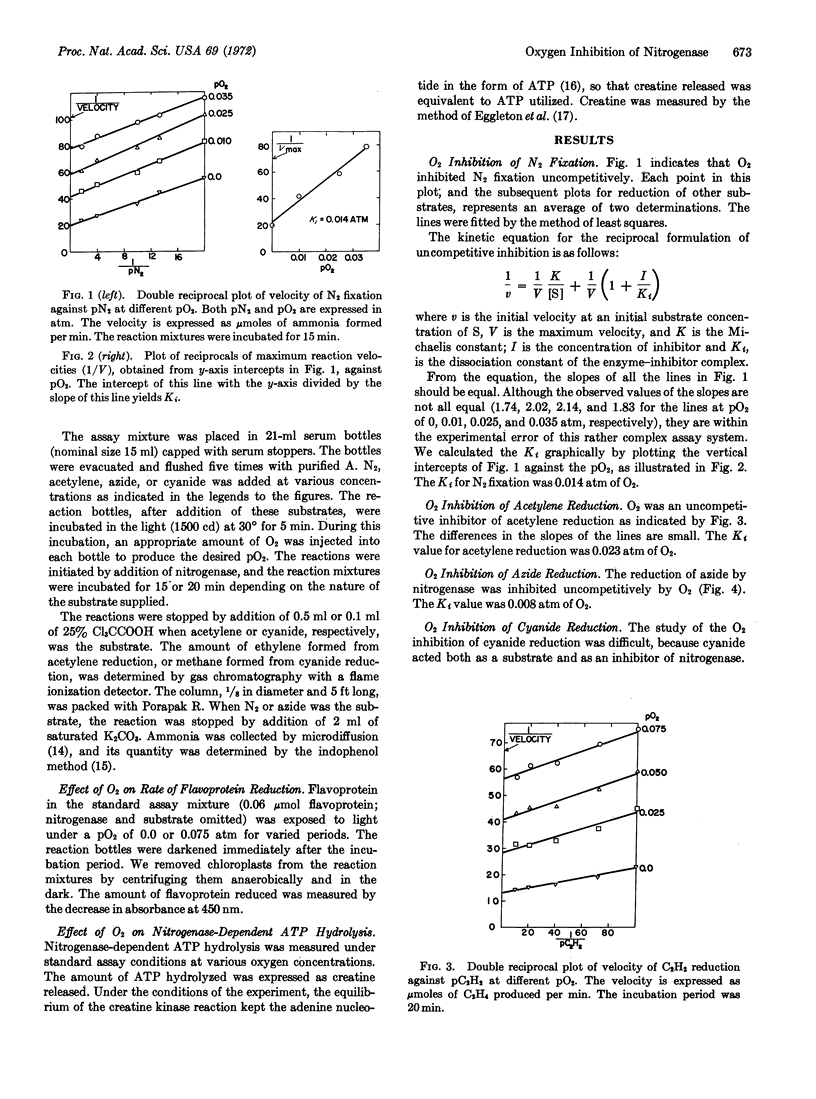
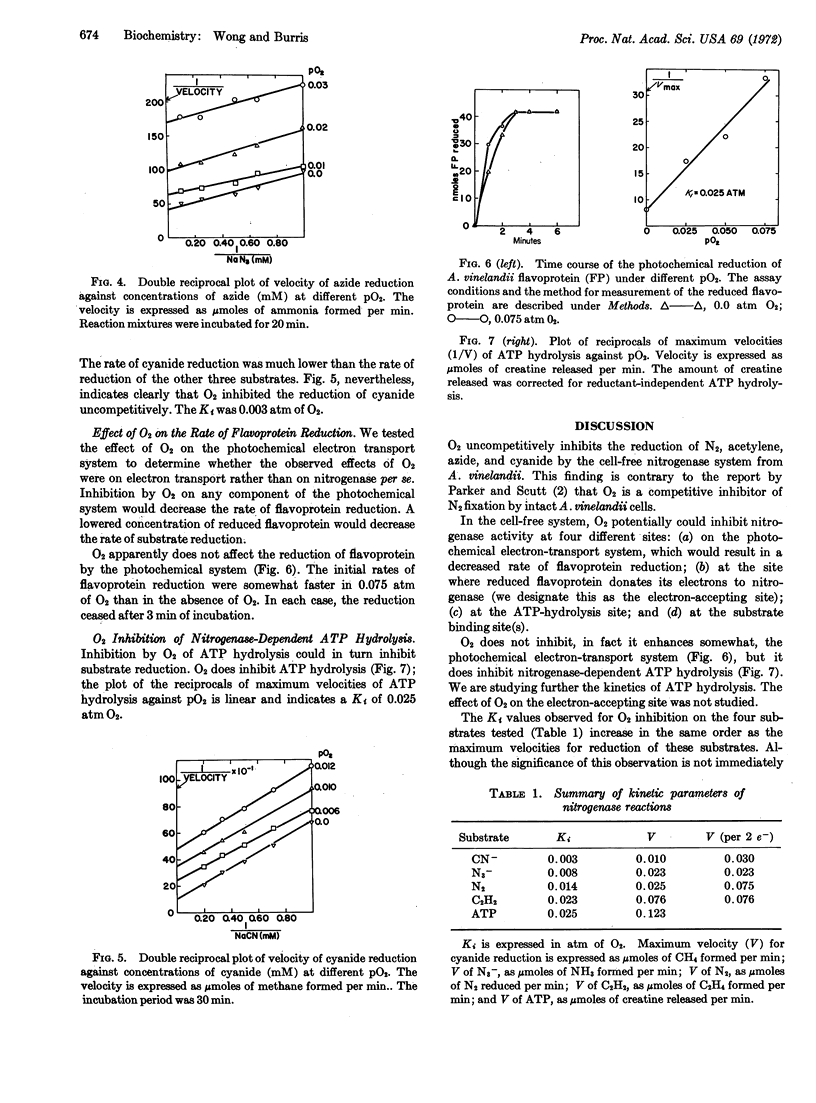
The reduction of nitrogen, acetylene, azide, and cyanide at various oxygen concentrations by nitrogenase from Azotobacter vinelandii was measured with a well-defined system. Oxygen inhibited the reduction of each substrate uncompetitively. The inhibition constants (Ki) were 0.014, 0.023, 0.008, and 0.003 atm of oxygen for reduction of nitrogen, acetylene, azide, and cyanide, respectively. The system used included ATP-generating components, subcellular particles from A. vinelandii with high nitrogenase specific activity, and illuminated spinach chloroplasts plus carriers to supply electrons. Oxygen did not affect the photochemical electron donating system, but it did inhibit nitrogenase-dependent ATP hydrolysis.
Keywords: kinetics, oxygen, uncompetitive inhibition
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benemann J. R., Yoch D. C., Valentine R. C., Arnon D. I. The electron transport system in nitrogen fixation by Azotobacter. I. Azotoflavin as an electron carrier. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1079–1086. doi: 10.1073/pnas.64.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaykin S. Assay of nicotinamide deamidase. Determination of ammonia by the indophenol reaction. Anal Biochem. 1969 Oct 1;31(1):375–382. doi: 10.1016/0003-2697(69)90278-4. [DOI] [PubMed] [Google Scholar]
- DILWORTH M. J. Oxygen inhibition in Azotobacter vinelandii pyruvate oxidation. Biochim Biophys Acta. 1962 Jan 1;56:127–138. doi: 10.1016/0006-3002(62)90533-4. [DOI] [PubMed] [Google Scholar]
- Dilworth M. J., Subramanian D., Munson T. O., Burris R. H. The adenosine triphosphate requirement for nitrogen fixation in cell-free extracts of Clostridium pasteurianum. Biochim Biophys Acta. 1965 Jun 22;99(3):486–503. doi: 10.1016/s0926-6593(65)80202-8. [DOI] [PubMed] [Google Scholar]
- Eggleton P., Elsden S. R., Gough N. The estimation of creatine and of diacetyl. Biochem J. 1943;37(5):526–529. doi: 10.1042/bj0370526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkson J. W., Bulen W. A. A free radical flavoprotein from Azotobacter. Isolation, crystallization, and properties. J Biol Chem. 1967 Jul 25;242(14):3345–3351. [PubMed] [Google Scholar]
- NEWTON J. W., WILSON P. W., BURRIS R. H. Direct demonstration of ammonia as an intermediate in nitrogen fixation by Azotobacter. J Biol Chem. 1953 Sep;204(1):445–451. [PubMed] [Google Scholar]
- PARKER C. A., SCUTT P. B. Competitive inhibition of nitrogen fixation by oxygen. Biochim Biophys Acta. 1958 Sep;29(3):662–662. doi: 10.1016/0006-3002(58)90037-4. [DOI] [PubMed] [Google Scholar]
- PARKER C. A., SCUTT P. B. The effect of oxygen on nitrogen fixation by Azotobacter. Biochim Biophys Acta. 1960 Feb 26;38:230–238. doi: 10.1016/0006-3002(60)91236-1. [DOI] [PubMed] [Google Scholar]
- Shethna Y. I., Wilson P. W., Beinert H. Purification of a non-heme iron protein and other electron transport components from Azotobacter extracts. Biochim Biophys Acta. 1966 Feb 14;113(2):225–234. doi: 10.1016/s0926-6593(66)80063-2. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Inhibition of chloroplasts by UV-irradiation and heat-treatment. Plant Physiol. 1968 Dec;43(12):2037–2040. doi: 10.1104/pp.43.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I. The nitrogen fixation system of photosynthetic bacteria. II. Chromatium nitrogenase activity linked to photochemically generated assimilatory power. Biochim Biophys Acta. 1970 Mar 3;197(2):180–184. doi: 10.1016/0005-2728(70)90029-0. [DOI] [PubMed] [Google Scholar]



