Abstract
Background
Malaria is endemic in sub-Saharan Africa with considerable burden for human health. Major insecticide resistance mechanisms such as kdrR and ace-1Ralleles constitute a hindrance to malaria vector control programs. Anopheles gambiae bearing both kdr and ace-1 resistant alleles are increasingly recorded in wild populations. In order to maintain the efficacy of vector control strategies, the characterization of concomitant kdr and ace-1 resistance, and their pleiotropic effects on malaria vector phenotype on insecticide efficacy are important.
Methods
Larval and adult bioassays were performed with different insecticide classes used in public health following WHO standard guidelines on four laboratory Anopheles gambiae strains, sharing the same genetic background but harboring distinct resistance status: KISUMU with no resistance allele; ACERKIS with ace-1R allele; KISKDR with kdrR allele and ACERKDRKIS with both resistance alleles’ ace-1R and kdrR.
Results
Larval bioassays indicate that the homozygote resistant strain harboring both alleles (ACERKDRKIS) displayed slightly but significantly higher resistance level to various insecticides like carbamates (bendiocarb, p < 0.001; propoxur, p = 0.02) and organophosphates (chlorpyriphos-methyl, p = 0.002; fenitrothion, p < 0.001) when compared to ACERKIS strain. However, no differences were recorded between ACERKDRKIS and KISKDR resistance level against permethrin (Pyrethroid, p = 0.7) and DDT (Organochlorine, p = 0.24). For adult bioassays, the percentages of mosquitoes knocked down were significantly lower for ACERKDRKIS than for KISKDR with permethrin (p = 0.003) but not with deltamethrin. The percentage of mortality from adult bioassays was similar between ACERKDRKIS and ACERKIS with carbamates and organophosphates, or between ACERKDRKIS and KISKDR with pyrethroid and DDT. Concerning acetylcholinesterase enzyme, ACERKDRKIS strain showed similarAChE1 activity than that of ACERKIS.
Conclusion
The presence of both kdrR and ace-1R alleles seems to increase the resistance levels to both carbamate and organophosphate insecticides and at operational level, may represent an important threat to malaria vector control programs in West Africa.
Keywords: Anopheles gambiae, Insecticide resistance genes, Concomitant effects, Malaria, Vector control
Background
It is considered that 207 million cases of malaria had been the cause of 627,000 deaths annually, mainly children under five years old, in sub-Saharan Africa [1]. In general, diseases control entails prevention and treatment of human infections. However, malaria vaccines are still under experimentation and not even being used at a program level. Furthermore, malaria parasites are now showing increased resistance to anti-malaria drugs [2,3] and populations from endemic countries struggle to get access to treatments due to economic impediments [4-7].
Human malaria parasites are exclusively transmitted by Anopheles mosquitoes (Diptera: Culicidae). Vector control represents one of the mainstay strategies for reducing the incidence of malaria [8]. Therefore, in most of African countries, the control of mosquito vectors is the only affordable measure for the fight against malaria [9,10].
Traditional strategies aimed at tackling malaria have often focused on reducing human-mosquito contact with insecticide treated bed nets and indoor residual spraying [11-16]. However, the rapid increase in insecticide resistance in vector species is jeopardizing the successfulness of the elimination and eradication campaigns [17-22].
Insecticide Treated Nets [9] were shown to efficiently protect vulnerable populations from endemic countries [1,22,23]. Until now, pyrethroids are the only insecticide class recommended for treating mosquito nets because of their excito-repellent properties, efficacy at low-doses, and good tolerance in humans and other mammals [24]. ITNs have been used on a large scale in the last decade but pyrethroids resistance in anopheline mosquitoes were reported in all sub-Saharan Africa [25-29].
The two main mechanisms responsible for pyrethroids resistance are target site insensitivity, known as knock down resistance kdrR, and metabolic resistance due to elevated levels of detoxifying enzymes [21,30]. kdrR resistance is caused by mutations in the sodium channel: leucine to phenylalanine substitution, originally observed in West Africa [31], and leucine to serine mutation in East Africa [32]. Recently, a new mutation in the sodium channel associated with kdr-west mutation conferring additional resistance to DDT and permethrin [33] was reported [34]. Experimental studies conducted in Southern Benin and in South Africa respectively with lambdacyhalothrin [33] on bednets and with deltamethrin [33] through indoor residual house spraying [35] suggested that PYRs resistance may have contributed to the failure of vector control endeavours in these areas [21,35-38].
As the main strategy for reducing malaria transmission is largely based on a limited number of insecticides [19], carbamates and organophosphates were suggested as potential alternative compounds to control pyrethroid-resistant populations [39-41]. Carbamates and organophosphates have shown a relatively good efficacy in ITNs and IRS [42-45] with high mortality of pyrethroids-resistant [46] An. gambiae s.s in Côte d’Ivoire [47]. However a particular concern for the use of carbamates and organophosphates is that resistance to these insecticides is already present in some An. gambiae s.s. populations from West Africa [29,48-53]. Carbamates and organophosphates resistance is associated with the G119S target site mutation in ace-1 gene causing insensitivity of the AChE1 enzyme to these insecticides and to over-expression of detoxification enzyme [49,51,54,55].
In Anopheles gambiae, kdrR and ace-1R insecticide resistance alleles were found concomitantly distributed in natural populations of An. gambiae s.s. from West Africa [29,35,37,52,56,57]. Moreover, some individuals were found carrying both resistant alleles and An. gambiae s.s. populations are becoming resistant to all classes of insecticides used in vector control strategies in West Africa [29,58,59]. A synergy between kdrR and ace-1R alleles has been previously observed in Culex pipiens [46] and An. gambiae s.s. individuals harboring both resistance alleles could appear phenotypically more resistant to pyrethroids and carbamates/organophosphates than those harboring only kdrR or ace-1R. Accordingly, the phenotypic effect associated with the interaction of these two resistance genes should be further investigated [29]. Moreover, ace-1R resistance gene is associated with a high fitness cost in An. gambiae [60] and this fitness cost could be used for the development of insecticide resistance management strategies [61]. Previous studies on Culex pipiens showed that mosquitoes harboring both kdrR and ace-1R resistant alleles showed enhanced fitness compared to the one carrying only ace-1R [46]. The synergy between kdrR and ace-1R resistant alleles could largely impede the expected success of using carbamates/organophosphates as alternative or complementary insecticides in areas where mosquitoes carry the pyrethroids resistance kdrR mutation. This represents a serious threat to malaria control in the near future.
In order to sustain the efficacy of insecticide-based vector control strategies, the characterization of concomitant kdr and ace-1 resistance and associated pleiotropic effects on malaria vector phenotype is relevantly important. In this study we have established a homozygote resistant strain harboring both alleles (kdrR and ace-1R) and compared its resistance level to various insecticides to strains carrying single resistance allele.
Methods
Mosquito strains
Four strains of An. gambiae s.s. were used in this study (Table 1): i) The KISUMU reference strain, susceptible to all insecticides used in this study [62]. ii) The ACERKIS strain, which is homozygous for the G119S mutation and resistant to both OPs and CXs insecticides [51]. iii) The KISKDR strain, which is homozygous for kdrR (L1014F) and confers resistance to both DDT and pyrethroids [63]. These strains have the same genetic background and metabolic resistance is not present within them. iv) The ACERKDRKIS strain was derived from the cross between ACERKIS and KISKDR strains selected with permethrin and chlorpyrifos-methyl from second to fourth generation in order to increase the frequency of individual carrying both ace-1R and kdrR alleles. At the fourth generation, the females blood fed were isolated in plastic cup for independently egg-laying. Their progeny were analyzed with kdrR and ace-1R specific molecular tests developed by Martinez-Torres et al. [31] and Weill et al. [49] respectively. When progenies displayed homozygous for ace-1R and kdrR alleles, they were mixed to constitute the ACERKDRKIS strain, homozygous for both resistance alleles’ace-1R and kdrR. All strains used in this study are An. gambiae s.s.
Table 1.
Resistance of the different strains to various insecticides
| Strains | Resistance mechanisms | |
|---|---|---|
| kdr R | ace-1 R | |
| Kisumu | 0 | 0 |
| Acerkis | 0 | 1 |
| KisKdr | 1 | 0 |
| AcerKdrKis | 1 | 1 |
0 = absent; 1 = present.
kdr R = mutation associated to pyrethroids and DDT resistance.
ace-1 R = mutation associated to carbamates and organophosphates resistance.
Larval bioassay
Resistance data of the four strains (KISUMU, ACERKIS, KISKDR and ACERKDRKIS) were obtained by conducting bioassays as previously described in Djogbenou et al. [51].The bioassays were done in plastic cups. Late third and early fourth instars larvae were used. Six insecticides of technical grade form Sigma-Aldrich®were used: two carbamates: propoxur (99.8% pure) and bendiocarb (99.5% pure); two organophosphates: chlorpyrifos-methyl (99.9% pure), fenitrothion (95.2% pure); one pyrethroid: permethrin (98.3% pure); and one organochlorine: DDT (99.7% pure). Insecticides were diluted in 70% ethanol to make a working solution and were stored at 4°C. A set of 25 larvae was incubated in 99 ml of distilled water, to which 1 ml of insecticide solution at the required concentration was added. Four replicates were used for each concentration. Six to twelve insecticide concentrations providing a range of mortality between 0 and 100% were used for each insecticide tested. Larval mortality was recorded after 24 hours exposure. Control bioassays were conducted by adding 1 ml of ethanol to 99 ml of distilled water. Temperature was maintained at 27°C ± 2°C during bioassays test (temperature measured using Waranet kit (Waranet Solutions SAS, Auch, France)).
WHO insecticide resistance tests on adult mosquitoes
Insecticide susceptibility tests were performed in WHO resistant test kits assays on 3–5 day old females from the four strains [64]. The tests were conducted using discriminating dosages of several insecticides used in public health as follows: two carbamates (0.4% propoxur and 0.1% bendiocarb); three organophosphates (0.4% chlorpyrifos methyl, 1.0% fenitrothion and 5% malathion); one pyrethroid (0.75% permethrin); and one organochlorine (4% DDT). Control tests were also set up by exposing adult females to untreated papers. WHO test and control papers were supplied by the WHO Collaborating Centre at University Sains Malaysia, Penang, Malaysia. Test papers were used no more than five times before being replaced. For each insecticide, six replicates were conducted. Twenty-five non blood fed females were exposed to the insecticide-impregnated test papers in the test tubes for one hour. For DDT, deltamethrin and permethrin, knockdown of females were reported every ten minutes. Mosquitoes were then transferred into holding tubes and supplied with a 10% sugar solution. Mortality was scored after 24 hours.
AChE1 activity measurement
AChE1 activity was measured in thirty mosquitoes of each An. gambiae ss strain: KISUMU, ACERKIS, KISKDR and ACERKDRDKIS as described in previous studies [65,66]. Each head was individually ground and homogenized in 400 μL phosphate buffer (0.25 M, pH7) containing 1% Triton X-100. Homogenates were centrifuged (10,000 rpm for 3 min at 4°C) and 100 μL of the supernatant were used with 10 μL of ethanol (95%) for AChE1 activity measure. We then added 100 μL of 1.6 mM substrate (acetylthiocholine, Sigma, France), and AChE1 activity was estimated by measuring changes in optical density as described by Ellman et al. [67]. Colour development was measured at 412 nm for 15 min with a microplate reader Multiskan® GO and the analysis software SkanIt 3.2 (Thermo Scientific). Part of each genotype was analyzed on each plate to avoid experimental artefacts.
Data analysis
The analyses of dose-mortality responses in bioassays were performed using the R software (v.3.0.0). The R script BioRssay (v. 5.1.1) was used; it is freely available on the website of the Institut des Sciences de l’Evolution de Montpellier [68]. This script computes the doses of insecticide killing 50% and 95% of the tested population or strain (Lethal Concentration 50 and 95, or LC50 and LC95) and the associated confidence intervals, tests for the linearity of the dose-mortality response (χ2 test). Finally, it allows the comparison of two or more strains or populations and calculates the resistance ratios, i.e. RR50 or RR95(= LC50 or LC95 of tested population/LC50 or LC95of the reference strain, resp.) and their 95% confidence intervals.
For adult bioassays, resistant/susceptible status was defined according to WHO criteria [1,64]. Mosquitoes were considered susceptible if the mortality rates were greater than 97% and resistant if mortality rates were less than 90%. Mortality rates between 90-97% suggested possible resistance. The knockdown times for permethrin, deltamethrin and DDT (KDT50 and KDT95) and their 95% confidence intervals were estimated using POLO-Plus (LeOra-Software 2006). The mortality difference between strains was tested using the prop.test function (based on a chi-square comparison) with the R software (v. 3.0.0).
Total acetylcholinesterase activities (Atot) conferred by each genotype [69] were compared using a generalized linear model as: Atot = geno + ε where geno is four level factor (KISUMU, ACERKIS, KISKDR, ACERKDRKIS), ε is the error parameter following a normal distribution to take over-dispersion into account, if present. We tested significance of the different levels by Likelihood ratio tests.
Results
Larval bioassays
We carried out 24 larval bioassays with four replicates for the four reference strains (Tables 1 and 2). With carbamates, organophosphates, pyrethroid and DDT, the result of chi-square test between the observed dead numbers (obtained) and the dead numbers predicted by the regression log-dose probit-mortality indicated that the data were well fitted by a straight line (p > 0.05, Table 2) excepted for ACERKIS to propoxur (p = 0.001). KISUMU and KISKDR susceptibility to CXs and OPs was not significantly different (p > 0.05). The same susceptibility was also recorded for KISUMU and ACERKIS to pyrethroid and DDT (p > 0.05). These results confirm that there were no other resistance alleles involved, except the specific target site mutations (Table 1). The homozygote resistant strain harboring both alleles (ACERKDRKIS) displayed slightly but significantly higher resistance level to various carbamates (bendiocarb, p < 0.001; propoxur, p = 0.02) and organophosphates (chlorpyriphos-methyl, p = 0.002; fenitrothion, p < 0.001) when compared to ACERKIS strain (Table 2). Both ACERKDRKIS and ACERKIS displayed lower resistance level to organophosphates than to carbamates. In contrary, we did not record a significant difference between ACERKDRKIS and KISKDR resistance levels against permethrin (p = 0.7) and DDT (p = 0.24).
Table 2.
Log-dose and probit-mortality data for different insecticides of reference strains from Anopheles gambiae s.s.
| Strains | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Insecticides | Kisumu | KisKdr | Acerkis | AcerKdrKis | |||||||
| LC 50 (mg/L) | Chi( p ) | LC 50 (mg/L) | RR 50 | Chi( p ) | LC 50 (mg/L) | RR 50 | Chi( p ) | LC 50 (mg/L) | RR 50 | Chi( p ) | |
| Bendiocarb | 0,22 | 0,88 | 0.23 | 1 | 0,92 | 62 | 290 | 0,17 | 83 | 385 | 0,88 |
| Propoxur | 0,12 | 0,08 | 0,12 | 1 | 0,4 | 268 | 2211 | <0.001 | 351 | 2899 | 0,42 |
| Chlorpyrifos-methyl | 0.004 | 0,8 | 0.004 | 1 | 0,4 | 0,053 | 12 | 0,33 | 0,065 | 15 | 0,6 |
| Fenitrothion | 0,004 | 0,13 | 0,004 | 1 | 0,11 | 0,067 | 16 | 0,77 | 0,11 | 27 | 0,48 |
| Permethrin | 0,006 | 0,24 | 0,055 | 9 | 0,89 | 0,006 | 1 | 0,17 | 0,067 | 11 | 0,26 |
| DDT | 0,01 | 0,66 | 0,12 | 12 | 0,34 | 0,01 | 1 | 0,059 | 0,21 | 21 | 0,47 |
LC50 is lethal concentration required to kill half of number larval tested after 24 hours.
RR50 is resistance ratio at LC50 = LC50(resistant strain)/LC50(Kisumu).
Chi(p) is indicated to judge whether the data are well fitted to the regression or not. The fits are acceptable when the p-value is over 0.05.
Adult susceptibility
Adult susceptibility tests with seven insecticides (propoxur, bendiocarb, malathion, chlorpyrifos methyl, fenitrothion, DDT and permethrin) were performed on the four An. gambiae strains. All tests used the WHO discriminating doses; 100–125 females were analyzed for each strain. Mortality in control groups was consistently <5%. Mortality in the susceptible strain KISUMU was above 99% for all tested insecticides. In addition, after one hour of exposure to the WHO diagnostic concentration for permethrin, the percentages of mosquitoes knocked down were significantly lower for ACERKDRKIS than KISKDR (p = 0.003) (Table 3). However, we did not record a significant difference between ACERKDRKIS and KISKDR knocked down percentages for deltamethrin (p = 1) (Table 3). DDT did not show any knockdown effect with both strains. Considering the percentage of mortality data from adult bioassays, no significant differences were recorded for bendiocarb (p = 0.14), propoxur (p = 0.56), chlorpyriphos-methyl (p = 0.99), fenitrothion (p = 0.51) and malathion (p = 1) between ACERKDRKIS and ACERKIS. Moreover, no significant differences were recorded between ACERKDRKIS and KISKDR mortality for permethrin (p = 1), deltamethrin (p = 0.66) and DDT (p = 1) (Figure 1).
Table 3.
Knock-down times (KdT50 and KdT95) of reference strains 1 h after exposed to insecticides
| Insecticides | Strains | N | % KD | KdT 50 | CI 95 | KdT 95 | CI 95 | KdT 50 R |
|---|---|---|---|---|---|---|---|---|
| Permethrin | Kisumu | 98 | 100 | 8.6 | 7.6-9.6 | 28.5 | 24.7-34 | - |
| Acerkis | 100 | 100 | 10.9 | 9.5-12.4 | 25.2 | 21-32 | 1,3 | |
| KisKDR | 99 | 23,23 | 178 | 114-414.5 | 2544 | 864-21000 | 20,6 | |
| AcerKdrkis | 100 | 7 | No KdT | - | No KdT- | - | - | |
| Deltamethrin | Kisumu | 101 | 100 | 7.7 | 6.8-8.5 | 21 | 18.3-25 | - |
| Acerkis | 96 | 100 | 8 | 7.2-8.7 | 17.8 | 15.4-21 | 1 | |
| KisKDR | 101 | 94 | 19.3 | 15-23.5 | 51 | 39.6-77.4 | 2,5 | |
| AcerKdrkis | 100 | 94 | 27 | 24-30 | 55.7 | 48-69.4 | 3,5 | |
| DDT | Kisumu | 99 | 98 | 33.6 | 31-36 | 50.1 | 45.6-57.7 | - |
| Acerkis | 100 | 77 | 45 | 43-47 | 77.5 | 70.7-88 | 1.3 | |
| KisKDR | 98 | 0 | No KdT | - | No KdT | - | - | |
| AcerKdrkis | 100 | 0 | No KdT | - | No KdT | - | - |
N = number of mosquitoes tested.
KdT50R, KdT50 of the tested population divided by KdT50 of the Kisumu (susceptible reference strain).
% KD = percentage of mosquitoes knock-down after 60 mn.
KdT50/95 = Knock-down times in minutes for 50 or 95% of adult mosquitoes after one hour of exposure to impregnated paper in WHO test Kits.
CI 95 = 95% confidence intervals.
No KdT = Complete loss of KD effect (less than 5% of KD mosquitoes after one hour exposure).
Figure 1.
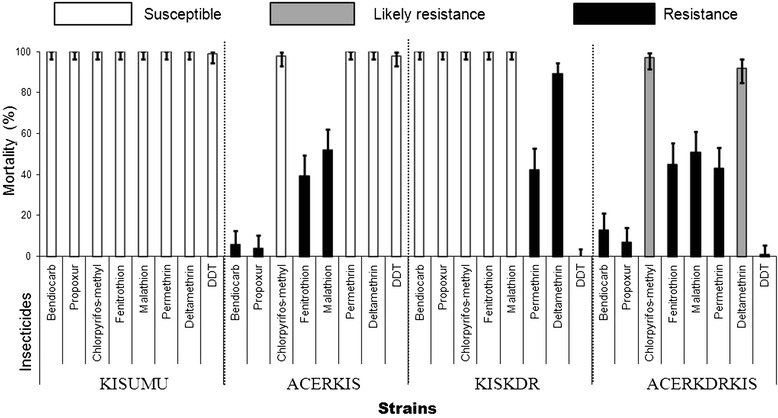
Mortality percentages and insecticide susceptibility status of different Anopheles gambiae s.s. strains to insecticide. The bars indicate the mortality percentage with 95% confidence intervals after one hour of exposure to impregnated paper in WHO test Kits and mortality reading after 24 hours.
Total AChE1 activity
The total AChE1 activity was measured for thirty individuals of each reference strains. We found a significant reduction of AChE1 activity for ACERKIS and ACERKDRKIS strains in comparison to KISUMU and KISKDR strains (p < 0.001, Figure 2). However the AChE1 activity for ACERKDRKIS (34 ± 9mOD/min) and ACERKIS (33 ± 5mOD/min) strains was not significantly different (p = 1) and KISKDR (85 ± 16mOD/min) strain displayed similar AChE1 activity as KISUMU (86 ± 5mOD/min) strain (p = 1).
Figure 2.
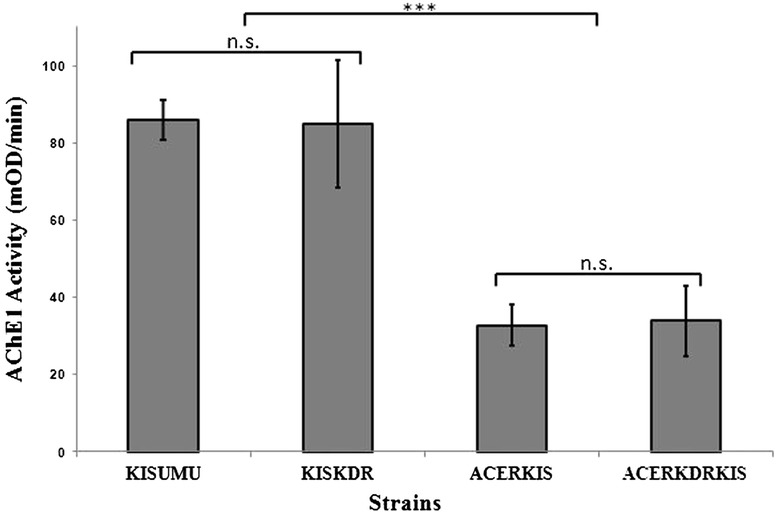
Total AChE1 activity for females of different Anopheles gambiae s.s. strains. The bar indicate the mean AChE1 activity in mosquito head from susceptible and resistant Anopheles gambiae s.s. strains (KISUMU (N = 30), KISKDR (N = 30), ACERKIS (N = 30), and ACERKDRKIS (N = 30). ***indicated significant difference (p < 0.001), n.s. indicated not significant difference (p > 0.05).
Discussion
The occurrence of multiple resistance mechanisms towards insecticides in single specimen of An. gambiae has been shown in several studies in West Africa [29,52]. In this study, we aimed at characterizing the impact of concomitant occurrence of kdrR (L1014F) and ace-1R (G119S) resistance alleles in single An. gambiae s.s. by establishing the ACERKDRKIS strain, which harbor the kdrR and ace-1R resistance alleles.
ACERKDRKIS strain displayed slightly but significant higher resistance levels to various carbamates and organophosphates when compared to ACERKIS strain. These observations were also shown in Culex quinquefasciatus where the BSCR strain harboring both alleles displayed a resistance level to carbosulfan (carbamates) higher than that of the SR strain (Culex quinquefasciatus homozygotes for the resistance allele ace-1R) [46]. These results suggest that the presence of kdrR mutation in individuals harboring ace-1R allele may contribute to the higher resistance levels observed against carbamates and organophosphates in An. gambiae s.s. We cannot clearly explain this data, although when the original targets of insecticides become insensitive and higher doses are required to achieve equivalent mortality in the strains harboring both insensitive targets of insecticides, secondary target sites may be involved [70-72].
Our study did not detect a significant difference for pyrethroids and DDT mortality rate between ACERKDRKIS and KISKDR in larval and adult stage suggesting that ace-1R mutation does not influence mosquito resistance to pyrethroids and DDT. However, our results detect a significant reduction of knockdown percentage for permethrin but not for deltamethrin (Table 3). This may depend on the types of pyrethroids compound because permethrin and deltamethrin are respectively from type I and II of pyrethroids, and type II have greater toxicity than type I [73-77]. However, this knockdown reduction in ace-1R and kdrR concomitant distribution area, may affect the malaria transmission risk in community.
We found a significant reduction of AChE1 activity from An. gambiae homozygous for ace-1R (resistant strain ACERKIS) in comparison to KISUMU, the susceptible control strain (Figure 2). Same results were observed in previous studies focused on comparison of An. gambiae and Culex pipiens acetylcholinesterase 1 biochemical properties [65]. This activity reduction of insensitive AChE1-R may be responsible for fitness cost associated with ace-1R mutation [60,65]. We did not find a significant difference between AChE1 activity of ACERKDRKIS individuals harboring both ace-1R and KdrR compared with ACERKIS individuals (homozygous for ace-1R) (Figure 2). Our findings showed as expected that kdrR mutation does not interact with AChE1 activity. Thus, if the fitness of double mutants is improved as it was showed for Culex pipiens [46], it will not be through the increase of AChE1 activity. Further studies on fitness cost in free insecticide environment should be conducted on An. gambiae strains harboring only one resistance allele (either ace-1R or kdrR), both alleles, or no resistance allele to answer this question.
Conclusion
This study demonstrated that ace-1R and kdrR alleles interact to enhance resistance to carbamates and organophosphates. These results show that the concomitant occurrence of acetylcholinesterase (ace-1R) and knockdown resistance (kdrR) in An. gambiae could be a great concern for carbamates and organophosphates use as alternatives against pyrethroids resistance. The cost reduction in An. gambiae double resistant could facilitate the spread of these targets resistance mechanisms in natural populations of vector. This represents a major threat for insecticide resistance management for malaria vector control. The results of this study should be carefully considered while elaborating malaria vector control programs in West Africa.
Acknowledgements
We are very grateful to MIM/TDR/WHO to support financially this study by the grant ID: B00040 which Dr. Djogbenou S. Luc was the principal investigator. This work was funded in part by French ANR program (project “AlterNET” SOC & ENV 2013-2015). Assogba S. Benoît was supported by a fellowship from the IRD. We thank Emadeldin Fawaz, Birkinesh Ameneshewa, Henry Rupp and Pierrick Labbé for critical reading on the manuscript, the anonymous reviewers for helpful comments on the manuscript. We thank also Koffi Dieudonné for his technical assistance.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BSA, LSD, FC and MW designed the study. BSA, LSD, LD, and AO carried out the laboratory activities. ID proved the KISKDR strain. BSA and PM analyzed the data. BSA drafted the manuscript. LSD, JS, PM, LD, ID, AO, FC, LBM, MW and MM critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Contributor Information
Benoît S Assogba, Email: assobe80@gmail.com.
Luc S Djogbénou, Email: ldjogbenou22002@yahoo.fr.
Jacques Saizonou, Email: saizonoujacques@yahoo.fr.
Pascal Milesi, Email: pascal.milesi@univ-montp2.fr.
Laurette Djossou, Email: djoslaure@yahoo.fr.
Innocent Djegbe, Email: djegbe1@yahoo.fr.
Welbeck A Oumbouke, Email: achiloswek@yahoo.fr.
Fabrice Chandre, Email: fabrice.chandre@ird.fr.
Lamine Baba-Moussa, Email: laminesaid@yahoo.fr.
Mylene Weill, Email: mylene.weill@univ-montp2.fr.
Michel Makoutodé, Email: makoutod@gmail.com.
References
- 1.Organization WH . World Malaria Report: 2013. Geneva: World Health Organization: Roll Back Malaria; 2013. [Google Scholar]
- 2.Malkin E, Dubovsky F, Moree M. Progress towards the development of malaria vaccines. Trends Parasitol. 2006;22(7):292–295. doi: 10.1016/j.pt.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz L, Brown GV, Genton B, Moorthy VS: A review of malaria vaccine clinical projects based on the WHO rainbow table.Malar J 2012, 11:11. [DOI] [PMC free article] [PubMed]
- 4.Russell S. The economic burden of illness for households in developing countries: a review of studies focusing on malaria, tuberculosis, and human immunodeficiency virus/acquired immunodeficiency syndrome. Am J Trop Med Hyg. 2004;71(2 Suppl):147–155. [PubMed] [Google Scholar]
- 5.Incardona S, Vong S, Chiv L, Lim P, Nhem S, Sem R, Khim N, Doung S, Mercereau-Puijalon O, Fandeur T: Large-scale malaria survey in Cambodia: novel insights on species distribution and risk factors.Malar J 2007, 6(1):37. [DOI] [PMC free article] [PubMed]
- 6.Yeung S, Van Damme W, Socheat D, White NJ, Mills A: Access to artemisinin combination therapy for malaria in remote areas of Cambodia.Malar J 2008, 7(1):96. [DOI] [PMC free article] [PubMed]
- 7.Chuma J, Okungu V, Molyneux C: Barriers to prompt and effective malaria treatment among the poorest population in Kenya.Malar J 2010, 9(1):144. [DOI] [PMC free article] [PubMed]
- 8.Pampana E. A Textbook of Malaria Eradication. 2. London: Oxford U.P; 1969. [Google Scholar]
- 9.Beier JC, Keating J, Githure JI, Macdonald MB, Impoinvil DE, Novak RJ: Integrated vector management for malaria control.Malar J 2008, 7(1):S4. [DOI] [PMC free article] [PubMed]
- 10.Michalakis Y, Renaud F. Malaria: evolution in vector control. Nature. 2009;462(7271):298–300. doi: 10.1038/462298a. [DOI] [PubMed] [Google Scholar]
- 11.Lengeler C: Insecticide-treated bed nets and curtains for preventing malaria.Cochrane Database Syst Rev 2004, 2(2):CD000363. [DOI] [PubMed]
- 12.Whalon M, Mota-Sanchez D, Hollingworth R. Analysis of global pesticide resistance in arthropods: global pesticide resistance in arthropods. CAB Int Camb. 2008;192:5–31. [Google Scholar]
- 13.Takken W, Knols BGJ. Malaria vector control: current and future strategies. Trends Parasitol. 2009;25(3):101–104. doi: 10.1016/j.pt.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Gu W, Novak RJ: Predicting the impact of insecticide-treated bed nets on malaria transmission: the devil is in the detail.Malar J 2009, 8:256. [DOI] [PMC free article] [PubMed]
- 15.Zhou G, Githeko AK, Minakawa N, Yan G: Community-wide benefits of targeted indoor residual spray for malaria control in the Western Kenya Highland.Malar J 2010, 9:67. [DOI] [PMC free article] [PubMed]
- 16.Pluess B, Tanser FC, Lengeler C, Sharp BL: Indoor residual spraying for preventing malaria.Cochrane Database Syst Rev 2010, 4(4):CD00665. [DOI] [PMC free article] [PubMed]
- 17.Raymond M, Callaghan A, Fort P, Pasteur N. Worldwide migration of amplified insecticide resistance genes in mosquitoes. Nature. 1991;350(6314):151–153. doi: 10.1038/350151a0. [DOI] [PubMed] [Google Scholar]
- 18.Raymond M, Berticat C, Weill M, Pasteur N, Chevillon C. Insecticide resistance in the mosquito Culex pipiens: what have we learned about adaptation? Genetica. 2001;112:287–296. doi: 10.1023/A:1013300108134. [DOI] [PubMed] [Google Scholar]
- 19.Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag Sci. 2007;63(7):628–633. doi: 10.1002/ps.1406. [DOI] [PubMed] [Google Scholar]
- 20.Baleta A. Insecticide resistance threatens malaria control in Africa. Lancet. 2009;374(9701):1581–1582. doi: 10.1016/S0140-6736(09)61933-4. [DOI] [PubMed] [Google Scholar]
- 21.Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27(2):91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Binka FN, Kubaje A, Adjuik M, Williams LA, Lengeler C, Maude G, Armah G, Kajihara B, Adiamah J, Smith PG. Impact of permethrin impregnated bednets on child mortality in Kassena‐Nankana District, Ghana: a randomized controlled trial. Tropical Med Int Health. 1996;1(2):147–154. doi: 10.1111/j.1365-3156.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 23.Eisele TP, Lindblade KA, Wannemuehler KA, Gimnig JE, Odhiambo F, HAWLEY WA, TER KUILE FO, Phillips-Howard P, Rosen DH, Nahlen BL. Effect of sustained insecticide-treated bed net use on all-cause child mortality in an area of intense perennial malaria transmission in western Kenya. Am J Trop Med Hyg. 2005;73(1):149–156. [PubMed] [Google Scholar]
- 24.Zaim M, Guillet P. Alternative insecticides: an urgent need. Trends Parasitol. 2002;18(4):161–163. doi: 10.1016/S1471-4922(01)02220-6. [DOI] [PubMed] [Google Scholar]
- 25.Brogdon WG, McAllister JC. Insecticide resistance and vector control. Emerg Infect Dis. 1998;4(4):605–613. doi: 10.3201/eid0404.980410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eritja R, Chevillon C. Interruption of chemical mosquito control and evolution of insecticide resistance genes in Culex pipiens (Diptera: Culicidae) J Med Entomol. 1999;36(1):41–49. doi: 10.1093/jmedent/36.1.41. [DOI] [PubMed] [Google Scholar]
- 27.Diabate A, Baldet T, Chandre F, Akogbeto M, Guiguemde TR, Darriet F, Brengues C, Guillet P, Hemingway J, Small GJ. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae sl in Burkina Faso. Am J Trop Med Hyg. 2002;67(6):617–622. doi: 10.4269/ajtmh.2002.67.617. [DOI] [PubMed] [Google Scholar]
- 28.Boyer S, Sérandour J, Lempérière G, Raveton M, Ravanel P. Do herbicide treatments reduce the sensitivity of mosquito larvae to insecticides? Chemosphere. 2006;65(4):721–724. doi: 10.1016/j.chemosphere.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 29.Dabiré KR, Diabaté A, Djogbenou L, Ouari A, N’Guessan R, Ouédraogo J-B, Hougard J-M, Chandre F, Baldet T: Dynamics of multiple insecticide resistance in the malaria vector Anopheles gambiae in a rice growing area in South-Western Burkina Faso.Malar J 2008, 7:188. [DOI] [PMC free article] [PubMed]
- 30.Hargreaves K, Hunt R, Brooke B, Mthembu J, Weeto M, Awolola T, Coetzee M. Anopheles arabiensis and An. quadriannulatus resistance to DDT in South Africa. Med Vet Entomol. 2003;17(4):417–422. doi: 10.1111/j.1365-2915.2003.00460.x. [DOI] [PubMed] [Google Scholar]
- 31.Martinez‐Torres D, Chandre F, Williamson M, Darriet F, Berge JB, Devonshire AL, Guillet P, Pasteur N, Pauron D. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae ss. Insect Mol Biol. 1998;7(2):179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 32.Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9(5):491–497. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 33.Adams KJ, Chavasse DC, Mount DL, Carneiro IA, Curtis CF. Comparative insecticidal power of three pyrethroids on netting. Med Vet Entomol. 2002;16(1):106–108. doi: 10.1046/j.0269-283x.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- 34.Jones CM, Liyanapathirana M, Agossa FR, Weetman D, Ranson H, Donnelly MJ, Wilding CS. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc Natl Acad Sci. 2012;109(17):6614–6619. doi: 10.1073/pnas.1201475109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corbel V, Raymond M, Chandre F, Darriet F, Hougard JM. Efficacy of insecticide mixtures against larvae of Culex quinquefasciatus (Say) (Diptera: Culicidae) resistant to pyrethroids and carbamates. Pest Manag Sci. 2004;60(4):375–380. doi: 10.1002/ps.809. [DOI] [PubMed] [Google Scholar]
- 36.Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu J, Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14(2):181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 37.N’Guessan R, Corbel V, Akogbéto M, Rowland M: Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin.Emerg Infect Dis 2007, 13(2):199. [DOI] [PMC free article] [PubMed]
- 38.Sharp BL, Ridl FC, Govender D, Kuklinski J, Kleinschmidt I: Malaria vector control by indoor residual insecticide spraying on the tropical island of Bioko, Equatorial Guinea.Malar J 2007, 6(1):52. [DOI] [PMC free article] [PubMed]
- 39.Guillet P, N’Guessan R, Darriet F, Traore-Lamizana M, Chandre F, Carnevale P. Combined pyrethroid and carbamate ‘two-in-one’ treated mosquito nets: field efficacy against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus. Med Vet Entomol. 2001;15(1):105–112. doi: 10.1046/j.1365-2915.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- 40.Najera JA, Zaim M. Malaria Vector Control. Decision Making Criteria and Procedures for Judicious use of Insecticides. Geneva: World health organization: Communicable disease control, Prevention and eradication/Who pesticide evaluation scheme; 2002. p. 116. [Google Scholar]
- 41.Hougard JM, Corbel V, N’Guessan R, Darriet F, Chandre F, Akogbeto M, Baldet T, Guillet P, Carnevale P, Traore-Lamizana M. Efficacy of mosquito nets treated with insecticide mixtures or mosaics against insecticide resistant Anopheles gambiae and Culex quinquefasciatus (Diptera: Culicidae) in Cote d’Ivoire. Bull Entomol Res. 2003;93(6):491–498. doi: 10.1079/BER2003261. [DOI] [PubMed] [Google Scholar]
- 42.Najera JA, Shidrawi G, Gibson F, Stafford J: A large-scale field trial of malathion as an insecticide for antimalarial work in southern Uganda.Bull World Health Organ 1967, 36(6):913. [PMC free article] [PubMed]
- 43.Fontaine R, Pull J, Payne D, Pradhan G, Joshi G, Pearson J, Thymakis M, Camacho MR: Evaluation of fenitrothion for the control of malaria.Bull World Health Organ 1978, 56(3):445. [PMC free article] [PubMed]
- 44.Molineaux L, Gramiccia G. Garki Project: Research on the Epidemiology and Control of Malaria in the Sudan Savanna of West Africa. Geneva: WHO; 1980. Garki Project: Research on the Epidemiology and Control of Malaria in the Sudan Savanna of West Africa. [Google Scholar]
- 45.Asidi AN, N’Guessan R, Koffi AA, Curtis CF, Hougard JM, Chandre F, Corbel V, Darriet F, Zaim M, Rowland MW: Experimental hut evaluation of bednets treated with an organophosphate (chlorpyrifos-methyl) or a pyrethroid (lambdacyhalothrin) alone and in combination against insecticide-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes.Malar J 2005, 4(1):25. [DOI] [PMC free article] [PubMed]
- 46.Berticat C, Bonnet J, Duchon S, Agnew P, Weill M, Corbel V: Costs and benefits of multiple resistance to insecticides for Culex quinquefasciatus mosquitoes.BMC Evol Biol 2008, 8:104. [DOI] [PMC free article] [PubMed]
- 47.Kolaczinski JH, Fanello C, Herve JP, Conway DJ, Carnevale P, Curtis CF. Experimental and molecular genetic analysis of the impact of pyrethroid and non-pyrethroid insecticide impregnated bednets for mosquito control in an area of pyrethroid resistance. Bull Entomol Res. 2000;90(2):125–132. doi: 10.1017/s0007485300000237. [DOI] [PubMed] [Google Scholar]
- 48.Weill M, Lutfalla G, Mogensen K, Chandre F, Berthomieu A, Berticat C, Pasteur N, Philips A, Fort P, Raymond M. Comparative genomics: Insecticide resistance in mosquito vectors. Nature. 2003;423(6936):136–137. doi: 10.1038/423136b. [DOI] [PubMed] [Google Scholar]
- 49.Weill M, Malcolm C, Chandre F, Mogensen K, Berthomieu A, Marquine M, Raymond M. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol Biol. 2004;13(1):1–7. doi: 10.1111/j.1365-2583.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 50.Corbel V, N’Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, Akogbéto M, Hougard J, Rowland M: Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa.Acta Trop 2007, 101(3):207. [DOI] [PubMed]
- 51.Djogbenou L, Weill M, Hougard JM, Raymond M, Akogbeto M, Chandre F. Characterization of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae (Diptera: Culicidae): resistance levels and dominance. J Med Entomol. 2007;44(5):805–810. doi: 10.1603/0022-2585(2007)44[805:COIAAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 52.Djogbenou L, Dabire R, Diabate A, Kengne P, Akogbeto M, Hougard JM, Chandre F. Identification and geographic distribution of the ACE-1R mutation in the malaria vector Anopheles gambiae in south-western Burkina Faso, West Africa. Am J Trop Med Hyg. 2008;78(2):298–302. [PubMed] [Google Scholar]
- 53.Dabire KR, Diabate A, Namontougou M, Djogbenou L, Kengne P, Simard F, Bass C, Baldet T. Distribution of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae s.l. populations from Burkina Faso (West Africa) Trop Med Int Health. 2009;14(4):396–403. doi: 10.1111/j.1365-3156.2009.02243.x. [DOI] [PubMed] [Google Scholar]
- 54.Hemingway J, Karunaratne SH. Mosquito carboxylesterases: a review of the molecular biology and biochemistry of a major insecticide resistance mechanism. Med Vet Entomol. 1998;12(1):1–12. doi: 10.1046/j.1365-2915.1998.00082.x. [DOI] [PubMed] [Google Scholar]
- 55.Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34(7):653–665. doi: 10.1016/j.ibmb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 56.Chandre F, Darriet F, Darder M, Cuany A, Doannio J, Pasteur N, Guillet P. Pyrethroid resistance in Culex quinquefasciatus from West Africa. Med Vet Entomol. 1998;12(4):359–366. doi: 10.1046/j.1365-2915.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- 57.Djogbenou L, Pasteur N, Akogbeto M, Weill M, Chandre F. Insecticide resistance in the Anopheles gambiae complex in Benin: a nationwide survey. Med Vet Entomol. 2011;25(3):256–267. doi: 10.1111/j.1365-2915.2010.00925.x. [DOI] [PubMed] [Google Scholar]
- 58.Essandoh J, Yawson AE, Weetman D: Acetylcholinesterase (Ace-1) target site mutation 119S is strongly diagnostic of carbamate and organophosphate resistance in Anopheles gambiae ss and Anopheles coluzzii across southern Ghana.Malar J 2013, 12(1):404. [DOI] [PMC free article] [PubMed]
- 59.Edi CV, Koudou BG, Jones CM, Weetman D, Ranson H: Multiple-insecticide resistance in Anopheles gambiae mosquitoes, Southern Cote d’Ivoire.Emerg Infect Dis 2012, 18(9):1508. [DOI] [PMC free article] [PubMed]
- 60.Djogbenou L, Noel V, Agnew P: Costs of insensitive acetylcholinesterase insecticide resistance for the malaria vector Anopheles gambiae homozygous for the G119S mutation.Malar J 2010, 9:12. [DOI] [PMC free article] [PubMed]
- 61.Brown ZS, Dickinson KL, Kramer RA: Insecticide resistance and malaria vector control: the importance of fitness cost mechanisms in determining economically optimal control trajectories.J Econ Entomol 2013, 106(1):366. [DOI] [PMC free article] [PubMed]
- 62.Shute G: A method of maintaining colonies of East African strains of Anopheles gambiae.Ann Trop Med Parasitol 1956, 50(1):92. [DOI] [PubMed]
- 63.Alout H, Ndam NT, Sandeu MM, Djégbe I, Chandre F, Dabiré RK, Djogbénou LS, Corbel V, Cohuet A: Insecticide resistance alleles affect vector competence of Anopheles gambiae ss for Plasmodium falciparum field isolates.PLoS One 2013, 8(5):e63849. [DOI] [PMC free article] [PubMed]
- 64.Organization WH: Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes. Edited by WHO. Geneva: World Health Organization; 2013.
- 65.Alout H, Djogbénou L, Berticat C, Chandre F, Weill M. Comparison of Anopheles gambiae and Culex pipiens acetycholinesterase 1 biochemical properties. Comp Biochem Physiol B. 2008;150(3):271–277. doi: 10.1016/j.cbpb.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Labbé P, Milesi P, Yébakima A, Pasteur N, Weill M, Lenormand T. Gene‐dosage effects on fitness in recent adaptive duplications: ace‐1 in the mosquito Culex pipiens. Evolution. 2014;68(7):2092–2101. doi: 10.1111/evo.12372. [DOI] [PubMed] [Google Scholar]
- 67.Ellman GL, Courtney KD, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 68.Milesi P, Labbé P. BioRssay: A R Script for Bioassay Analyses v. 5.1.1. 2013. [Google Scholar]
- 69.Johnson GC, Esposito L, Barratt BJ, Smith AN, Heward J, Di Genova G, Ueda H, Cordell HJ, Eaves IA, Dudbridge F, Twells RCJ, Payne F, Hughes W, Nutland S, Stevens H, Carr P, Tuomilehto-Wolf E, Tuomilehto J, Gough SCL, Clayton DG, Todd JA. Haplotype tagging for the identification of common disease genes. Nat Genet. 2001;29(2):233–237. doi: 10.1038/ng1001-233. [DOI] [PubMed] [Google Scholar]
- 70.Van Den Beukel I, Dijcks FA, Vanderheyden P, Vauquelin G, Oortgiesen M. Differential muscarinic receptor binding of acetylcholinesterase inhibitors in rat brain, human brain and Chinese hamster ovary cells expressing human receptors. J Pharmacol Exp Ther. 1997;281(3):1113–1119. [PubMed] [Google Scholar]
- 71.Raymond-Delpech V, Matsuda K, Sattelle BM, Rauh JJ, Sattelle DB. Ion channels: molecular targets of neuroactive insecticides. Invert Neurosci. 2005;5(3–4):119–133. doi: 10.1007/s10158-005-0004-9. [DOI] [PubMed] [Google Scholar]
- 72.O’Reilly AO, Khambay BP, Williamson MS, Field LM, Wallace BA, Davies TG. Modelling insecticide-binding sites in the voltage-gated sodium channel. Biochem J. 2006;396(2):255–263. doi: 10.1042/BJ20051925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gammon DW, Brown MA, Casida JE. Two classes of pyrethroid action in the cockroach. Pestic Biochem Physiol. 1981;15(2):181–191. doi: 10.1016/0048-3575(81)90084-5. [DOI] [Google Scholar]
- 74.Lund AE, Narahashi T. Kinetics of sodium channel modification as the basis for the variation in the nerve membrane effects of pyrethroids and DDT analogs. Pestic Biochem Physiol. 1983;20(2):203–216. doi: 10.1016/0048-3575(83)90025-1. [DOI] [Google Scholar]
- 75.Casida JE, Gammon DW, Glickman AH, Lawrence LJ. Mechanisms of selective action of pyrethroid insecticides. Annu Rev Pharmacol Toxicol. 1983;23(1):413–438. doi: 10.1146/annurev.pa.23.040183.002213. [DOI] [PubMed] [Google Scholar]
- 76.Scott JG, Matsumura F. Evidence for two types of toxic actions of pyrethroids on susceptible and DDT-resistant German cockroaches. Pestic Biochem Physiol. 1983;19(2):141–150. doi: 10.1016/0048-3575(83)90133-5. [DOI] [Google Scholar]
- 77.Schleier JJ, III, Peterson RK. The joint toxicity of type I, II, and nonester pyrethroid insecticides. J Econ Entomol. 2012;105(1):85–91. doi: 10.1603/EC11267. [DOI] [PubMed] [Google Scholar]


