Abstract
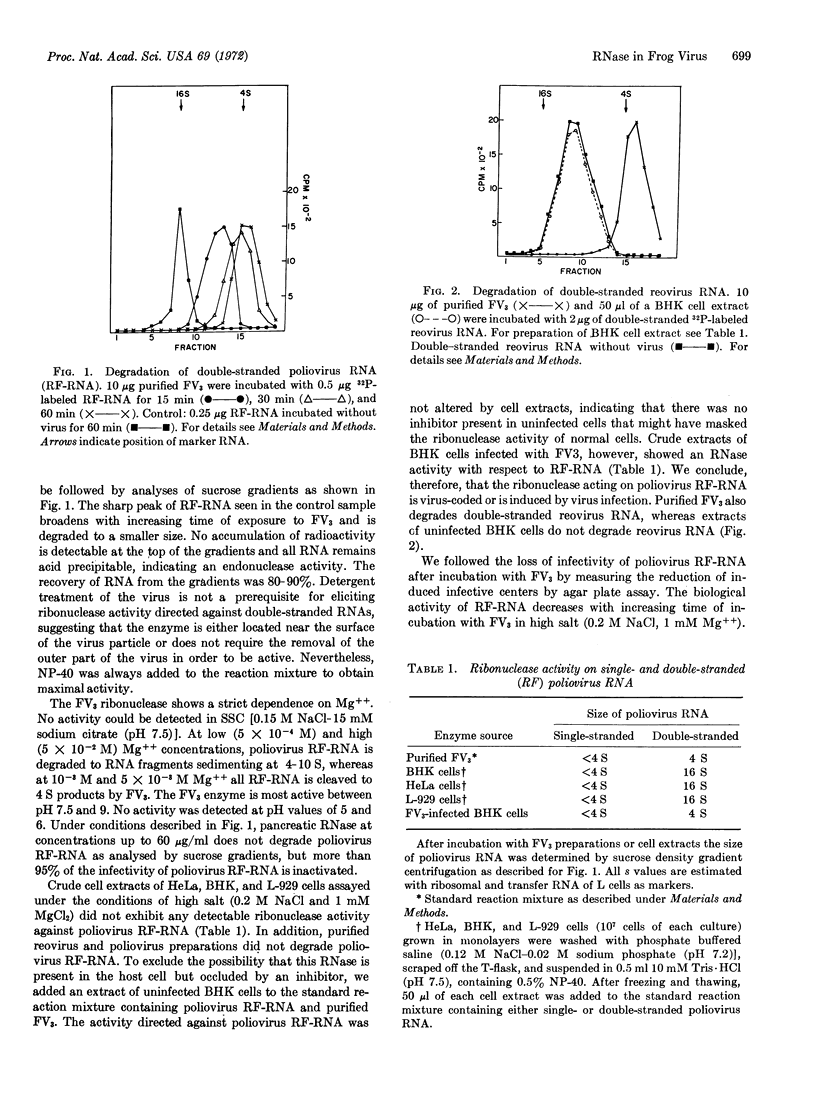
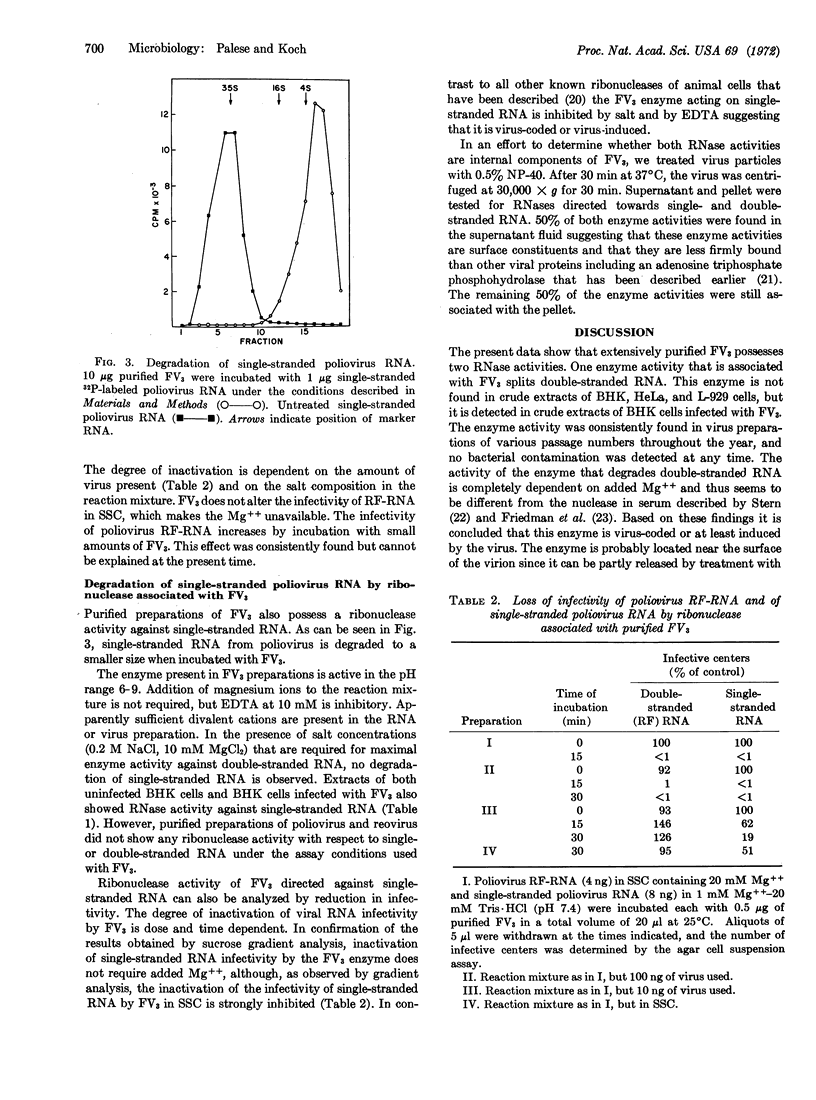
Purified preparations of frog virus 3 possess ribonuclease activities directed against single-and double-stranded RNA. Double-stranded RNAs isolated from purified reovirus type 3 and from HeLa cells infected with poliovirus and single-stranded poliovirus RNA from purified virus are readily degraded by incubation with frog virus 3. The mode of action of the nucleases is endonucleolytic. Under the assay conditions used for the viral enzyme, crude extracts of uninfected HeLa, L, and baby hamster kidney cells did not show enzyme activity against double-stranded RNA but exhibited activity against single-stranded RNA. The dependence of the viral nucleases on divalent cations for optimal activity and the inhibition of the cleavage of single-stranded RNA by 0.2 M NaCl suggests that the enzymes are either virus-coded or virus-induced.
Keywords: virus-associated enzymes, DNA virus, nuclease
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubertin A., Palese P., Tan K. B., Vilagines R., McAuslan B. R. Proteins of a polyhedral cytoplasmic deoxyvirus. 3. Structure of frog virus 3 and location ov virus-associated adenosine triphosphate phosphohydrolase. J Virol. 1971 Nov;8(5):643–648. doi: 10.1128/jvi.8.5.643-648.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALTIMORE D. IN VITRO SYNTHESIS OF VIRAL RNA BY THE POLIOVIRUS RNA POLYMERASE. Proc Natl Acad Sci U S A. 1964 Mar;51:450–456. doi: 10.1073/pnas.51.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Barnard E. A. Ribonucleases. Annu Rev Biochem. 1969;38:677–732. doi: 10.1146/annurev.bi.38.070169.003333. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Koch G. Purification and characterization of poliovirus-induced infectious double-stranded ribonucleic acid. J Biol Chem. 1967 Apr 25;242(8):1736–1743. [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W., Pettersson U., Philipson L. Adenovirus endonuclease: association with the penton of adenovirus type 2. J Mol Biol. 1971 Aug 28;60(1):45–64. doi: 10.1016/0022-2836(71)90446-3. [DOI] [PubMed] [Google Scholar]
- Chow N. L., Simpson R. W. RNA-dependent RNA polymerase activity associated with virions and subviral particles of myxoviruses. Proc Natl Acad Sci U S A. 1971 Apr;68(4):752–756. doi: 10.1073/pnas.68.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Colby C. On the biosynthesis and structure of double-stranded RNA in vaccinia virus-infected cells. Proc Natl Acad Sci U S A. 1969 Sep;64(1):396–403. doi: 10.1073/pnas.64.1.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Barth R. F., Stern R. Anomalous effects of heterologous normal serum on interferon production in mice. Nat New Biol. 1971 Mar 3;230(1):17–18. doi: 10.1038/newbio230017a0. [DOI] [PubMed] [Google Scholar]
- Hausen P., Stein H. Ribonuclease H. An enzyme degrading the RNA moiety of DNA-RNA hybrids. Eur J Biochem. 1970 Jun;14(2):278–283. doi: 10.1111/j.1432-1033.1970.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Bratt M. A. Ribonucleic acid polymerase in virions of Newcastle disease virus: comparison with the vesicular stomatitis virus polymerase. J Virol. 1971 Mar;7(3):389–394. doi: 10.1128/jvi.7.3.389-394.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball P. C., Duesberg P. H. Virus interference by cellular double-stranded ribonucleic acid. J Virol. 1971 Jun;7(6):697–706. doi: 10.1128/jvi.7.6.697-706.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G. Differential effect of phleomycin on the infectivity of poliovirus and poliovirus-induced ribonucleic acids. J Virol. 1971 Jul;8(1):28–34. doi: 10.1128/jvi.8.1.28-34.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Quintrell N., Bishop J. M. An agar cell-suspension plaque assay for isolated viral RNA. Biochem Biophys Res Commun. 1966 Aug 12;24(3):304–309. doi: 10.1016/0006-291x(66)90155-0. [DOI] [PubMed] [Google Scholar]
- Libonati M. Degradation of poly A and double-stranded RNA by aggregates of pancreatic ribonuclease. Biochim Biophys Acta. 1971 Jan 28;228(2):440–445. doi: 10.1016/0005-2787(71)90049-9. [DOI] [PubMed] [Google Scholar]
- Libonati M., Floridi A. Breakdown of double-stranded RNA by bull semen ribonuclease. Eur J Biochem. 1969 Mar;8(1):81–87. doi: 10.1111/j.1432-1033.1969.tb00498.x. [DOI] [PubMed] [Google Scholar]
- MONTAGNIER L., SANDERS F. K. REPLICATIVE FORM OF ENCEPHALOMYOCARDITIS VIRUS RIBONUCLEIC ACID. Nature. 1963 Aug 17;199:664–667. doi: 10.1038/199664a0. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintrell N., Fanshier L., Evans B., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerase(s) of Rous sarcoma virus: effects of virion-associated endonuclease on the enzymatic product. J Virol. 1971 Jul;8(1):17–27. doi: 10.1128/jvi.8.1.17-27.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- Robinson W. S. Ribonucleic acid polymerase activity in Sendai virions and nucleocapsid. J Virol. 1971 Jul;8(1):81–86. doi: 10.1128/jvi.8.1.81-86.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. Simultaneous detection of reverse transcriptase and high molecular weight RNA unique to oncogenic RNA viruses. Science. 1971 Nov 19;174(4011):840–843. doi: 10.1126/science.174.4011.840. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Rada B. Reovirus-directed ribonucleic acid synthesis in infected L cells. J Virol. 1967 Feb;1(1):24–35. doi: 10.1128/jvi.1.1.24-35.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. doi: 10.1073/pnas.61.4.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R. A nuclease from animal serum which hydrolyzes double-stranded RNA. Biochem Biophys Res Commun. 1970 Nov 9;41(3):608–614. doi: 10.1016/0006-291x(70)90056-2. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]



