Abstract
Aims
We investigated clinical characteristics and outcomes of patients with significant valvular disease (SVD) in the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) trial.
Methods and results
ROCKET AF excluded patients with mitral stenosis or artificial valve prostheses. We used Cox regression to adjust comparisons for potential confounders. Among 14 171 patients, 2003 (14.1%) had SVD; they were older and had more comorbidities than patients without SVD. The rate of stroke or systemic embolism with rivaroxaban vs. warfarin was consistent among patients with SVD [2.01 vs. 2.43%; hazard ratio (HR) 0.83, 95% confidence interval (CI) 0.55–1.27] and without SVD (1.96 vs. 2.22%; HR 0.89, 95% CI 0.75–1.07; interaction P = 0.76). However, rates of major and non-major clinically relevant bleeding with rivaroxaban vs. warfarin were higher in patients with SVD (19.8% rivaroxaban vs. 16.8% warfarin; HR 1.25, 95% CI 1.05–1.49) vs. those without (14.2% rivaroxaban vs. 14.1% warfarin; HR 1.01, 95% CI 0.94–1.10; interaction P = 0.034), even when controlling for risk factors and potential confounders. In intracranial haemorrhage, there was no interaction between patients with and without SVD where the overall rate was lower among those randomized to rivaroxaban.
Conclusions
Many patients with ‘non-valvular atrial fibrillation’ have significant valve lesions. Their risk of stroke is similar to that of patients without SVD after controlling for stroke risk factors. Efficacy of rivaroxaban vs. warfarin was similar in patients with and without SVD; however, the observed risk of bleeding was higher with rivaroxaban in patients with SVD but was the same among those without SVD. Atrial fibrillation patients with and without SVD experience the same stroke-preventive benefit of oral anticoagulants.
Keywords: Fibrillation, Anticoagulants, Heart diseases, Regurgitation, Stenosis
See page 3323 for the editorial comment on this article (doi:10.1093/eurheartj/ehu386)
Introduction
Recent trials have assessed the efficacy and safety of novel, oral, direct-acting anticoagulants in patients with non-valvular atrial fibrillation (AF).1–10 There has been some variation in the inclusion and exclusion criteria of these trials (Table 1).1–12 In general, non-valvular AF was considered to be present if there was no mitral stenosis, no heart valve prosthesis, and no valvular disease requiring surgery, whereas only one trial1,2 also excluded patients with any type of haemodynamically relevant valvular disease. Thus, exclusion criteria of most trials would allow for inclusion of patients with other significant valvular lesions. However, the frequency of including patients in these trials who had significant valvular disease (SVD), but who do not meet these exclusion requirements, has not been reported.
Table 1.
Selected exclusion criteria regarding valvular disease in recent oral anticoagulation trials to identify patients with non-valvular atrial fibrillation
| Trial | Exclusion criteria |
|---|---|
| ROCKET AF5,6 | Haemodynamically significant mitral valve stenosis. Prosthetic heart valve. Annuloplasty with or without prosthetic ring, commissurotomy, and/or valvuloplasty are permitted. Planned invasive procedure with potential for uncontrolled bleeding, including major surgery |
| RE-LY1,2 | History of heart valve disorder (i.e. prosthetic valve or haemodynamically relevant valve disease) |
| AVERROES3,4 | Valvular disease requiring surgery. Prosthetic mechanical heart valve. Conditions other than atrial fibrillation that required chronic anticoagulation |
| ARISTOTLE7,8 | Moderate or severe mitral stenosis, conditions other than atrial fibrillation that required anticoagulation (e.g. a prosthetic heart valve) |
| ENGAGE9,10 | Moderate or severe mitral stenosis, unresected atrial myxoma, or a mechanical heart valve (subjects with bioprosthetic heart valves and/or valve repair could be included) |
| ACTIVE W and A11 | Requirement for clopidogrel or for oral anticoagulant (such as prosthetic mechanical heart valve), and mitral stenosis |
| SPORTIF III12 | Mitral stenosis or previous valvular heart surgery |
The clinical characteristics and the outcomes of patients with SVD when treated with oral anticoagulants like vitamin K antagonists, especially warfarin or novel factor IIa or Xa inhibitors, are unknown. Therefore, this analysis focuses on AF patients with significant valvular lesions who were not considered to have valvular AF, as defined by haemodynamically significant mitral valve stenosis or prosthetic heart valve (Table 1). For this purpose, we retrospectively analysed the data of the large randomized trial Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) that compared warfarin and rivaroxaban, a novel factor Xa inhibitor, in patients with non-valvular AF.5,6
Methods
The rationale and design of the ROCKET AF study have been published (ClinicalTrials.gov number, NCT00403767).5 In brief, ROCKET AF was a multicentre, international, double-blind, double-dummy, randomized trial comparing fixed-dose rivaroxaban with dose-adjusted warfarin for prevention of all stroke (ischaemic or haemorrhagic) or systemic embolism. The study was funded by Johnson & Johnson Pharmaceutical Research & Development (Raritan, NJ, USA) and Bayer HealthCare AG (Leverkusen, Germany). The Duke Clinical Research Institute (Durham, NC, USA) coordinated the trial and performed the statistical analyses for this article independent of the sponsors. An international executive steering committee designed the study and takes responsibility for the accuracy and completeness of the analyses. All appropriate national regulatory authorities and ethics committees at participating centres approved the study.
This analysis included 14 171 of 14 264 patients in the ROCKET AF trial. Due to one site's violations of Good Clinical Practice guidelines that made the data unreliable, 93 patients were excluded from all efficacy analyses before unblinding but were included in the safety analyses.6
A total of 2003 patients had SVD. Eleven of these patients were at the site that violated Good Clinical Practice guidelines, and an additional 4 patients were randomized but did not receive study drug. Therefore, 1992 SVD patients were used in the analysis of efficacy endpoints [intention-to-treat (ITT) population], and 1999 SVD patients were used in the analysis of safety endpoints (safety population). None of the patients had undergone transcutaneous aortic valve replacement.
Five patients had been randomized despite the presence of a prosthetic valve. The sites were immediately contacted and instructed to discontinue these patients from study drug. These patients were included in the ITT population but not in the safety population. Three of these five patients died before the end of the study in 1 year to about 3 years after enrolment; the other two patients remained alive and were followed until the end of the study.
Patients were randomized to fixed-dose rivaroxaban [20 mg once daily; 15 mg daily for those with moderately impaired renal function (creatinine clearance 30–49 mL/min)] or dose-adjusted warfarin (target international normalized ratio 2.0–3.0), in a double-blind fashion.
Definitions and endpoints
Non-valvular AF was defined5,6 as the presence of AF and the absence of mitral stenosis or prosthetic heart valves, whereas annuloplasty with or without a prosthetic ring, commissurotomy, and/or valvuloplasty were permitted. In addition, a planned invasive procedure with a potential for uncontrolled bleeding, including major surgery, was an exclusion criterion. Patients with other types of valvular disease could be included in the trial. Specifically, the case report form asked whether there was ‘significant valvular disease,’ and if so, it asked for ‘valve location and abnormality’ and ‘etiology.’ Thus, for the purpose of this study, any type of valvular lesion that did not meet the above exclusion criteria was included in SVD if it was considered to be significant by the recruiting physician(s) in order to reflect clinical practice (external validity).
Additional exclusion criteria were transient or self-limited AF caused by reversible conditions, severe renal insufficiency (creatinine clearance <30 mL/min), active liver disease, or serum enzyme levels >2× the upper limit of normal, conditions associated with increased bleeding risk, and concurrent antithrombotic drugs other than aspirin (≤100 mg/day as monotherapy was allowed).
The primary efficacy endpoint in ROCKET AF was the composite of all stroke (both ischaemic and haemorrhagic) and systemic embolism. Detection of primary endpoints was enhanced by a standardized stroke symptom questionnaire and additional evaluation by local study-affiliated neurologists or stroke specialists blinded to treatment. A full description of the endpoints in ROCKET AF has been published.5,6 Secondary efficacy endpoints included cardiovascular death; all-cause death; the composite of stroke, systemic embolism, or cardiovascular death; and the composite of stroke, systemic embolism, or all-cause death. The safety endpoint was major or non-major clinically relevant bleeding or intracranial haemorrhage. All suspected primary endpoint events and causes of death were adjudicated by an independent clinical endpoint committee.
Patients were evaluated at a minimum of every 4 weeks throughout the trial for study drug management, ascertainment of adverse events, and surveillance for primary endpoints and other clinical events.
Statistical analysis
We used Cox proportional hazards models to assess the association with the risk of outcomes for (1) patients with SVD vs. patients without SVD and (2) rivaroxaban vs. warfarin within subgroups of patients with and without SVD. All models included a term for the interaction between randomized treatment and SVD as well as covariates identified as predictive of outcomes by modelling in the full ROCKET AF cohort. The efficacy endpoint models used the ITT population and time period to site notification, and they contained the following as covariates: age, sex, body mass index, prior stroke/transient ischaemic attack, vascular disease (myocardial infarction, peripheral artery disease, carotid occlusive disease), chronic heart failure, hypertension, chronic obstructive pulmonary disease, diabetes mellitus, paroxysmal AF, diastolic blood pressure, creatinine clearance (Cockcroft–Gault), heart rate, and abstinence from alcohol use. The safety endpoint model (safety model I) used the safety population and time period while on treatment, defined as on therapy plus 2 days, and contained the following as covariates: age, sex, prior stroke/transient ischaemic attack, anaemia, prior gastrointestinal bleeding, chronic obstructive pulmonary disease, diastolic blood pressure, creatinine clearance (Cockcroft–Gault), platelets, albumin, and prior aspirin, vitamin K antagonist, or thienopyridine use. In a second step (safety model II), heart failure was added to this model as a covariate on an exploratory basis since it was hypothesized that congestion and hypoperfusion of the gastrointestinal tract and of the liver might increase the propensity to bleeding.
Categorical variables are summarized as percentages (counts), and differences were tested with the Pearson χ2-test. Continuous variables are summarized as medians (25th, 75th percentiles), and differences were tested with the Wilcoxon rank-sum test. Outcomes are presented as events per 100 patient-years (pt-yrs). Risk relationships are presented as adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) derived from the adjusted Cox models. The time to event for each group was assessed using the Kaplan–Meier method. All statistical analyses were performed by the Duke Clinical Research Institute using SAS software (version 9.2, SAS Institute, Cary, NC, USA).
Results
Clinical characteristics of patients with and without significant valvular disease
Among 14 171 patients included in this analysis, 2003 (14.1%) patients had SVD as classified by the investigator. Data from 1992 patients were used for analysis of efficacy endpoints, and data from 1999 patients were used for safety analysis. Table 2 presents information on type of SVD. Mitral regurgitation predominated (89.6%), followed by aortic regurgitation (24.8%), and aortic stenosis (11.0%), exceeding 100% due to cases with more than one type of valvular lesion. The etiology was considered as calcific or degenerative in 40.4% of cases, post-infarction and/or ischaemic in 12.9%, rheumatic in 3.2%, and other, unknown, or having no data in 15.7, 15.9, and 13.7% of cases, respectively. A prior cardiac valvular procedure had been performed in 106 cases (5.3%), which had been a valvuloplasty in 64 of these cases (60.4%) or designated as ‘other cardiac valvular procedure’ in the remaining 42 cases.
Table 2.
Type of valvular disease in patients assessed as having significant valvular disease
| Characteristic | N (%) |
|---|---|
| Valve location/abnormalitya | |
| Aortic stenosis | 215 (11.0%) |
| Aortic regurgitation | 486 (24.8%) |
| Mitral regurgitation | 1756 (89.6%) |
| Other (without any of preceding) | 11 (0.6%) |
| Etiologya | |
| Rheumatic | 62 (3.2%) |
| Congenital | 15 (0.8%) |
| Calcific/degenerative | 791 (40.4%) |
| Post-infarction and/or ischaemic | 253 (12.9%) |
| Other | 307 (15.7%) |
| Unknown | 312 (15.9%) |
| No data | 268 (13.7%) |
| Prior cardiac valvular procedures | 106 (5.3%) |
| Valvuloplasty | 64 (60.4%) |
| Other cardiac valvular procedure | 42 (39.6%) |
In 1960 of 2003 patients, detailed information was available. Percentages for valve location/abnormality and etiology calculated among patients with history of significant valvular disease. Percentages for valvular procedures calculated among patients with prior procedure.
aA patient could be in more than one category.
The clinical characteristics of patients in the overall trial population and of patients separated according to the presence or absence of SVD are presented in Table 3. Significant valvular disease patients were older than patients without SVD (median 75 vs. 72 years; P < 0.0001). There was no difference in sex (female 39.4 vs. 39.6%). There was also no difference in the CHADS2 and HAS-BLED scores or in the prevalence of diabetes mellitus. Patients with SVD had persistent AF slightly more often but had paroxysmal and newly diagnosed or new-onset AF less often than patients without SVD (P = 0.049). The time since AF diagnosis was significantly longer in patients with vs. without SVD (median 4 and 3 years, respectively, P < 0.0001). Prior stroke, embolism, or transient ischaemic attack was less prevalent in SVD patients (48.2 vs. 55.9%, P < 0.0001). Significant valvular disease patients also more often had previously received vitamin K antagonists (72.5 vs. 60.8%, P < 0.0001) and more often had congestive heart failure (70.4 vs. 61.2%, P < 0.0001), prior myocardial infarction (24.2 vs. 16.1%, P < 0.0001), peripheral vascular disease (8.0 vs. 5.5%, P < 0.0001), chronic obstructive pulmonary disease (14.4 vs. 9.8%, P < 0.0001), reduced creatinine clearance (62 vs. 68 mL/min, P < 0.0001), and previous coronary artery bypass surgery (11.9 vs. 6.5%, P < 0.0001). Significant valvular disease was relatively more frequent in North America and Eastern Europe, and was less frequent in Western Europe and Latin America. There was no substantial difference in race, although the comparison did reach statistical significance (P < 0.0001), driven largely by a slight shift between Asian and ‘other’ groups. There were fewer patients of Hispanic origin in the SVD group vs. the no-SVD group (7.8 vs. 17.9%, P < 0.0001).
Table 3.
Baseline characteristics for all intention-to-treat patients and for patients grouped by the absence or presence of significant valvular disease
| Variable | Overall trial population (N = 14 171) | SVD (N = 1992) | No SVD (N = 12 179) | P-value |
|---|---|---|---|---|
| Age, years | 73 (65, 78) | 75 (68, 79) | 72 (65, 78) | <0.0001 |
| Female | 5605 (39.6%) | 785 (39.4%) | 4820 (39.6%) | 0.89 |
| Race | ||||
| White | 11 786 (83.2%) | 1672 (83.9%) | 10 114 (83.0%) | <0.0001 |
| Black | 180 (1.3%) | 20 (1.0%) | 160 (1.3%) | |
| Asian | 1786 (12.6%) | 273 (13.7%) | 1513 (12.4%) | |
| Other | 419 (3.0%) | 27 (1.4%) | 392 (3.2%) | |
| Hispanic | 2331 (16.4%) | 156 (7.8%) | 2175 (17.9%) | <0.0001 |
| Region | ||||
| Asia/Pacific Islands | 2109 (14.9%) | 301 (15.1%) | 1808 (14.8%) | <0.0001 |
| Eastern Europe | 5407 (38.2%) | 582 (29.2%) | 4825 (39.6%) | |
| Latin America | 1878 (13.3%) | 76 (3.8%) | 1802 (14.8%) | |
| North America | 2681 (18.9%) | 653 (32.8%) | 2028 (16.7%) | |
| Western Europe | 2096 (14.8%) | 380 (19.1%) | 1716 (14.1%) | |
| Body mass index, kg/m2 | 28.2 (25.1, 32.0) | 27.7 (24.8, 31.2) | 28.3 (25.2, 32.1) | <0.0001 |
| Systolic blood pressure, mm Hg | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | |
| Diastolic blood pressure, mm Hg | 80 (70, 85) | 80 (70, 84) | 80 (70, 86) | |
| Type of atrial fibrillation | ||||
| Persistent | 11 485 (81.0%) | 1653 (83.0%) | 9832 (80.7%) | 0.049 |
| Paroxysmal | 2490 (17.6%) | 317 (15.9%) | 2173 (17.8%) | |
| Newly diagnosed or new onset | 196 (1.4%) | 22 (1.1%) | 174 (1.4%) | |
| Years since AF diagnosis | 3 (1, 7) | 4 (1, 8) | 3 (1, 7) | <0.0001 |
| Prior chronic aspirin use | 5184 (36.6%) | 690 (34.6%) | 4494 (36.9%) | 0.052 |
| Prior vitamin K antagonist use | 8853 (62.5%) | 1444 (72.5%) | 7409 (60.8%) | <0.0001 |
| CHADS2 score, mean (SD) | 3.5 (0.9) | 3.5 (1.0) | 3.5 (0.9) | 0.98 |
| HAS-BLED score, mean (SD) | 2.8 (0.9) | 2.8 (1.0) | 2.8 (0.9) | 0.18 |
| Previous stroke, embolism, or TIA | 7767 (54.8%) | 961 (48.2%) | 6806 (55.9%) | <0.0001 |
| Congestive heart failure | 8851 (62.5%) | 1402 (70.4%) | 7449 (61.2%) | <0.0001 |
| Hypertension | 12 824 (90.5%) | 1775 (89.1%) | 11 049 (90.7%) | 0.023 |
| Diabetes mellitus | 5647 (39.8%) | 798 (40.1%) | 4849 (39.8%) | 0.84 |
| Previous myocardial infarction | 2446 (17.3%) | 482 (24.2%) | 1964 (16.1%) | <0.0001 |
| Peripheral vascular disease | 832 (5.9%) | 160 (8.0%) | 672 (5.5%) | <0.0001 |
| COPD | 1481 (10.5%) | 287 (14.4%) | 1194 (9.8%) | <0.0001 |
| Previous CABG | 1029 (7.3%) | 238 (11.9%) | 791 (6.5%) | <0.0001 |
| Current smoker | 4760 (33.6%) | 768 (38.6%) | 3992 (32.8%) | <0.0001 |
| Creatinine clearance,a mL/min | 67 (52, 87) | 62 (49, 80) | 68 (53, 88) | <0.0001 |
Patients with significant vascular disease were from the intention-to-treat population that was used for analysis of efficacy outcomes. Continuous variables are shown as median (25th, 75th percentiles). Categorical variables are shown as n (%). P-values are from Wilcoxon rank sum tests for continuous variables and Pearson Chi-square tests for categorical variables.
CABG, indicates coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; HAS-BLED, score for assessing bleeding risk (Hypertension; Abnormal renal/liver function; Stroke history; Bleeding predisposition; Labile international normalized ratio; Elderly, age ≥65; Drug/alcohol usage); TIA, transient ischaemic attack.
aCockcroft–Gault.
Efficacy and safety endpoints in patients with and without significant valvular disease
Event rates for efficacy and safety outcomes according to the SVD status are shown in Table 4. Although stroke or systemic embolism (Figure 1) or other combined secondary endpoints were slightly more frequent in patients with SVD than in those without, these differences were not significant after multivariable adjustments, except for systemic embolism, which occurred more often in SVD patients (0.32 vs. 0.14 events per 100 pt-yrs; P = 0.049). Major or non-major clinically relevant bleeding and major bleeding alone occurred significantly more frequently in patients with SVD. The composite endpoint of stroke and major bleeding was significantly (P = 0.0099) more frequent in patients with than in those without SVD [adjusted HR 1.22 (1.05, 1.42); Table 4].
Table 4.
Efficacy and safety outcomes as a function of the absence or presence of significant valvular disease
| SVD events/100 pt-yrs (total events) | No SVD events/100 pt-yrs (total events) | HR (95% CI) SVD vs. no SVD | P-value | |
|---|---|---|---|---|
| Efficacy outcomes (ITT population) n = 1992 | ||||
| Stroke or SE | 2.23 (88) | 2.09 (487) | 1.07 (0.85–1.35) | 0.58 |
| Stroke, SE, or vascular death | 5.20 (199) | 4.31 (982) | 1.09 (0.93–1.27) | 0.28 |
| Stroke, SE, vascular death, or MI | 6.36 (240) | 4.99 (1128) | 1.14 (0.99–1.31) | 0.072 |
| Stroke | 1.92 (76) | 1.96 (458) | 0.98 (0.77–1.26) | 0.89 |
| Systemic embolism | 0.32 (13) | 0.14 (34) | 2.02 (1.00–4.08) | 0.049 |
| MI | 1.51 (60) | 0.90 (212) | 1.32 (0.98–1.78) | 0.065 |
| All-cause death | 5.54 (212) | 4.39 (1002) | 1.09 (0.93–1.26) | 0.29 |
| Safety outcomes (safety on-treatment population) n = 1999 | ||||
| Major or NMCR bleeding | 18.24 (493) | 14.16 (2431) | 1.14 (1.03–1.25) | 0.011 |
| Major bleeding | 5.11 (156) | 3.27 (625) | 1.32 (1.10–1.57) | 0.0027 |
| GI bleeding | 44% | 40% | n.s. | |
| ICH | 0.80 (25) | 0.59 (114) | 1.35 (0.87–2.09) | 0.18 |
| Composite endpoint: stroke/major bleeding | 7.06 (211) | 5.25 (982) | 1.22 (1.05, 1.42) | 0.0099 |
HR estimates are based on multivariable analysis (see Methods).
CI, confidence interval; GI, gastrointestinal; HR, hazard ratio; ICH, intracranial haemorrhage: NMCR, non-major clinically relevant; MI, myocardial infarction; pt-yrs, patient-years; SE, systemic embolism; SVD, significant vascular disease.
Figure 1.
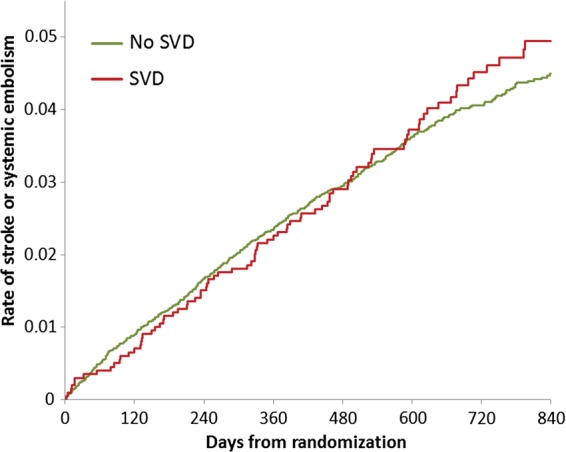
Unadjusted primary combined outcome parameters of stroke or systemic embolism in patients without (no SVD) and with (SVD) significant valvular disease.
Efficacy and safety of rivaroxaban vs. warfarin in patients with and without significant valvular disease
The rate of stroke or systemic embolism in patients treated with rivaroxaban compared with warfarin was consistent among patients with SVD (2.01% rivaroxaban vs. 2.43% warfarin; HR 0.83, 95% CI 0.55–1.27) and without SVD (1.96% rivaroxaban vs. 2.22% warfarin; HR 0.89, 95% CI 0.75–1.07; interaction P = 0.76) (Figure 2 and Table 5). However, the rates of major and non-major clinically relevant bleeding in patients with SVD were higher among those treated with rivaroxaban compared with warfarin (19.8% rivaroxaban vs. 16.8% warfarin; HR 1.25, 95% CI 1.05–1.49), whereas there was no difference among those without SVD (14.2 vs. 14.1%; HR 1.01, 95% CI 0.94–1.10; interaction P = 0.034). There was no difference in HRs whether heart failure was included in the safety model or not (Supplementary material online), and there was also no difference in the new use of any antiplatelet drugs or the duration of aspirin use during follow-up (Supplementary material online). Median time in therapeutic range of INR values (TTR) was not significantly different in warfarin patients with and without SVD (60.8 vs. 57.4). The rate of intracranial haemorrhage was lower with rivaroxaban than with warfarin among those without SVD but was about the same among those with SVD. This difference in interaction of SVD and treatment did not achieve statistical significance (P = 0.084), however.
Figure 2.
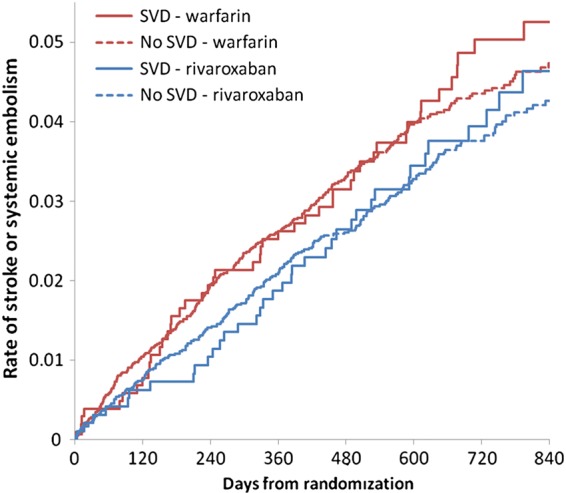
Unadjusted primary combined outcome parameters of stroke or systemic embolism in patients with and without significant valvular disease (SVD) randomized to either rivaroxaban or warfarin.
Table 5.
Efficacy (intention-to-treat population) and safety (on-treatment population) outcomes in patients with and without significant valvular disease in patients randomized to rivaroxaban and warfarin
| SVD |
No SVD |
P-value for interaction of SVD and treatment | |||||
|---|---|---|---|---|---|---|---|
| Rivaroxaban events/100 pt-yrs (total events) | Warfarin events/100 pt-yrs (total events) | Rivaroxaban vs. Warfarin HR (95% CI) | Rivaroxaban events/100 pt-yrs (total events) | Warfarin events/100 pt-yrs (total events) | Rivaroxaban vs. Warfarin HR (95% CI) | ||
| Efficacy outcomes | |||||||
| Stroke or SE | 2.01 (38) | 2.43 (50) | 0.83 (0.55–1.27) | 1.96 (231) | 2.22 (256) | 0.89 (0.75–1.07) | 0.76 |
| Stroke, SE, or vascular death | 5.14 (94) | 5.26 (105) | 0.99 (0.75–1.31) | 4.16 (478) | 4.47 (504) | 0.94 (0.83–1.06) | 0.72 |
| Stroke, SE, vascular death, or MI | 6.09 (110) | 6.62 (130) | 0.94 (0.73–1.21) | 4.81 (549) | 5.17 (579) | 0.94 (0.83–1.05) | 0.98 |
| All-cause death | 5.48 (100) | 5.60 (112) | 0.98 (0.75–1.29) | 4.19 (482) | 4.60 (520) | 0.91 (0.80–1.03) | 0.60 |
| Safety outcomes | |||||||
| Major or NMCR bleeding | 19.81 (253) | 16.83 (240) | 1.25 (1.05–1.49) | 14.19 (1222) | 14.14 (1209) | 1.01 (0.94–1.10) | 0.034 |
| Major bleeding | 6.14 (88) | 4.20 (68) | 1.56 (1.14–2.14) | 3.22 (307) | 3.33 (318) | 0.98 (0.84–1.15) | 0.010 |
| ICH | 0.88 (13) | 0.73 (12) | 1.27 (0.58–2.79) | 0.43 (42) | 0.74 (72) | 0.59 (0.40–0.86) | 0.084 |
Data are based on multivariable analysis (see Methods). The overall ICH-adjusted hazard ratio (95% confidence interval) were 0.67 (0.47–0.93) P = 0.02.6
CI, confidence interval; HR, hazard ratio; ICH, intracranial haemorrhage: NMCR, non-major clinically relevant; MI, myocardial infarction; pt-yrs, patient-years; SE, systemic embolism; SVD, significant vascular disease.
Discussion
Large, multicentre randomized trials1–10 have recently established the efficacy and safety of novel direct-acting oral anticoagulants for the prevention of stroke and systemic embolism in patients with non-valvular AF. In these trials, non-valvular AF was defined as the absence of (rheumatic) mitral stenosis and prosthetic heart valves (Table 1), but haemodynamically relevant valve disease was also generally excluded.1 However, uncertainty and even confusion exists among physicians as to whether patients with other types of SVD and AF paradoxically fall under the category of non-valvular AF and may be treated by novel anticoagulants. Based on the inclusion and exclusion criteria used in ROCKET AF, a large fraction of included patients had SVD.
Definition of non-valvular atrial fibrillation
The American College of Cardiology/American Heart Association/European Society of Cardiology 2006 guidelines stated that ‘The term “non-valvular AF” is restricted to cases in which the rhythm disturbance occurs in the absence of rheumatic mitral valve disease, a prosthetic heart valve, or mitral valve repair.’13 Similarly, the recent update of the European Society of Cardiology guidelines continue to divide AF into ‘valvular’ or ‘non-valvular’ categories. ‘Valvular AF’ was used to imply that AF is related to rheumatic valvular disease (predominantly mitral stenosis) or prosthetic heart valves.13,14 The pinpointing of rheumatic mitral stenosis and prosthetic heart valves as separate entities is based on the generally accepted higher risk of thromboembolic stroke in these patients.15
Prevalence of valvular heart disease in clinical practice: results from clinical registries
Several recent registries have shown that patients with valvular disease are relatively prevalent in clinical practice.16–21 However, the prevalence of rheumatic valve disease varied between 2 and 4.3% of patients included in such registries.17,19 Non-rheumatic valve disease was reported in 19–48%16,17 or even 81%, if minor degrees of mitral regurgitation were also considered (Bosch R, Cardio Centrum Ludwigsburg-Bietigheim, Ludwigsburg, Germany; Personal communication; 1 July 2013, referring to ref.19).
Although these registries show some variability, the generally high prevalence of patients with any type of valvular disease in clinical practice underlines the need for a better understanding of the benefits and risks of treating patients with SVD with a novel anticoagulant like rivaroxaban. To our knowledge, there has not been a fully published analysis of patients with SVD among the population traditionally designated as ‘non-valvular.’ Several observations suggest that the incidence and localization of thrombus formation in patients with ‘rheumatic’ AF (i.e. in mitral stenosis) may be different from that in non-valvular AF. Among patients with rheumatic AF, about half of thrombi were found in the left atrial appendage and the rest in its cavity, whereas in non-valvular AF, thrombi were predominantly found in the left atrial appendage.22 This difference in location of thrombi may influence outcomes and efficacy of anticoagulant therapy.
Clinical characteristics and outcomes of patients with and without significant valvular disease in ROCKET AF
The ROCKET AF protocol was designed and conducted specifically to ensure that patients in the trial were similar to those in the clinical trials upon which the non-inferiority margin was based.
Apart from patients with haemodynamically significant mitral valve stenosis, prosthetic heart valves, or planned invasive procedures with potential for uncontrolled bleeding, including major surgery (Table 1), SVD of other types occurred in 14% of patients in ROCKET AF (Table 2), most frequently mitral (89.6%) or aortic regurgitation (24.8%), and aortic stenosis (11.0%).
Mitral regurgitation of rheumatic origin has become a relatively rare entity since mitral regurgitation nowadays is mostly due to (i) the presence of coronary artery disease and its complications or (ii) left ventricular dysfunction due to other causes. This is consistent with the higher prevalence of heart failure and prior myocardial infarction observed in patients with mitral regurgitation in ROCKET AF and substantiates the observation that SVD patients were more prevalent in North America and Eastern Europe (Table 3).
The major finding in this post hoc analysis was that after adjusting for differences in baseline prognostic factors, participants with SVD shared an incidence of thromboembolic ischaemic events and mortality that was similar to that of participants without SVD. In line with identical CHADS2 scores, the rates of stroke were similar among patients with and without SVD. However, the rate of systemic embolism was twice as high in SVD patients and reached marginal statistical significance (P = 0.049). All other efficacy outcomes were not significantly different, although they also tended to be numerically higher in SVD patients. The differences in the rates of systemic embolism may reflect the play of chance.
We observed a significantly higher prevalence of major or non-major clinically relevant bleeding, and of major bleeding alone after multivariable adjustment, which included (among other factors) prior aspirin, vitamin K antagonist, or thienopyridine use, and renal function. There were no significant differences between patients with and without SVD with regard to gastrointestinal bleeding. The HAS-BLED23,24 score as a measure of the risk of bleeding did not differ between patients with and without SVD.
The question arises as to whether the adjusted differences in extra-cranial bleeding events are a play of chance, are a true biological effect, or may reflect hitherto unidentified residual confounding effects (e.g. differences in practice patterns). Since patients with SVD more frequently had vascular disease, deviations from baseline concomitant drug therapy, especially antiplatelet drugs, during the course of the trial would be a possible explanation. However, there was no difference in the new use of antiplatelet drugs in those who were not on any one drug at baseline, or of the duration of aspirin use in all patients or in those on rivaroxaban or warfarin, nor in the presence of heart failure (as hypothesized above; Supplementary material online). Although a higher proportion of patients in the SVD group had been on vitamin K antagonism therapy, which beforehand could have influenced the outcomes in the SVD group in favour of warfarin, prior vitamin K antagonist use was adjusted for in the analysis and, thus, is improbable as an explanation. Overall, the clinical importance of these post hoc observations requires further study.
Efficacy and safety of rivaroxaban vs. warfarin in patients with and without significant valvular disease
While the above observations are based on patients with and without SVD independent of treatment allocation to either warfarin or rivaroxaban, the question arises as to whether any differences might exist with regard to the outcomes on either drug. The results of our present analyses show that the effects of warfarin and rivaroxaban on thromboembolic and ischaemic cardiovascular outcomes did not differ among AF patients with and without SVD. With regard to bleeding, a statistically significant quantitative interaction was observed between treatment and SVD, with HRs for major or non-major clinically relevant bleeding combined and major bleeding alone that were higher with rivaroxaban than with warfarin in patients with (1.25 and 1.56, respectively, with 95% CIs above 1.0) and without SVD (1.1 and 0.98, respectively). Whether this effect is real or simply the result of multiple post hoc reviews of the data is debatable.
Is non-valvular atrial fibrillation a misnomer?
The generally accepted term ‘non-valvular AF’ is misleading since the ROCKET AF trial, as well as other trials (Table 1), has allowed the inclusion of patients who, although not having mitral stenosis or artificial heart valves or valve repair, could have other types of valvular heart disease. In ROCKET AF, these valvular lesions were considered by the recruiting investigator and his or her team as significant. As long as there is no agreed-upon new term, ‘non-valvular AF’ will continued to be used, but one should always stress that this does not exclude patients with other types of valvular heart disease from therapy with novel anticoagulants like rivaroxaban.
Limitations
The protocol did not include precise quantification of valve disease. However, the term ‘significant’ valvular lesion implied that the physician did not consider it as less than moderate. On the other hand, it could also not be of such haemodynamic significance that cardiac surgery would be necessary in the foreseeable future since this was an exclusion criterion. Thus, the majority of patient can be suspected to have had moderate valve disease.
Some baseline characteristics of patients with SVD differed significantly from those of patients without SVD. Thus, despite statistical adjustments, substantial bias might remain, and, therefore, results should be regarded as hypothesis generating. Finally, the number of events in the group of patients with SVD, especially intracranial bleeding, was particularly low, which may indicate that these differences may have occurred by chance.
Conclusions
In the ROCKET AF trial, every seventh patient had SVD. This proportion is probably a low estimate of the prevalence of SVD in clinical practice.16–21 Importantly, AF patients with SVD experienced the same stroke-prevention benefit from oral anticoagulants as did AF patients without SVD. The quantitative interaction in bleeding rates that were higher with rivaroxaban than with warfarin in patients with SVD requires special attention and careful use of rivaroxaban in these patients. Therefore, SVD patients, except for those with haemodynamically significant mitral valve stenosis or prosthetic heart valves, represent an important part of the spectrum of non-valvular AF to which the results of trials of oral anticoagulants apply, at least as far as the use of rivaroxaban is concerned.
The type of patients included in this trial corresponds to the definition of non-valvular atrial fibrillation as was just re-emphasized in the very recent AHA/ACC/HRS guidelines.25 Since these patients are encompassed in the prescription labels of the drug regulatory authorities, it is important for the physician to know that there might be some differences between patients with and without SVD, even if patients meet the criteria for non-valvular AF.
Previous presentation
These data were partly presented at the Scientific Sessions of the American College of Cardiology 2013: G. Breithardt et al. Characteristics and Outcomes of Patients with Atrial Fibrillation and Significant Valvular Lesions: Experience from the ROCKET AF Trial. J Am College Cardiol 2013;61:Supplement, E282; G. Breithardt et al. Outcomes of Patients with Atrial Fibrillation and Significant Valvular Lesions: Comparison of the Effects of Rivaroxaban and Warfarin in the ROCKET AF Trial. J Am College Cardiol 2013;61:Supplement, E339.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work (the ROCKET AF trial) was supported by research grants from Janssen Research & Development (Raritan, NJ, USA) and Bayer HealthCare AG (Leverkusen, Germany). Funding to pay the Open Access publication charges for this article was provided by Janssen Pharmaceuticals Research & Development and Bayer HealthCare.
Conflict of interest: G.B.: Consultant to Bayer Health Care, J&J, BoehringerIngelheim, Sanofi-Aventis, MSD, 3M. H.B.: None. S.D.B.: Employed by Bayer HealthCare Pharmaceuticals. A.S.H.: None. J.P.P.: Consulting fees/honoraria for Johnson & Johnson, Medtronic, Forest Laboratories, BMS/Pfizer, Spectranetics; research grants from Johnson & Johnson. S.R.S.: None. Y.L.: Consulting fees from Johnson & Johnson. M.R.P.: Consulting fees from Bayer Healthcare, Ortho McNeil Jansen, Medscape—theheart.org, Ikaria; research grants from Johnson and Johnson, Maquet, Astra Zeneca, National Heart Lung and Blood Institute, Genzyme. J.L.H.: Consulting fees from BoehringerIngelheim, Sanofi-Aventis, Biotronik, Inc., Ortho-McNeil-Janssen Pharmaceuticals, Inc., Johnson & Johnson, Medtronik, Inc., AstraZeneca, Daiichi Sankyo Pharma, Pfizer, Inc., Bristol Meyers Squibb, Bayer HealthCare AG. D.E.S.: Consulting fees from BoehringerIngelheim, Bristol-Myers Squibb, Johnson and Johnson, Inc., Pfizer, Daiichi Sankyo, Medtronic, Inc., Bayer Healthcare. G.J.H.: Consulting fees from Duke Clinical Research Institute, Bayer Healthcare, and Medscape—the heart.org. W.H.: Consulting fees from Sygnis Pharma Germany, Photothera USA, BoehringerIngelheim, Codman USA, and Bayer; research grants from BoehringerIngelheim. R.C.B.: Consulting fees from Bristol-Myers Squibb, Sanofi-Aventis, BoehringerIngelheim; research grants from BMS, Bayer Pharmaceuticals, Johnson and Johnson, Regado Biosciences, AstraZeneca. C.C.N.: Employed by Johnson & Johnson Pharmaceutical Research and Development. K.W.M.: Full disclosures prior to 1 August 2013 available at www.dcri.org. Disclosures after August 1, 2013 available at http://med.stanford.edu/profiles/kenneth_mahaffey. K.A.A.F.: Consulting fees from BoehringerIngelheim, Sanofi-Aventis, Astra Zeneca, Johnson & Johnson/Bayer; research grants from Eli Lilly. R.M.C.: Consulting fees from KOWA, Eli Lilly, GlaxoSmithKline, WebMD, Bristol Myers Squibb, Nitrox LLC, Bayer, Orexigen Therapeutics, Sanofi-Aventis, Medtronic, BoehringerIngelheim, Gilead; research grants from BMS, Roche, Merck, Merck, Novartis, Scios/Johnson and Johnson, Amilyn, Bristol Myers Squibb, Bayer.
References
- 1.Ezekowitz MD, Connolly S, Parekh A, Reilly PA, Varrone J, Wang S, Oldgren J, Themeles E, Wallentin L, Yusuf S. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805–810. doi: 10.1016/j.ahj.2009.02.005. 810.e1–2. [DOI] [PubMed] [Google Scholar]
- 2.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 3.Eikelboom JW, O'Donnell M, Yusuf S, Diaz R, Flaker G, Hart R, Hohnloser S, Joyner C, Lawrence J, Pais P, Pogue J, Synhorst D, Connolly SJ. Rationale and design of AVERROES: apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. Am Heart J. 2010;159:348–353.e1. doi: 10.1016/j.ahj.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O'Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 5.ROCKET AF Study Investigators. Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159:340–347.e1. doi: 10.1016/j.ahj.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 7.Lopes RD, Alexander JH, Al-Khatib SM, Ansell J, Diaz R, Easton JD, Gersh BJ, Granger CB, Hanna M, Horowitz J, Hylek EM, McMurray JJ, Verheugt FW, Wallentin L ARISTOTLE Investigators. Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J. 2010;159:331–339. doi: 10.1016/j.ahj.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 9.Ruff CT, Giugliano RP, Antman EM, Crugnale SE, Bocanegra T, Mercuri M, Hanyok J, Patel I, Shi M, Salazar D, McCabe CH, Braunwald E. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48) Am Heart J. 2010;160:635–641. doi: 10.1016/j.ahj.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 10.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM the ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 11.Connolly S, Yusuf S, Budaj A, Camm J, Chrolavicius S, Commerford PJ, Flather M, Fox KA, Hart R, Hohnloser S, Joyner C, Pfeffer M, Anand I, Arthur H, Avezum A, Bethala-Sithya M, Blumenthal M, Ceremuzynski L, De Caterina R, Diaz R, Flaker G, Frangin G, Franzosi MG, Gaudin C, Golitsyn S, Goldhaber S, Granger C, Halon D, Hermosillo A, Hunt D, Jansky P, Karatzas N, Keltai M, Lanas F, Lau CP, Le Heuzey JY, Lewis BS, Morais J, Morillo C, Oto A, Paolasso E, Peters RJ, Pfisterer M, Piegas L, Pipillis T, Proste C, Sitkei E, Swedberg K, Synhorst D, Talajic M, Trégou V, Valentin V, van Mieghem W, Weintraub W, Varigos J Active Steering Committee, ACTIVE Investigators. Rationale and design of ACTIVE: the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events. Am Heart J. 2006;151:1187–1193. doi: 10.1016/j.ahj.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Olsson SB Executive Steering Committee of the SPORTIF III Investigators. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with nonvalvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362:1691–1698. doi: 10.1016/s0140-6736(03)14841-6. [DOI] [PubMed] [Google Scholar]
- 13.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL American College of Cardiology; American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:651–745. doi: 10.1093/europace/eul097. [DOI] [PubMed] [Google Scholar]
- 14.Camm AJ, Lip GYH, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P ESC Committee for Practice Guidelines (CPG) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 15.Sherman DG, Dyken ML, Fisher M, Harrison MJG, Hart RG. Cerebral embolism. Chest. 1986;89:82S–98S. doi: 10.1378/chest.89.2_supplement.82s. [DOI] [PubMed] [Google Scholar]
- 16.Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, Cobbe S, Breithardt G, Le Heuzey JY, Prins MH, Lévy S, Crijns HJ European Heart Survey Investigators. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26:2422–2434. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 17.Nabauer M, Gerth A, Limbourg T, Schneider S, Oeff M, Kirchhof P, Goette A, Lewalter T, Ravens U, Meinertz T, Breithardt G, Steinbeck G. The Registry of the German Competence NETwork on Atrial Fibrillation: patient characteristics and initial management. Europace. 2009;11:423–434. doi: 10.1093/europace/eun369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meinertz T, Kirch W, Rosin L, Pittrow D, Willich SN, Kirchhof P ATRIUM Investigators. Management of atrial fibrillation by primary care physicians in Germany: baseline results of the ATRIUM registry. Clin Res Cardiol. 2011;100:897–905. doi: 10.1007/s00392-011-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosch RF, Kirch W, Theuer J-D, Pittrow D, Kohlhaußen A, Willich SN, Bonnemeier H. Atrial fibrillation management, outcomes and predictors of stable disease in daily practice: prospective non-interventional study. Int J Cardiol. 2013;167:750–756. doi: 10.1016/j.ijcard.2012.03.053. [DOI] [PubMed] [Google Scholar]
- 20.Chiang CE, Naditch-Brûlé N, Murin J, Goethals M, Inoue H, O'Neill J, Silva-Cardoso J, Zharinov O, Gamra H, Alam S, Ponikowski P, Lewalter T, Rosenqvist M, Steg PG. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol. 2012;5:632–639. doi: 10.1161/CIRCEP.112.970749. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey J-Y, Schilling RJ, Schmitt J, Zamorano JL. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboembolic events—European Registry in Atrial Fibrillation (PREFER in AF) Europace. 2014;16:6–14. doi: 10.1093/europace/eut263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 23.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 24.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–180. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 25.January CT, Wann LS, Alpert JS, Field ME, Calkins H, Murray KT, Cleveland JC, Jr, Sacco RL, Cigarroa JE, Stevenson WG, Conti JB, Tchou PJ, Ellinor PT, Tracy CM, Ezekowitz MD, Yancy CW. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary. J Am College Cardiol. 2014 doi: 10.1016/j.jacc.2014.03.022. doi:10.1016/j.jacc.2014.03.021. [DOI] [PubMed] [Google Scholar]


