Abstract
Most ionic liquids (ILs) are either water soluble or present a non-negligible miscibility with water that may cause some harmful effects upon their release into the environment. Among other methods, adsorption of ILs onto activated carbon (AC) has shown to be an effective technique to remove these compounds from aqueous solutions. However, this method has proved to be viable only for hydrophobic ILs rather than for the hydrophilic that, being water soluble, have a larger tendency for contamination. In this context, an alternative approach using the salting-out ability of inorganic salts is here proposed to enhance the adsorption of hydrophilic ILs onto activated carbon. The effect of the concentrations of Na2SO4 on the adsorption of five ILs onto AC was investigated. A wide range of ILs that allow the inspection of the IL cation family (imidazolium- and pyridinium-based) and the anion nature (accounting for its hydrophilicity and fluorination) through the adsorption onto AC was studied. In general, it is shown that the use of Na2SO4 enhances the adsorption of ILs onto AC. In particular, this effect is highly relevant when dealing with hydrophilic ILs that are those that are actually poorly removed by AC. In addition, the COnductor like Screening MOdel for Real Solvents (COSMO-RS) was used aiming at complementing the experimental data obtained. This work contributes with the development of novel methods to remove ILs from water streams aiming at creating “greener” processes.
Keywords: ionic liquids, adsorption, activated carbon, inorganic salt, salting-out
1. Introduction
The use of ionic liquids (ILs) as novel solvents or fluids for a diverse range of applications has become increasingly relevant [1,2]. ILs are salts with melting temperatures below 100°C, and that are usually formed by large organic cations and organic or inorganic anions. Since ILs are constituted by ionic species, most of them present particular properties not common in molecular solvents, such as a negligible vapour pressure, general non flammability, high thermal and chemical stabilities, among others. Moreover, the possibility of controlling their physicochemical properties by the adequate manipulation of the cation and/or the anion allows the design of these solvents for target applications [3]. This tailoring ability makes of ILs excellent alternative solvents for diverse extraction purposes [4].
The intrinsic non-volatile nature of ILs provides an opportunity to reduce, or even completely eliminate, hazardous and toxic emissions to the atmosphere. Nevertheless, although ILs cannot contribute to air pollution, some of them (even those considered hydrophobic) present a non-negligible solubility in water [5-10], and their release into aquatic media raises serious environmental concerns. Therefore, the search of novel methods/techniques to remove ILs from aqueous environments is of outmost importance. There are some previous studies regarding the removal of ILs from aqueous streams [11-31]. Amongst those, some of them use destructive methods such as advanced oxidation [11-13] or biological treatments [14,21,22]. However, when envisaging sustainable technologies, the degradation of ILs should be avoided and they should be recovered and recycled instead [32]. There are also some non-destructive methods already reported in the literature, such as distillation [23], crystallization [24], nanofiltration [25], pervaporation [26], phase separation [15] and adsorption [17]. Among the non-destructive techniques, the adsorption onto activated carbon (AC) proved to be able to remove different ILs from aqueous solutions [17,18,31]. Nonetheless, although adsorption has provide good results for hydrophobic ILs, it fails when dealing with hydrophilic ILs that have a lower affinity to AC.
It is well-known that the addition of salting-out species to aqueous media, like inorganic or organic salt ions, leads to a decrease on the solubility of hydrophobic ILs in water [33], and can even induce phase separation with hydrophilic or completely water-soluble ILs [34]. Therefore, in this work, the salting-out ability of an inorganic salt, Na2SO4, was used to improve the adsorption of different ILs (hydrophobic and hydrophilic) onto AC. The adsorption of different ILs, in the presence of salt solutions at different concentrations, onto AC was investigated. The effect of the IL chemical structure and its ability to adsorb onto AC was also studied. In order to complement the experimental measurements, the COnductor like Screening MOdel for Real Solvents (COSMO-RS), a quantum chemical-based prediction model, was finally used to better evaluate the salt effect in the adsorption of ILs onto AC.
2. Experimental
2.1. Materials
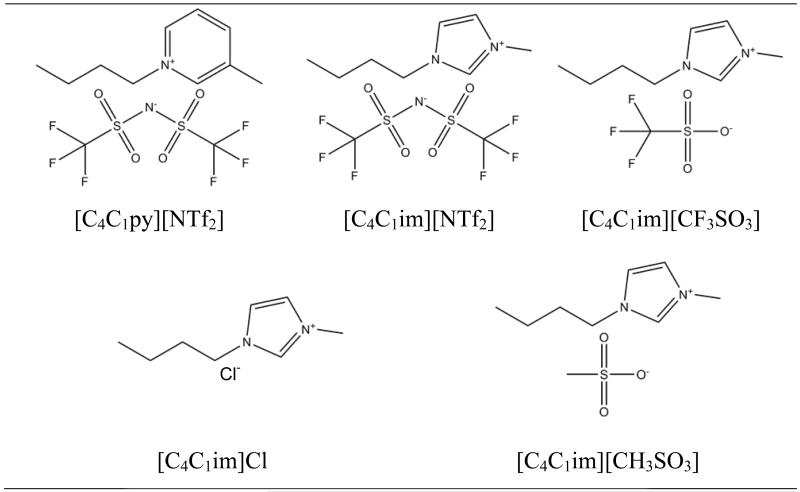
The study on the adsorption onto AC was carried out for the following ILs: 1-butyl-3-methylimidazolium chloride, [C4C1im]Cl; 1-butyl-3-methylimidazolium methanesulfonate, [C4C1im][CH3SO3]; 1-butyl-3-methylimidazolium trifluoromethanesulfonate (triflate), [C4C1im][CF3SO3]; 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide, [C4C1im][NTf2]; 1-butyl-3-methylpyridinium bis(trifluoromethylsulfonyl)imide, [C4C1py][NTf2]. All ILs were acquired from Iolitec with a mass fraction purity of 99 %. The purity of all ILs was also confirmed by 1H and 13C NMR.
The inorganic salt sodium sulfate (Na2SO4) was acquired from Sigma-Aldrich with a mass fraction purity above 99 %. The commercial AC was supplied by Merck (AC-MkU) and was used as adsorbent. The AC used in this work has a BET area of 927 m2·g−1 with high micropore volume contribution and a low concentration of surface functional groups, as reported before [31].
2.2. Method
The experiments were conducted in glass bottles (100 mL) at 308.15 K, using 50 mL of each salt solution with the IL in a concentration ranging from 100 to 500 mg·L−1. The concentrations of the different salt solutions varied between 0.28 and 1.76 mol·kg−1. The AC was then added to the aqueous solutions and placed in an orbital incubator (Julabo Shake Temp, model SW-22) at 200 rpm, and left for, at least, four days. This time proved to be the minimum time necessary to reach the equilibrium [17]. After this period, the samples were removed and the ILs content was quantified. The concentration of imidazolium- and pyridinium-based ILs were determined by UV spectroscopy (Varian, model Cary 1E) at 212 nm for the imidazolium and at 266 nm for the pyridinium aromatic ILs and using calibration curves previously established. The data obtained was fitted with the Langmuir model (Eq. (1)),
| (1) |
where B (L·mmol−1) and qmax (mmol·g−1) are empirical coefficients of the Langmuir equation, Ce (mmol·L−1) is the equilibrium concentration of adsorbate in the fluid phase and qe (mmol·g−1) is the equilibrium concentration of adsorbate in the solid phase [17]. The empirical coefficients obtained and the respective correlation coefficients are reported in Table 1.
Table 1.
Empirical coefficients obtained from the Langmuir model fitting and Kd coefficients estimated at Ce = 1.2 mmol·L−1 for the different ILs studied.
| IL | [Na2SO4] (mol·kg−1) | qmax (mmol·g−1) | B (L·mmol−1) | R2 | Kd (L·kg−1) |
|---|---|---|---|---|---|
| |C4C1im|CI | No salt | 0.26 | 0.64 | 0.900 | 95 |
| 0.28 | 0.47 | 4.52 | 0.999 | 332 | |
| 0.70 | 0.54 | 3.02 | 0.997 | 353 | |
| 1.06 | 0.58 | 5.76 | 0.997 | 422 | |
| 1.41 | 0.74 | 3.54 | 0.961 | 498 | |
| 1.76 | 0.78 | 3.31 | 0.993 | 522 | |
|
| |||||
| |C4C1im||CH3SO3| | No salt | 0.13 | 3.94 | 0.973 | 92 |
| 0.28 | 0.72 | 0.13 | 0.924 | 80 | |
| 0.70 | 0.34 | 7.54 | 0.939 | 254 | |
| 1.06 | 0.60 | 2.94 | 0.969 | 388 | |
| 1.41 | 0.45 | 78.04 | 0.977 | 374 | |
| 1.76 | 0.53 | 39.79 | 0.899 | 433 | |
|
| |||||
| |C4C1im||CF3SO3| | No salt | 0.58 | 2.56 | 0.990 | 367 |
| 0.28 | 0.67 | 1.12 | 0.988 | 318 | |
| 0.70 | 0.57 | 3.52 | 0.997 | 385 | |
| 1.06 | 0.81 | 2.96 | 0.999 | 525 | |
| 1.41 | 0.93 | 2.10 | 0.995 | 556 | |
| 1.76 | 1.02 | 3.17 | 0.978 | 670 | |
|
| |||||
| |C4C1im||NTf2| | No salt | 1.04 | 8.71 | 0.992 | 794 |
| 0.28 | 0.93 | 5.91 | 0.981 | 680 | |
| 0.70 | 0.99 | 5.73 | 0.999 | 719 | |
| 1.06 | 1.04 | 4.57 | 0.933 | 734 | |
| 1.41 | 1.02 | 16.99 | 0.964 | 809 | |
| 1.76 | 1.13 | 20.86 | 0.988 | 908 | |
|
| |||||
| |C4C1py||NTf2| | No salt | 1.10 | 75.71 | 0.995 | 906 |
| 0.28 | 1.20 | 8.41 | 0.997 | 909 | |
| 0.70 | 1.47 | 5.69 | 0.997 | 1068 | |
| 1.06 | 1.15 | 23.72 | 0.991 | 929 | |
| 1.41 | 1.32 | 61.70 | 0.989 | 1088 | |
| 1.76 | 1.41 | 406.48 | 0.458 | 1173 | |
The apparent distribution coefficients (Kd, L·kg−1) of each system were calculated taking into consideration Eq. (2):
| (2) |
This coefficient evaluates the capacity of AC for the adsorption of different ILs at the same equilibrium concentration. The apparent distribution coefficients are reported in Table 1.
The AC/water/salt partition coefficient (P) of each IL at infinite dilution was calculated by COSMO-RS at 308 K. The molecular geometry of all molecular models (AC, ions of the Na2SO4 salt, water and ion-paired structures of ILs) were optimized at the B3LYP/6-31++G** computational level. The ideal screening charges on the molecular surface for each species were calculated by the continuum solvation COSMO model using the BVP86/TZVP/DGA1 level of theory. According to the chosen quantum method, the functional and basis sets, the corresponding parameterization (BP_TZVP_C30_1201) in the COSMOtherm code was used.
3. Results and Discussion
In Fig. 1 are represented the chemical structures of the ILs studied.
Fig. 1.
Chemical structure of the ILs studied.
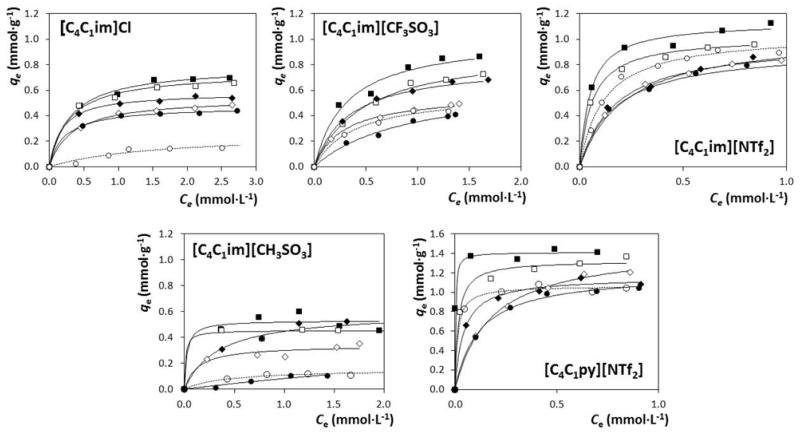
The adsorption of the ILs [C4C1im]Cl, [C4C1im][CH3SO3], [C4C1im][CF3SO3], [C4C1im][NTf2] and [C4C1py][NTf2] onto commercial AC was measured for Na2SO4 aqueous solutions with concentrations ranging from 0.28 to 1.76 mol·kg−1. The adsorption isotherms for all ILs at 308 K onto AC-MkU are depicted in Fig. 2.
Fig. 2.
Experimental data (symbols) and Langmuir fitting (Eq. (1)) (lines) for the adsorption equilibrium of the ILs onto AC-MkU at 308 K, at several salt concentrations: (○), no salt; (•), [Na2SO4] = 0.28 mol·kg−1; (◇), [Na2SO4] = 0.70 mol·kg−1; (◆), [Na2SO4] = 1.06mol·kg−1; (□),[Na2SO4] = 1.41 mol·kg−1; (■), [Na2SO4] = 1.76 mol·kg−1.
Adsorption of ILs onto AC proved to be a useful and non-destructive way to remove low concentrations of ILs from water [17,18,20,31]. However, unlike for hydrophobic ILs, for hydrophilic compounds this method is not effective due to the low polarity of the AC surface [17,31].
In a previous work [15], the formation of aqueous biphasic systems by the addition of strong salting-out species revealed to be a highly efficient methodology for removing hydrophilic ILs from water, at high concentrations. The addition of salting-out species, like inorganic salts, to a water solution containing hydrophobic ILs was previously shown to led to a significant decrease of their solubility in water [33,34]. Therefore, with the goal of improving the adsorption of hydrophilic ILs onto AC, the salting-out ability of Na2SO4 was here investigated. For that purpose, several concentrations of Na2SO4, a strong salting-out inducing salt that does not change the medium pH when dissolved in aqueous medium [33,35], were tested. It was already shown that Na2SO4 can promote the phase separation when combined with hydrophilic ILs in aqueous solutions [35], and that it can decrease the solubility of highly hydrophobic and fluorinated ILs in water [33].
The results here obtained show different adsorption capacities attending to both the nature of the IL and the amount of salt in solution. For comparison purposes, B and qmax empirical coefficients of the Langmuir model (Eq. (1)) and the apparent distribution coefficients (Kd, Eq. (2)), obtained from the experimental adsorption data, are reported in Table 1.
In general, and for the imidazolium-based ILs, the anion influence on their adsorption capacity onto AC follows the order: Cl− ≈ [CH3SO]− < [CF3SO3]− < [NTf2]−. This trend is in good agreement with the results already reported in the literature [17], and reveals that the adsorption of ILs onto AC is progressively more favourable with the increase on the anion hydrophobicity. On the other hand, taking into consideration the IL cation family, the pyridinium adsorbs more onto AC than the imidazolium-based counterpart. This pattern is also related to the higher hydrophobic character of the pyridinium cation that although being aromatic consists on a 6-sided ring whereas the imidazolium cation is a 5-sided aromatic ring [31].
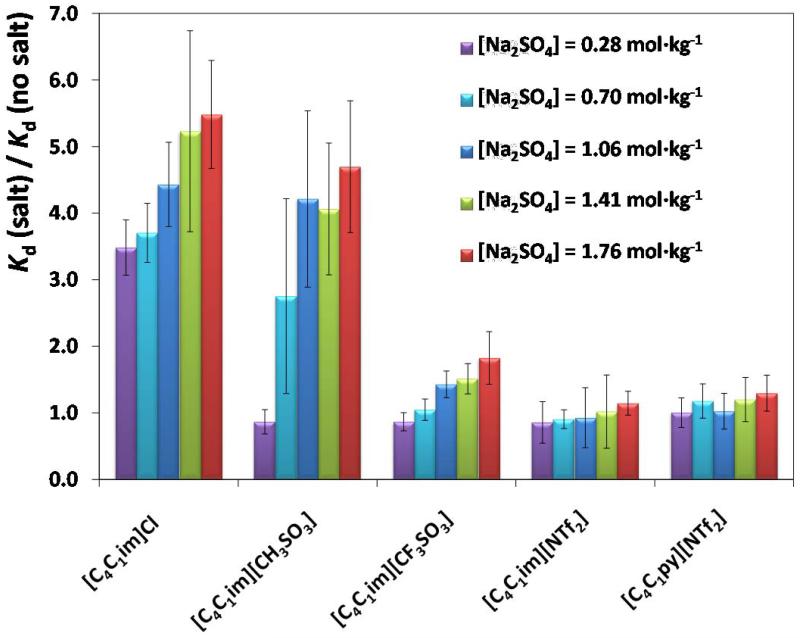
In Fig. 3 it is shown the ratio between the Kd obtained in mixtures with salt to that with no salt. This ratio quantifies thus the relative influence of the salt, and salt concentration, on the adsorption of the different ILs onto AC.
Fig. 3.
Ratio between the Kd values in the presence of salt to that with no salt at different concentrations and for the several ILs investigated.
It is remarkable that, in general, the presence of the inorganic and salting-out salt in aqueous solution enhances the adsorption of the different ILs onto AC. In addition, the salt influence is much more noticeable in the systems including ILs with a more hydrophilic nature, and following the trend: [C4C1im]Cl > [C4C1im][CH3SO3] > [C4C1im][CF3SO3] > [C4C1im][NTf2] > [C4C1py][NTf2]. With the [C4C1im]Cl and [C4C1im][CH3SO3] ILs, the Kd value increases up to 5.5 times by the salt addition. Furthermore, it was also found that increasing the salt concentration leads to an increase on the adsorption of hydrophilic ILs (such as [C4C1im]Cl and [C4C1im][CH3SO3]). The salting-out phenomenon, particularly for the more hydrophilic ILs, leads to a decrease on the solubility of the ILs in water and therefore favours the partition to, and enhance their adsorption onto AC [33]. Although mainly an entropic effect, the formation of salt-ion-hydration complexes leads to the dehydration of the IL solute and to an increase on the surface tension of the cavity that is responsible for the observed salting-out phenomenon of the salt over the IL [33,36]. Nevertheless, at low salt concentrations, the opposite behaviour is sometimes observed, and the adsorption of some ILs decrease in presence of the salt. This salting-in effect was previously observed before for other ILs, and is common with proteins or other charged molecules, at low salt concentrations [33]. In contrast, the presence of salt does not improve significantly the adsorption of the most hydrophobic ILs ([NTf2]-based ILs) onto AC. As reported before [19], the available pore volume of the AC-MkU adsorbent at maximum capacity should be almost nearly filled by these hydrophobic ILs. For this reason, the presence of salt does not improve the adsorption of the [NTf2]-based ILs onto this specific AC since its saturation was already reached.
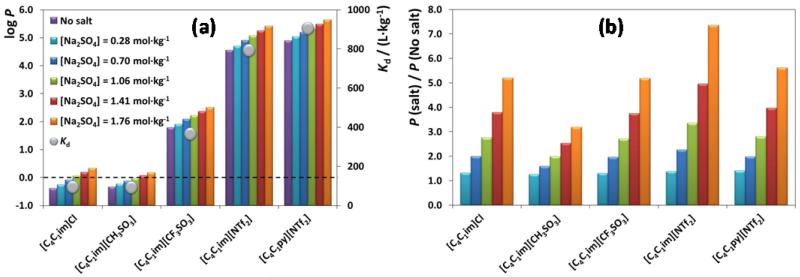
To complete the current analysis and to better interpret the gathered experimental data, the COSMO-RS was used to estimate the partition coefficient (P) of the different ILs, at infinite dilution, and between AC and the diverse aqueous phases at increasing concentrations of Na2SO4. The log(P) has been already reported as a reference quantitative parameter of the affinity of each IL for AC when in aqueous solutions [31]. Fig. 4 (A) depicts the log(P) values for the five studied ILs obtained by COSMO-RS while specifying in all the calculations the increasing salt concentration used in the experiments. For comparison purposes, Fig. 4 (A) also includes the experimental Kd values obtained for each IL in the absence of salt. In Fig. 4 (B) it is depicted the ratio between the P value in the presence and in absence of salt obtained by COSMO-RS.
Fig. 4.
(a) log(P) predicted by COSMO-RS for the ILs studied with different salt concentrations using the AC molecular model and experimental Kd values for these ILs on AC-MkU without salt; (b) effect of the salt concentration on the P ratio predicted by COSMO-RS.
COSMO-RS correctly predicts the influence of the IL chemical structure onto their AC adsorption capacity since log(P) values increase with the increase on the Kd values and with the hydrophobicity of the IL solute. In addition, COSMO-RS estimations reasonably reproduce the influence of the Na2SO4 salt on the ILs adsorption onto AC. Higher values of log(P) are obtained when increasing the salt concentration in aqueous media. However, as expectable, the COSMO-RS approach is not able to correctly describe the effects of lower salt concentrations on hydrophobic ILs ([NTf2]-based), since aspects as the available pore volume of exhausted AC adsorbent cannot be included in its respective calculations.
In summary, it is here demonstrated, for the first time, that AC can be also used as an effective adsorbent for hydrophilic ILs present at low concentrations in aqueous media if inorganic salts are concomitantly used to promote a salting-out phenomenon and enhance the partition towards the AC.
4. Conclusions
In the past years, ILs have been proposed as potential substituents for volatile organic compounds (VOCs) mainly due to their negligible vapour pressures avoiding thus the atmospheric pollution. However, they display some or even complete miscibility with water and can be dispersed by aqueous streams leading to serious environmental concerns. In this context, it is of crucial relevance to develop novel methods capable of removing ILs from wastewater streams. The adsorption onto AC proved to be a potential non-destructive method to remove low concentrations of ILs from aqueous solutions. However, this method is not so effective for hydrophilic ILs. Therefore, in this work, the addition of a salting-out inorganic salt (Na2SO4) is used to improve the adsorption of different ILs onto AC. The results obtained show that the Kd values of hydrophilic ILs, such as [C4C1im]Cl and [C4C1im][CH3SO3], increase up to 5.5 times in the presence of the salt. This new approach can be envisaged as a promising route for reducing the pollution of aqueous streams resulting from industrial processes where ILs are employed.
Supplementary Material
Acknowledgements
The authors acknowledge FCT- Fundação para a Ciência e a Tecnologia, through the projects Pest-C/CTM/LA0011/2013 and PTDC/AAC-AMB/119172/2010. Catarina M. S. S. Neves also acknowledges FCT for the doctoral grant SFRH/BD/70641/2010.The authors are also grateful to the Spanish “Ministerio de Ciencia e Innovación (MICINN)” and “Comunidad de Madrid” for financial support (projects CTQ2011-26758 and S2009/PPQ-1545). M. G. Freire acknowledges the European Research Council (ERC) for the Starting Grant ERC-2013-StG-337753.
References
- [1].Plechkova NV, Seddon KR. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008;37:123–150. doi: 10.1039/b006677j. [DOI] [PubMed] [Google Scholar]
- [2].Earle MJ, Esperanca JMSS, Gilea MA, Canongia Lopes JN, Rebelo LPN, Magee JW, Seddon KR, Widegren JA. The distillation and volatility of ionic liquids. Nature. 2006;439:831–834. doi: 10.1038/nature04451. [DOI] [PubMed] [Google Scholar]
- [3].Rogers RD, Seddon KR. Chemistry. Ionic liquids - solvents of the future? Science. 2003;302:792–793. doi: 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- [4].Bosmann A, Datsevich L, Jess A, Lauter A, Schmitz C, Wasserscheid P. Deep desulfurization of diesel fuel by extraction with ionic liquids. Chem. Commun. 2001:2494–2495. doi: 10.1039/b108411a. [DOI] [PubMed] [Google Scholar]
- [5].Freire MG, Carvalho PJ, Gardas RL, Santos LMNBF, Marrucho IM, Coutinho JAP. Solubility of water in tetradecyltrihexylphosphonium-based ionic liquids. J. Chem. Eng. Data. 2008;53:2378–2382. [Google Scholar]
- [6].Freire MG, Carvalho PJ, Gardas RL, Marrucho IM, Santos LMNBF, Coutinho JAP. Mutual solubilities of water and the [Cnmim][Tf2N] hydrophobic ionic liquids. J. Phys. Chem. B. 2008;112:1604–1610. doi: 10.1021/jp7097203. [DOI] [PubMed] [Google Scholar]
- [7].Freire MG, Neves CMSS, Carvalho PJ, Gardas RL, Fernandes AM, Marrucho IM, Santos LMNBF, Coutinho JAP. Mutual solubilities of water and hydrophobic ionic liquids. J. Phys. Chem. B. 2007;111:13082–13089. doi: 10.1021/jp076271e. [DOI] [PubMed] [Google Scholar]
- [8].Freire MG, Neves CMSS, Shimizu K, Bernardes CES, Marrucho IM, Coutinho JAP, Lopes JNC, Rebelo LPN. Mutual solubility of water and structural/positional isomers of N-alkylpyridinium-based ionic liquids. J. Phys. Chem. B. 2010;114:15925–15934. doi: 10.1021/jp1093788. [DOI] [PubMed] [Google Scholar]
- [9].Freire MG, Neves CMSS, Ventura SPM, Pratas MJ, Marrucho IM, Oliveira J, Coutinho JAP, Fernandes AM. Solubility of non-aromatic ionic liquids in water and correlation using a QSPR approach. Fluid Phase Equilib. 2010;294:234–240. [Google Scholar]
- [10].Neves CMSS, Batista MLS, Cláudio AFM, Santos LMNBF, Marrucho IM, Freire MG, Coutinho JAP. Thermophysical Properties and Water Saturation of [PF6]-Based Ionic Liquids. J. Chem. Eng. Data. 2010;55:5065–5073. [Google Scholar]
- [11].Czerwicka M, Stolte S, Müller A, Siedlecka EM, Golebiowski M, Kumirska J, Stepnowski P. Identification of ionic liquid breakdown products in an advanced oxidation system. J. Hazard. Mater. 2009;171:478–483. doi: 10.1016/j.jhazmat.2009.06.027. [DOI] [PubMed] [Google Scholar]
- [12].Stepnowski P, Zaleska A. Comparison of different advanced oxidation processes for the degradation of room temperature ionic liquids. J. Photochem. Photobiol. A Chem. 2005;170:45–50. [Google Scholar]
- [13].Siedlecka EM, Czerwicka M, Stolte S, Stepnowski P. Stability of ionic liquids in application conditions. Curr. Org. Chem. 2011;15:1974–1991. [Google Scholar]
- [14].Neumann J, Grundmann O, Thoming J, Schulte M, Stolte S. Anaerobic biodegradability of ionic liquid cations under denitrifying conditions. Green Chem. 2010;12:620–627. [Google Scholar]
- [15].Neves CMSS, Freire MG, Coutinho JAP. Improved recovery of ionic liquids from contaminated aqueous streams using aluminium-based salts. RSC Adv. 2012;2:10882–10890. [Google Scholar]
- [16].Anthony JL, Maginn EJ, Brennecke JF. Solution thermodynamics of imidazolium-based ionic liquids and water. J. Phys. Chem. B. 2001;105:10942–10949. [Google Scholar]
- [17].Palomar J, Lemus J, Gilarranz MA, Rodriguez JJ. Adsorption of ionic liquids from aqueous effluents by activated carbon. Carbon N. Y. 2009;47:1846–1856. [Google Scholar]
- [18].Lemus J, Palomar J, Heras F, Gilarranz MA, Rodriguez JJ. Developing criteria for the recovery of ionic liquids from aqueous phase by adsorption with activated carbon. Sep. Purif. Technol. 2012;97:11–19. [Google Scholar]
- [19].Lemus J, Palomar J, Gilarranz MA, Rodriguez JJ. Characterization of supported ionic liquid phase (SILP) materials prepared from different supports. Adsorption. 2011;17:561–571. [Google Scholar]
- [20].Lemus J, Palomar J, Gilarranz MA, Rodriguez JJ. On the kinetics of ionic liquid adsorption onto activated carbons from aqueous solution. Ind. Eng. Chem. Res. 2013;52:2969–2976. [Google Scholar]
- [21].Stolte S, Arning J, Thöming J. Biologische abbaubarkeit von ionischen flüssigkeiten - testverfahren und strukturelles design. Chemie Ing. Tech. 2011;15:1946–1973. [Google Scholar]
- [22].Abrusci C, Palomar J, Pablos JL, Rodriguez F, Catalina F. Efficient biodegradation of common ionic liquids by Sphingomonas paucimobilis bacterium. Green Chem. 2011;13:709. [Google Scholar]
- [23].Meindersma GW, de Haan AB. Conceptual process design for aromatic/aliphatic separation with ionic liquids. Chem. Eng. Res. Des. 2008;86:745–752. [Google Scholar]
- [24].Nockemann P, Binnemans K, Driesen K. Purification of imidazolium ionic liquids for spectroscopic applications. Chem. Phys. Lett. 2005;415:131–136. [Google Scholar]
- [25].Kröckel J, Kragl U. Nanofiltration for the separation of nonvolatile products from solutions containing ionic liquids. Chem. Eng. Technol. 2003;26:1166–1168. [Google Scholar]
- [26].Schäfer T, Rodrigues CM, Afonso CAM, Crespo JG. Selective recovery of solutes from ionic liquids by pervaporation - a novel approach for purification and green processing. Chem. Commun. 2001:1622–1623. doi: 10.1039/b104191f. [DOI] [PubMed] [Google Scholar]
- [27].Mahmoud ME, Al-Bishri HM. Supported hydrophobic ionic liquid on nano-silica for adsorption of lead. Chem. Eng. J. 2011;166:157–167. [Google Scholar]
- [28].Farooq A, Reinert L, Levêque J-M, Papaiconomou N, Irfan N, Duclaux L. Adsorption of ionic liquids onto activated carbons: effect of pH and temperature. Microporous Mesoporous Mater. 2012;158:55–63. [Google Scholar]
- [29].Reinert L, Batouche K, Lévêque J-M, Muller F, Bény J-M, Kebabi B, Duclaux L. Adsorption of imidazolium and pyridinium ionic liquids onto montmorillonite: characterisation and thermodynamic calculations. Chem. Eng. J. 2012;209:13–19. [Google Scholar]
- [30].Qi X, Li L, Tan T, Chen W, Smith RL. Adsorption of 1-butyl-3-methylimidazolium chloride ionic liquid by functional carbon microspheres from hydrothermal carbonization of cellulose. Environ. Sci. Technol. 2013;47:2792–8. doi: 10.1021/es304873t. [DOI] [PubMed] [Google Scholar]
- [31].Lemus J, Neves CMSS, Marques CFC, Freire MG, Coutinho JAP, Palomar J. Composition and structural effects on the adsorption of ionic liquids onto activated carbon. Environ. Sci. Process. Impacts. 2013;15:1752–1759. doi: 10.1039/c3em00230f. [DOI] [PubMed] [Google Scholar]
- [32].Fernandez JF, Neumann J, Thoming J. Regeneration, recovery and removal of ionic liquids. Curr. Org. Chem. 2011;15:1992–2014. [Google Scholar]
- [33].Freire MG, Carvalho PJ, Silva AMS, Santos LMNBF, Rebelo LPN, Marrucho IM, Coutinho JAP. Ion specific effects on the mutual solubilities of water and hydrophobic ionic liquids. J. Phys. Chem. B. 2009;113:202–211. doi: 10.1021/jp8080035. [DOI] [PubMed] [Google Scholar]
- [34].Freire MG, Cláudio AFM, Araújo JMM, Coutinho JAP, Marrucho IM, Canongia Lopes JN, Rebelo LPN. Aqueous biphasic systems: a boost brought about by using ionic liquids. Chem. Soc. Rev. 2012;41:4966–95. doi: 10.1039/c2cs35151j. [DOI] [PubMed] [Google Scholar]
- [35].Cláudio AFM, Ferreira AM, Shahriari S, Freire MG, Coutinho JAP. Critical assessment of the formation of lonic-liquid-based aqueous two-phase systems in acidic media. J. Phys. Chem. B. 2011;115:11145–11153. doi: 10.1021/jp204865a. [DOI] [PubMed] [Google Scholar]
- [36].Freire MG, Neves CMSS, Silva AMS, Santos LMNBF, Marrucho IM, Rebelo LPN, Shah JK, Maginn EJ, Coutinho JAP. 1H NMR and molecular dynamics evidence for an unexpected interaction on the origin of salting-in/salting-out phenomena. J. Phys. Chem. B. 2010;114:2004–2014. doi: 10.1021/jp9095634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.