Abstract
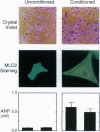
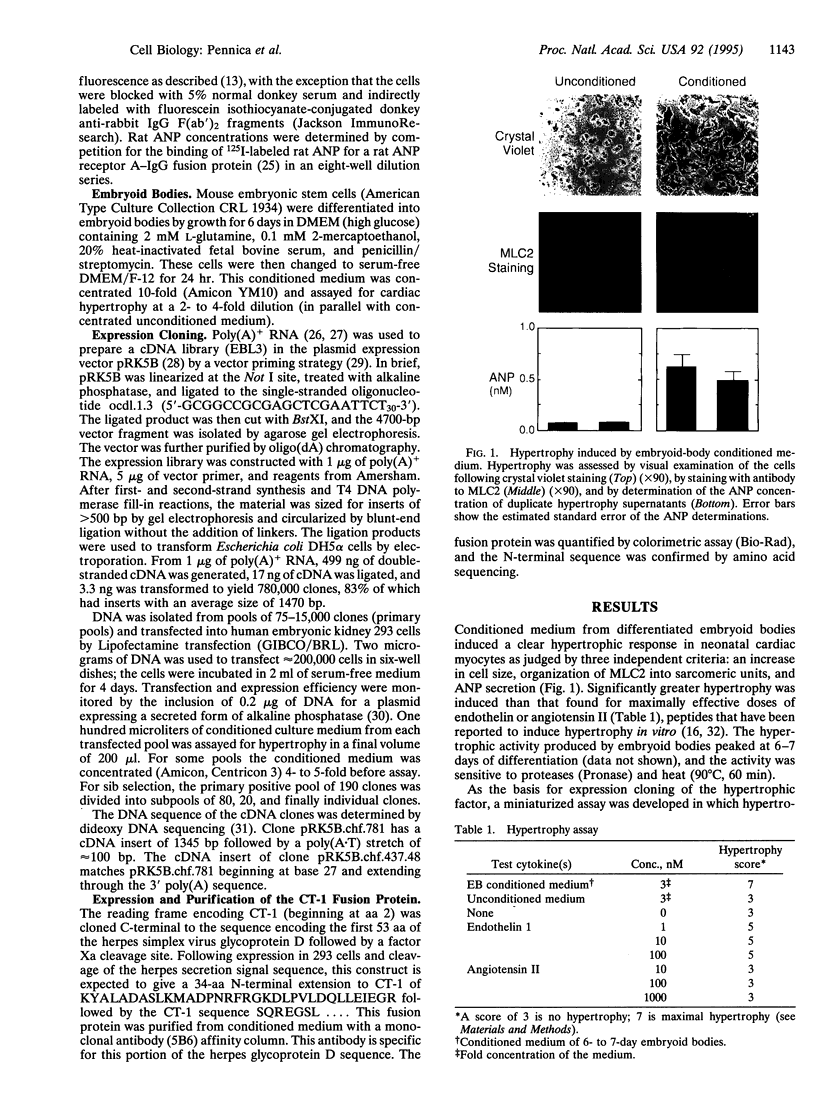
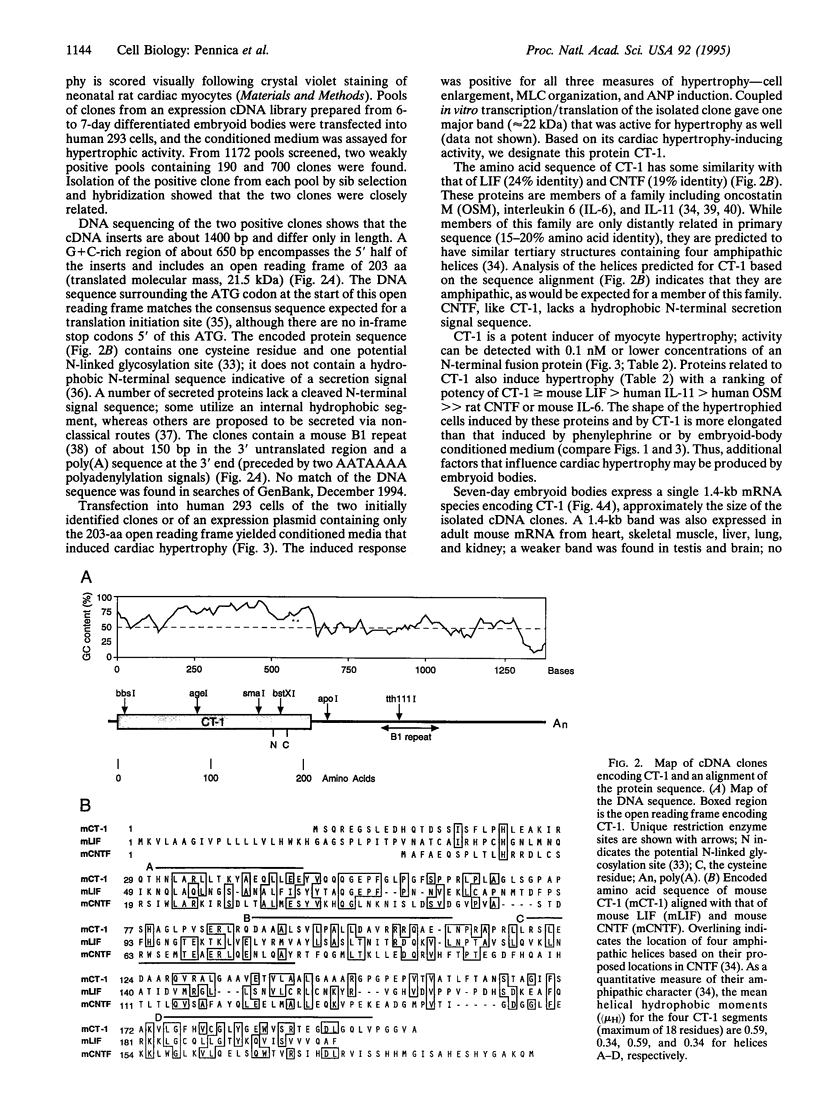
Heart failure continues to be a leading cause of mortality worldwide. A hallmark of this disease is dilated cardiac hypertrophy, which is accompanied by a reactivation of genes expressed in fetal heart development. Reasoning that fetal or embryonic growth factors may mediate the onset of cardiac hypertrophy, we have coupled expression cloning with an embryonic stem cell-based model of cardiogenesis to isolate a 21.5-kDa protein, cardiotrophin 1, that potently induces cardiac myocyte hypertrophy in vitro. Amino acid similarity data indicate that cardiotrophin 1 is a member of the leukemia inhibitory factor/ciliary neurotrophic factor/oncostatin M/interleukin 6/interleukin 11 family of cytokines. Several members of this family that are known to signal through the transmembrane protein gp130 stimulate cardiac myocyte hypertrophy, like cardiotrophin 1, suggesting that the gp130 signaling pathway may play a role in cardiac hypertrophy. A 1.4-kb cardiotrophin 1 mRNA is expressed in the heart and several other mouse tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Neuropoietic cytokines in the hematopoietic fold. Neuron. 1991 Aug;7(2):197–208. doi: 10.1016/0896-6273(91)90258-2. [DOI] [PubMed] [Google Scholar]
- Bishopric N. H., Simpson P. C., Ordahl C. P. Induction of the skeletal alpha-actin gene in alpha 1-adrenoceptor-mediated hypertrophy of rat cardiac myocytes. J Clin Invest. 1987 Oct;80(4):1194–1199. doi: 10.1172/JCI113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chamow S. M., Peers D. H., Byrn R. A., Mulkerrin M. G., Harris R. J., Wang W. C., Bjorkman P. J., Capon D. J., Ashkenazi A. Enzymatic cleavage of a CD4 immunoadhesin generates crystallizable, biologically active Fd-like fragments. Biochemistry. 1990 Oct 23;29(42):9885–9891. doi: 10.1021/bi00494a019. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Knowlton K. U., Zhu H., Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J. 1991 Dec;5(15):3037–3046. doi: 10.1096/fasebj.5.15.1835945. [DOI] [PubMed] [Google Scholar]
- Chien K. R. Molecular advances in cardiovascular biology. Science. 1993 May 14;260(5110):916–917. doi: 10.1126/science.8493528. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Zhu H., Knowlton K. U., Miller-Hance W., van-Bilsen M., O'Brien T. X., Evans S. M. Transcriptional regulation during cardiac growth and development. Annu Rev Physiol. 1993;55:77–95. doi: 10.1146/annurev.ph.55.030193.000453. [DOI] [PubMed] [Google Scholar]
- Davis S., Yancopoulos G. D. The molecular biology of the CNTF receptor. Curr Opin Cell Biol. 1993 Apr;5(2):281–285. doi: 10.1016/0955-0674(93)90117-9. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Holmes W. E., Lee J., Kuang W. J., Rice G. C., Wood W. I. Structure and functional expression of a human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- Iwaki K., Sukhatme V. P., Shubeita H. E., Chien K. R. Alpha- and beta-adrenergic stimulation induces distinct patterns of immediate early gene expression in neonatal rat myocardial cells. fos/jun expression is associated with sarcomere assembly; Egr-1 induction is primarily an alpha 1-mediated response. J Biol Chem. 1990 Aug 15;265(23):13809–13817. [PubMed] [Google Scholar]
- Jones R. L., Miller J. C., Hagler H. K., Chien K. R., Willerson J. T., Buja L. M. Association between inhibition of arachidonic acid release and prevention of calcium loading during ATP depletion in cultured rat cardiac myocytes. Am J Pathol. 1989 Sep;135(3):541–556. [PMC free article] [PubMed] [Google Scholar]
- Kalb V. F., Glasser S., King D., Lingrel J. B. A cluster of repetitive elements within a 700 base pair region in the mouse genome. Nucleic Acids Res. 1983 Apr 11;11(7):2177–2184. doi: 10.1093/nar/11.7.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Knowlton K. U., Baracchini E., Ross R. S., Harris A. N., Henderson S. A., Evans S. M., Glembotski C. C., Chien K. R. Co-regulation of the atrial natriuretic factor and cardiac myosin light chain-2 genes during alpha-adrenergic stimulation of neonatal rat ventricular cells. Identification of cis sequences within an embryonic and a constitutive contractile protein gene which mediate inducible expression. J Biol Chem. 1991 Apr 25;266(12):7759–7768. [PubMed] [Google Scholar]
- Knowlton K. U., Michel M. C., Itani M., Shubeita H. E., Ishihara K., Brown J. H., Chien K. R. The alpha 1A-adrenergic receptor subtype mediates biochemical, molecular, and morphologic features of cultured myocardial cell hypertrophy. J Biol Chem. 1993 Jul 25;268(21):15374–15380. [PubMed] [Google Scholar]
- Komuro I., Yazaki Y. Control of cardiac gene expression by mechanical stress. Annu Rev Physiol. 1993;55:55–75. doi: 10.1146/annurev.ph.55.030193.000415. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMorte V. J., Thorburn J., Absher D., Spiegel A., Brown J. H., Chien K. R., Feramisco J. R., Knowlton K. U. Gq- and ras-dependent pathways mediate hypertrophy of neonatal rat ventricular myocytes following alpha 1-adrenergic stimulation. J Biol Chem. 1994 May 6;269(18):13490–13496. [PubMed] [Google Scholar]
- Lee H. R., Henderson S. A., Reynolds R., Dunnmon P., Yuan D., Chien K. R. Alpha 1-adrenergic stimulation of cardiac gene transcription in neonatal rat myocardial cells. Effects on myosin light chain-2 gene expression. J Biol Chem. 1988 May 25;263(15):7352–7358. [PubMed] [Google Scholar]
- Long C. S., Henrich C. J., Simpson P. C. A growth factor for cardiac myocytes is produced by cardiac nonmyocytes. Cell Regul. 1991 Dec;2(12):1081–1095. doi: 10.1091/mbc.2.12.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- Meidell R. S., Sen A., Henderson S. A., Slahetka M. F., Chien K. R. Alpha 1-adrenergic stimulation of rat myocardial cells increases protein synthesis. Am J Physiol. 1986 Nov;251(5 Pt 2):H1076–H1084. doi: 10.1152/ajpheart.1986.251.5.H1076. [DOI] [PubMed] [Google Scholar]
- Miller-Hance W. C., LaCorbiere M., Fuller S. J., Evans S. M., Lyons G., Schmidt C., Robbins J., Chien K. R. In vitro chamber specification during embryonic stem cell cardiogenesis. Expression of the ventricular myosin light chain-2 gene is independent of heart tube formation. J Biol Chem. 1993 Nov 25;268(33):25244–25252. [PubMed] [Google Scholar]
- Morgan H. E., Baker K. M. Cardiac hypertrophy. Mechanical, neural, and endocrine dependence. Circulation. 1991 Jan;83(1):13–25. doi: 10.1161/01.cir.83.1.13. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Gordon E. E., Kira Y., Chua H. L., Russo L. A., Peterson C. J., McDermott P. J., Watson P. A. Biochemical mechanisms of cardiac hypertrophy. Annu Rev Physiol. 1987;49:533–543. doi: 10.1146/annurev.ph.49.030187.002533. [DOI] [PubMed] [Google Scholar]
- Muesch A., Hartmann E., Rohde K., Rubartelli A., Sitia R., Rapoport T. A. A novel pathway for secretory proteins? Trends Biochem Sci. 1990 Mar;15(3):86–88. doi: 10.1016/0968-0004(90)90186-f. [DOI] [PubMed] [Google Scholar]
- Neben S., Turner K. The biology of interleukin 11. Stem Cells. 1993 Jul;11 (Suppl 2):156–162. doi: 10.1002/stem.5530110825. [DOI] [PubMed] [Google Scholar]
- Parker T. G., Schneider M. D. Growth factors, proto-oncogenes, and plasticity of the cardiac phenotype. Annu Rev Physiol. 1991;53:179–200. doi: 10.1146/annurev.ph.53.030191.001143. [DOI] [PubMed] [Google Scholar]
- Patterson P. H. The emerging neuropoietic cytokine family: first CDF/LIF, CNTF and IL-6; next ONC, MGF, GCSF? Curr Opin Neurobiol. 1992 Feb;2(1):94–97. doi: 10.1016/0959-4388(92)90169-l. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Robbins J., Gulick J., Sanchez A., Howles P., Doetschman T. Mouse embryonic stem cells express the cardiac myosin heavy chain genes during development in vitro. J Biol Chem. 1990 Jul 15;265(20):11905–11909. [PubMed] [Google Scholar]
- Rockman H. A., Ross R. S., Harris A. N., Knowlton K. U., Steinhelper M. E., Field L. J., Ross J., Jr, Chien K. R. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J., Xu Y., Slayter H. S., Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993 Dec 3;75(5):977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubeita H. E., McDonough P. M., Harris A. N., Knowlton K. U., Glembotski C. C., Brown J. H., Chien K. R. Endothelin induction of inositol phospholipid hydrolysis, sarcomere assembly, and cardiac gene expression in ventricular myocytes. A paracrine mechanism for myocardial cell hypertrophy. J Biol Chem. 1990 Nov 25;265(33):20555–20562. [PubMed] [Google Scholar]
- Simpson P., McGrath A., Savion S. Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and by catecholamines. Circ Res. 1982 Dec;51(6):787–801. doi: 10.1161/01.res.51.6.787. [DOI] [PubMed] [Google Scholar]
- Starksen N. F., Simpson P. C., Bishopric N., Coughlin S. R., Lee W. M., Escobedo J. A., Williams L. T. Cardiac myocyte hypertrophy is associated with c-myc protooncogene expression. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8348–8350. doi: 10.1073/pnas.83.21.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Gavish H., Shannon W. R., Buchwald M. Cloning of cDNAs for Fanconi's anaemia by functional complementation. Nature. 1992 Apr 30;356(6372):763–767. doi: 10.1038/356763a0. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Urade R., Micanovic R., Gerber L., Udenfriend S. Secreted alkaline phosphatase: an internal standard for expression of injected mRNAs in the Xenopus oocyte. FASEB J. 1990 Feb 1;4(2):227–231. doi: 10.1096/fasebj.4.2.2298343. [DOI] [PubMed] [Google Scholar]
- Thorburn A., Thorburn J., Chen S. Y., Powers S., Shubeita H. E., Feramisco J. R., Chien K. R. HRas-dependent pathways can activate morphological and genetic markers of cardiac muscle cell hypertrophy. J Biol Chem. 1993 Jan 25;268(3):2244–2249. [PubMed] [Google Scholar]