Abstract
Partial reactions designed to study the individual steps in the initiation of protein synthesis may be divided into two groups: artificial models using artificial mRNA templates and natural models using naturally occurring mRNA. The requirements for each of the reticulocyte initiation factors, IF-M1, IF-M2, and IF-M3, and for GTP are examined using these models in order to determine if either type of model is a valid representation of the events occurring in natural initiation.
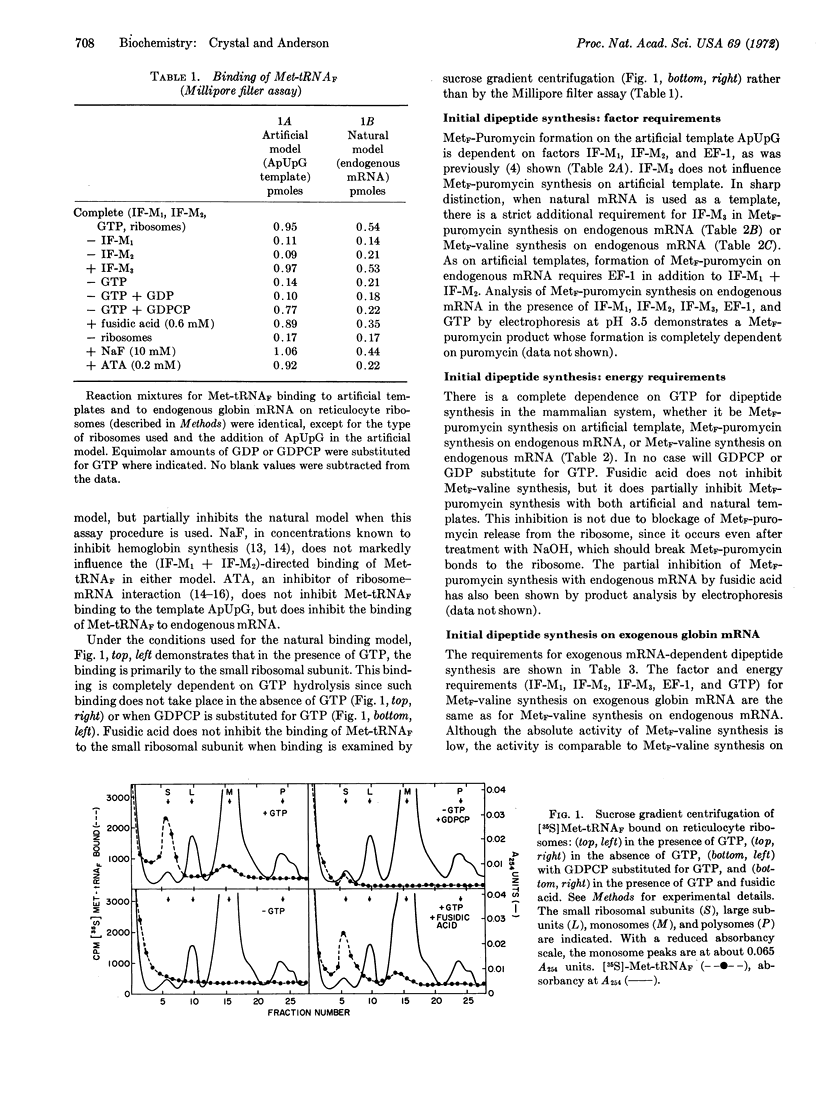
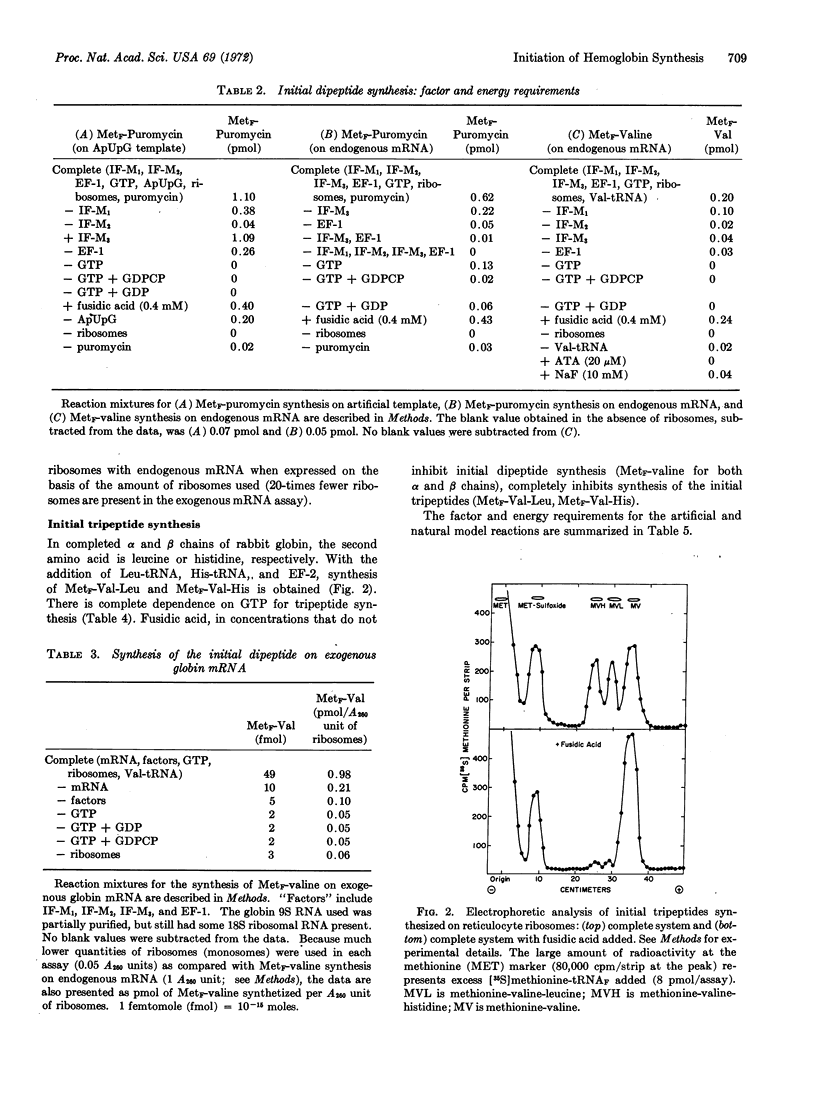
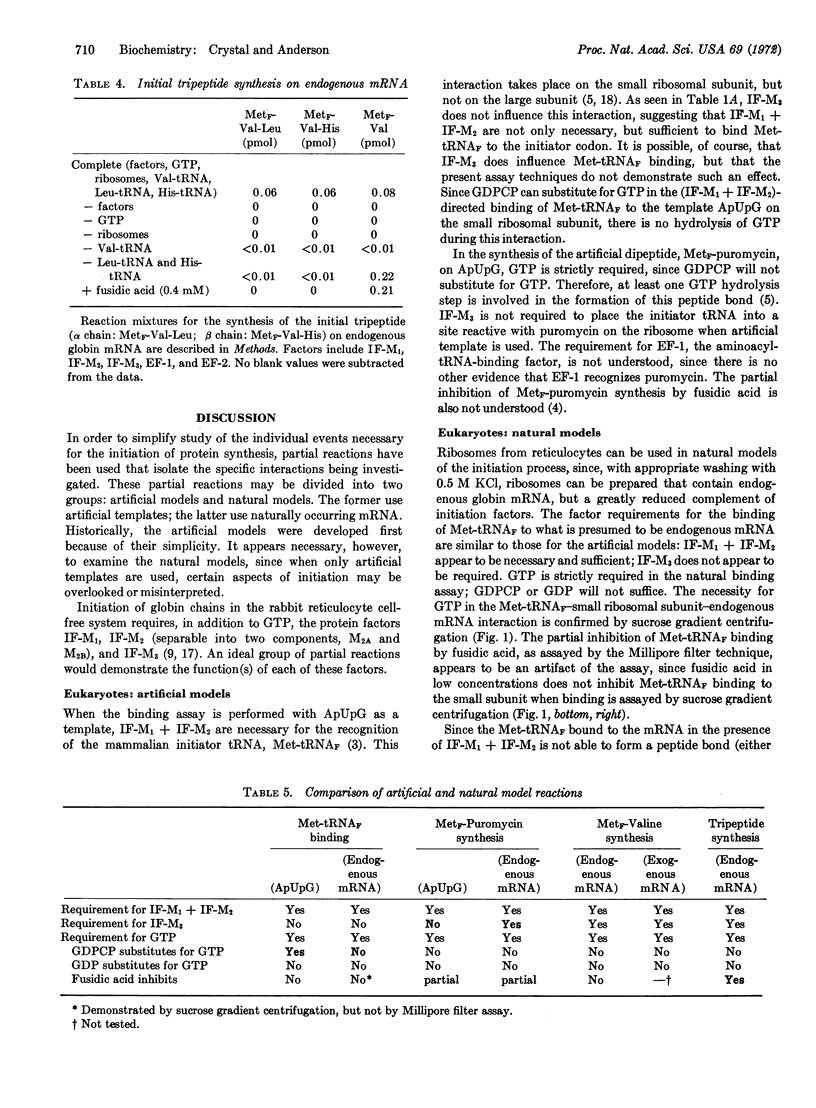
Binding of the initiator tRNA, Met-tRNAF, to endogenous mRNA requires IF-M1, IF-M2, and GTP. A requirement for hydrolysis of GTP is not found when the artificial template ApUpG is used since GDPCP may be substituted for GTP. Met-tRNAF bound to the template ApUpG by IF-M1 + IF-M2 can form a peptide bond with the aminoacyl-tRNA analog, puromycin. Met-tRNAF bound by IF-M1 + IF-M2 to the initiator codon of natural globin mRNA, however, cannot form a peptide bond with either puromycin or valine-tRNA unless IF-M3 is also present. The requirements for MetF-valine synthesis on exogenous globin mRNA are the same as the requirements on endogenous mRNA. Synthesis of the initial tripeptides of the α and β chains of rabbit globin, MetF-Val-Leu and MetF-Val-His, requires, in addition, leucyl-tRNA and histidyl-tRNA.
It appears, therefore, that model systems that use natural messenger RNA can duplicate the factor and energy requirements of natural initiation, but that the model systems thus far studied that use artificial messengers as templates do not.
Keywords: initiation factors, elongation factors, fusidic acid, GDPCP, puromycin
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess A. B., Mach B. Formation of an initiation complex with purified mammalian ribosomal subunits. Nature. 1971 Oct 13;233(5320):209–210. [PubMed] [Google Scholar]
- Crystal R. G., Shafritz D. A., Prichard P. M., Anderson W. F. Initial dipeptide formation in hemoglobin biosynthesis. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1810–1814. doi: 10.1073/pnas.68.8.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J. M., Anderson W. F. Cell-free hemoglobin synthesis. II. Characteristics of the transfer ribonucleic acid-dependent assay system. J Biol Chem. 1970 May 10;245(9):2342–2349. [PubMed] [Google Scholar]
- Hoerz W., McCarty K. S. Evidence for a proposed initiation complex for protein synthesis in reticulocyte polyribosome profiles. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1206–1213. doi: 10.1073/pnas.63.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebleu B., Marbaix G., Wérenne J., Burny A., Huez G. Effect of aurintricarboxylic acid and of NaF on the binding of globin messenger RNA to reticulocyte 40S ribosomal subunits. Biochem Biophys Res Commun. 1970 Aug 11;40(3):731–739. doi: 10.1016/0006-291x(70)90964-2. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- Malkin M., Lipmann F. Fusidic acid: inhibition of factor T2 in reticulocyte protein synthesis. Science. 1969 Apr 4;164(3875):71–72. doi: 10.1126/science.164.3875.71. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Laycock D. G., Anderson W. F. Translation of rabbit haemoglobin messenger RNA by thalassaemic and non-thalassaemic ribosomes. Nat New Biol. 1971 Jun 16;231(24):205–208. doi: 10.1038/newbio231205a0. [DOI] [PubMed] [Google Scholar]
- Prichard P. M., Gilbert J. M., Shafritz D. A., Anderson W. F. Factors for the initiation of haemoglobin synthesis by rabbit reticulocyte ribosomes. Nature. 1970 May 9;226(5245):511–514. doi: 10.1038/226511a0. [DOI] [PubMed] [Google Scholar]
- Prichard P. M., Picciano D. J., Laycock D. G., Anderson W. F. Translation of exogenous messenger RNA for hemoglobin on reticulocyte and liver ribosomes. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2752–2756. doi: 10.1073/pnas.68.11.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K., Coleman W. H. Polyuridylic acid binding by protein from Ehrlich ascites cell ribosomes and its inhibition by aurintricarboxylic acid. Biochemistry. 1971 Nov;10(23):4304–4312. doi: 10.1021/bi00799a023. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Factor dependent binding of methionyl-tRNAs to reticulocyte ribosomes. Nature. 1970 Aug 29;227(5261):918–920. doi: 10.1038/227918a0. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Isolation and partial characterization of reticulocyte factors M1 and M2. J Biol Chem. 1970 Nov 10;245(21):5553–5559. [PubMed] [Google Scholar]
- Shafritz D. A., Laycock D. G., Anderson W. F. Puromycin-peptide bond formation with reticulocyte initiation factors M1 and M2. Proc Natl Acad Sci U S A. 1971 Feb;68(2):496–499. doi: 10.1073/pnas.68.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz D. A., Laycock D. G., Crystal R. G., Anderson W. F. Requirement for GTP in the initiation process on reticulocyte ribosomes and ribosomal subunits. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2246–2251. doi: 10.1073/pnas.68.9.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. L., Grollman A. P., Huang M. T. Aurintricarboxylic acid: inhibitor of initiation of protein synthesis. Proc Natl Acad Sci U S A. 1971 Jan;68(1):97–101. doi: 10.1073/pnas.68.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Nishimura T., Kinoshita T. Inhibition by fusidic acid of transferase II in reticulocyte protein synthesis. J Biochem. 1970 Mar;67(3):459–463. doi: 10.1093/oxfordjournals.jbchem.a129268. [DOI] [PubMed] [Google Scholar]
- Weiss J. F., Pearson R. L., Kelmers A. D. Two additional reversed-phase chromatographic systems for the separation of transfer ribonucleic acids and their application to the preparation of two formylmethionine and a valine transfer ribonucleic acid from Escherichia coli B. Biochemistry. 1968 Oct;7(10):3479–3487. doi: 10.1021/bi00850a024. [DOI] [PubMed] [Google Scholar]



