Abstract
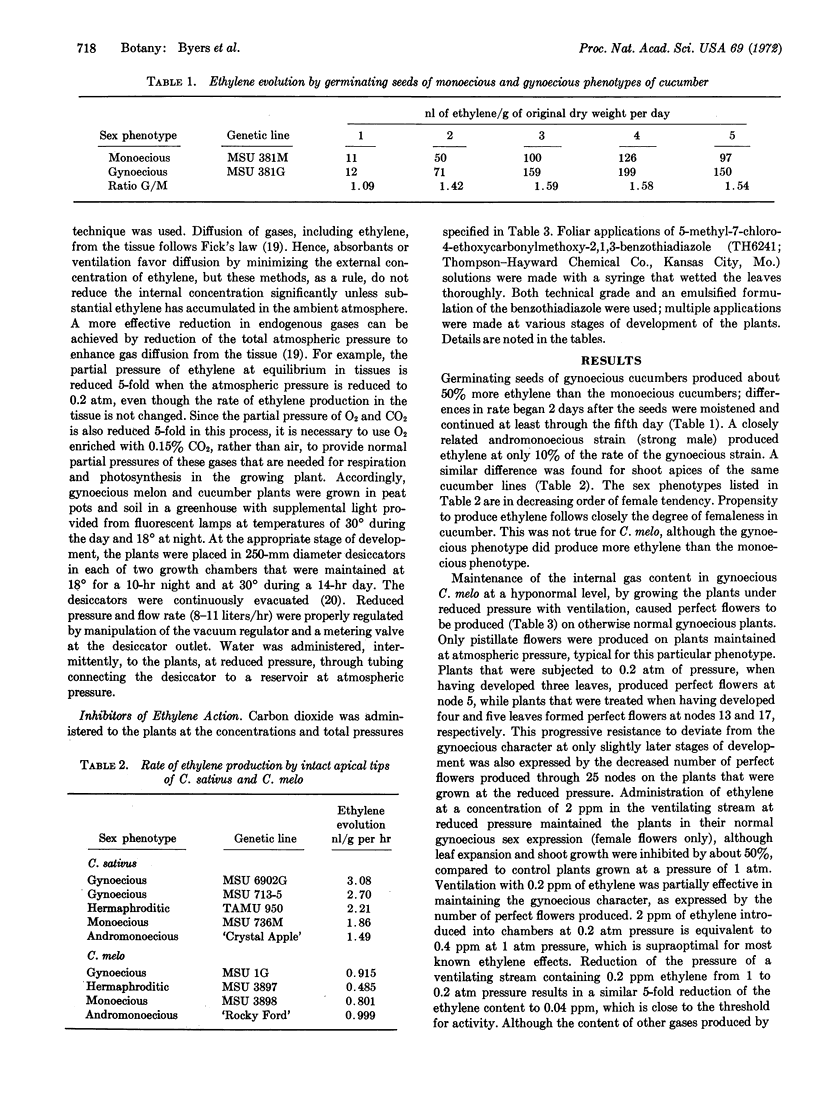
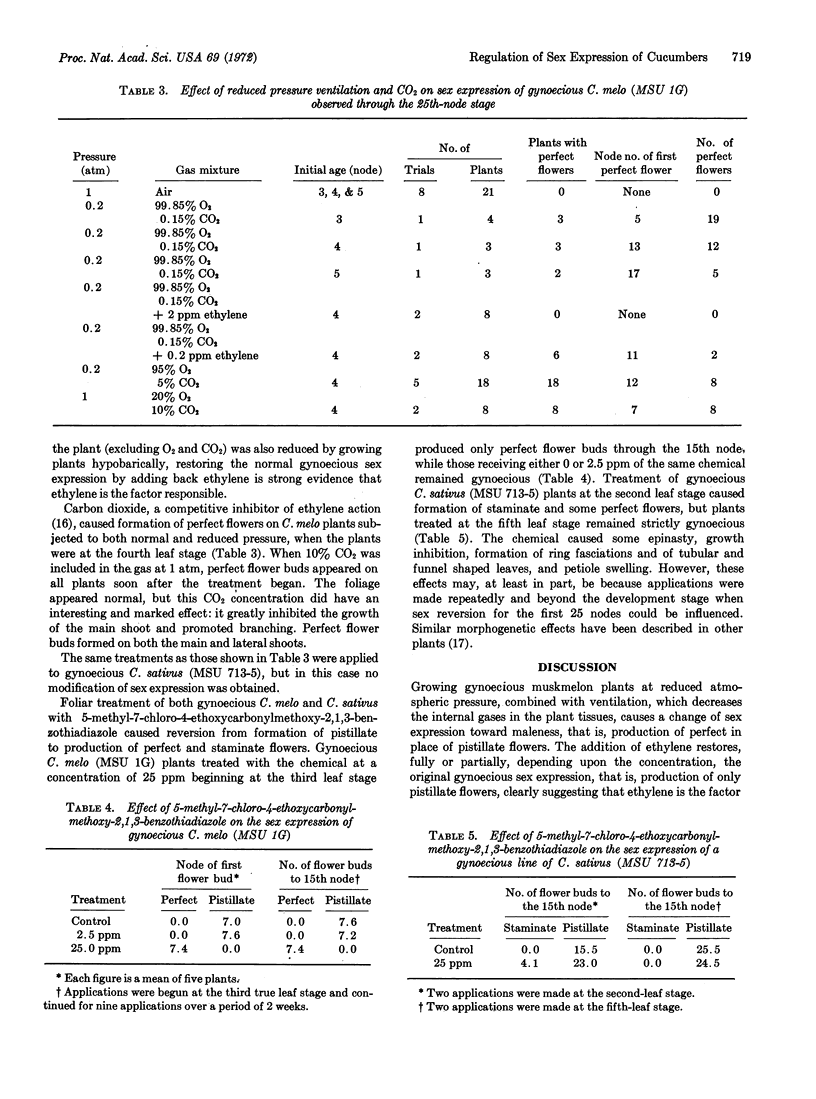
Sex expression in cucumber (Cucumis sativus L.) and muskmelon (C. melo L.) was correlated with endogenous ethylene production. Plants of gynoecious (all female) sex types of the two species produced more ethylene than monoecius (male-female) plants. C. melo plants of a gynoecious sex type that normally produce only pistillate (female) flowers, when grown with hypobaric ventilation to facilitate removal of endogenous gases by diffusion, produced perfect (hermaphroditic) flowers. When either the plant was returned to atmospheric pressure or when the reduced-pressure ventilating stream was supplemented with ethylene, the same plants produced pistillate flowers. Enrichment of the atmosphere at either normal or reduced pressure with CO2, a competitive inhibitor of ethylene action, also resulted in development of perfect flowers. Foliar application of a benzothiadiazole, a postulated inhibitor of ethylene action, resulted in formation of perfect flowers on gynoecious plants of C. melo and of staminate (male) flowers on gynoecious C. sativus. Based on these findings, it is proposed that ethylene is an endogenous regulator of sex expression in C. sativus and C. melo.
Keywords: cucumbers, gynoecious, monoecious, perfect flowers, muskmelon
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atsmon D., Lang A., Light E. N. Contents and recovery of gibberellins in monoecious and gynoecious cucumber plants. Plant Physiol. 1968 May;43(5):806–810. doi: 10.1104/pp.43.5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Fruit storage at subatmospheric pressures. Science. 1966 Jul 15;153(3733):314–315. doi: 10.1126/science.153.3733.314. [DOI] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galun E., Izhar S., Atsmon D. Determination of Relative Auxin Content in Hermaphrodite and Andromonoecious Cucumis sativus L. Plant Physiol. 1965 Mar;40(2):321–326. doi: 10.1104/pp.40.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahori S., Lyons J. M., Smith O. E. Sex expression in cucumber plants as affected by 2-chloroethylphosphonic Acid, ethylene, and growth regulators. Plant Physiol. 1970 Sep;46(3):412–415. doi: 10.1104/pp.46.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C. E., Anhder L. D. Induction of Staminate Flowers on Gynoecious Cucumbers with Gibberellin A3. Science. 1960 Jun 3;131(3414):1673–1674. doi: 10.1126/science.131.3414.1673. [DOI] [PubMed] [Google Scholar]



