Abstract
Background
Published findings indicate that acetaldehyde (ACD; the first metabolite of EtOH) and salsolinol (SAL; formed through the non-enzymatic condensation of ACD and dopamine) can be formed following ethanol (EtOH) consumption. Both ACD and SAL exhibit reinforcing properties within the posterior ventral tegmental area (pVTA) and both exhibit an inverted “U-shaped” dose-response curve. The current study was undertaken to examine the dose-response effects of microinjections of ACD or SAL into the pVTA on DA efflux in the nucleus accumbens shell (AcbSh).
Methods
For the first experiment, separate groups of male Wistar rats received pulse microinjections of aCSF or 12, 23 or 90 µM ACD into the pVTA while extracellular DA levels were concurrently measured in the AcbSh. The second experiment was similarly conducted, except rats were given microinjections of aCSF or 0.03, 0.3, 1.0 or 3.0 µM SAL, while extracellular levels of DA were measured in the AcbSh.
Results
Both ACD and SAL produced a dose-dependent inverted “U-shaped” response on DA release in the AcbSh, with 23 µM ACD (200% baseline) and 0.3 µM SAL (300% baseline) producing maximal peak responses with higher concentrations of ACD (90 µM) and SAL (3.0 µM) producing significantly lower DA efflux.
Conclusions
The findings from the current study indicate that local application of intermediate concentrations of ACD and SAL stimulated DA neurons in the pVTA, whereas higher concentrations may be having secondary effects within the pVTA that inhibit DA neuronal activity. The present results parallel studies on the reinforcing effects of ACD and SAL in the pVTA and support the idea that the reinforcing effects of ACD and SAL within the pVTA are mediated by activating DA neurons.
Keywords: Acetaldehyde, Salsolinol, Dopamine, Posterior Ventral Tegmental Area, Nucleus Accumbens Shell
Introduction
Acetaldehyde (ACD) and salsolinol (SAL) are two biologically active compounds that can be formed following the consumption of ethanol (EtOH). ACD is the first metabolite of EtOH and is formed in the brain mainly through a catalase-mediated reaction (Jamal et al., 2007). SAL, on the other hand, can be formed in the brain through the non-enzymatic condensation of ACD and dopamine (DA) (Melchior and Collins, 1982). Over the past four decades, several studies have suggested that ACD and SAL may have significant roles in the behavioral and neurobiological effects of EtOH, and the development of EtOH abuse and alcoholism (for review see: Deehan et al., 2012).
In rats, ACD has been found to increase the consumption of EtOH (Brown et al., 1980; Myers et al., 1984b; Tampier & Quintanilla, 2002), as well as produce a conditioned place preference following both central and peripheral administration (Peana et al., 2008; Quintanilla & Tampier, 2003; Smith et al., 1984). Rats will readily self-administer ACD intravenously (Myers et al., 1984b), as well as into the lateral ventricle (Amit et al., 1977; Brown et al., 1980). SAL appears to be reinforcing in rats to a similar extent, as microinjections of SAL or tetrahydropapaveroline (THP; formed via the condensation of DA and dopaldehyde) into the lateral ventricle produce enduring increases in EtOH intake and preference (Duncan & Deitrich, 1980; Myers & Melchior, 1977). Further, rats will exhibit a conditioned place preference following SAL exposure (Hipolito et al., 2011).
Several studies, utilizing pharmacological agents capable of inhibiting brain catalase activity (Aragon & Amit 1992; Koechling & Amit 1994; Aragon et al., 1985, 1989; Escarabajal et al., 2000; Karahanian et al., 2011) or sequestering aldehydes (Font et al., 2005, 2006a,b; Peana et al., 2008), have suggested that some of the behavioral and pharmacological effects of EtOH can be attributed to the formation of ACD. Additionally, compelling evidence for the involvement ACD in the central actions of EtOH comes from data generated through the use of lentiviral vectors. Administration of a lentiviral vector that inhibits the formation of catalase synthesis within the VTA decreased both the voluntary consumption of EtOH as well as EtOH stimulated DA release (Karahanian et al., 2011; Quintanilla et al., 2012). While the formation of SAL may not be directly altered by the aforementioned manipulations, suppression of the formation of ACD would subsequently result in the suppression of the formation of SAL.
Behavioral data indicate that both ACD and SAL exhibit potent reinforcing properties within the mesolimbic DA reward pathway. Utilization of the intracranial self-administration (ICSA) paradigm, whereby rats receive response contingent infusions of a compound directly into a discrete brain region, established that rats will readily self-administer both ACD and SAL directly into the pVTA (Rodd et al., 2005; 2008). It appears that the pVTA is significantly more sensitive to the reinforcing properties of both ACD and SAL compared to EtOH. Alcohol-preferring (P) rats display the highest levels of ICSA for 23 µM ACD and 0.1 µM SAL, doses that are approximately 2,000 fold and 400,000 fold lower than the optimum dose of EtOH (200 mg%; 44 mM), respectively (Rodd et al., 2005, 2008; Rodd-Henricks et al., 2002). Responding/infusion data from the ICSA experiments exhibit an inverted “U-shaped” dose response curve for both ACD and SAL, in which lower and higher doses do not produce reliable responding/self-infusions (Rodd et al., 2005, 2008; Rodd-Henricks et al., 2002). Pharmacological manipulations suggest that the reinforcing effects of both ACD and SAL within the pVTA appear to involve the activation of DA neurons (Rodd et al., 2005; 2008). This is further supported by neurobiological data showing that ACD and/or SAL stimulate DA neurons within the pVTA (Melis et al., 2009; Xie et al., 2012)
To date, the limited research completed on the effect of the local administration of ACD or SAL within the pVTA on DA efflux within the AcbSh suggests that both compounds act to increase DA efflux. Melis et al. (2007) reported that the reverse microdialysis of 75 uM ACD in the pVTA produced an increase in DA release in the AcbSh to 150% that of baseline. A microinjection of 150 uM SAL into the pVTA increased DA levels in the AcbSh to 130% of baseline (Hipolito et al., 2011). However, the literature lacks an adequate examination of a dose response for both ACD and SAL at a range of pharmacologically relevant concentrations that have been shown to be reinforcing/rewarding (Rodd et al., 2003; 2008) as well as stimulate DA neuronal activity (Xie et al., 2012) within the pVTA. Therefore, the current study was designed to examine the DA stimulatory properties of both ACD and SAL on the projection from the pVTA to the AcbSh for the range of concentrations observed to exhibit an inverted “U-shaped” dose response curve during ICSA testing (Rodd et al., 2005; Rodd-Henricks et al., 2002). The hypothesis of the current study is that microinjections of ACD or SAL into the pVTA will dose-dependently alter DA efflux in the AcbSh in a manner consistent with previous ICSA observations. Specifically, doses of either compound that fall within the middle of the dose range will produce the largest increases in DA release with lower and higher doses producing significantly smaller or negligible changes in DA efflux.
Methods
Animals
Male adult Wistar rats (weight 350 to 400 g, Harlan, Indianapolis, IN) were used in this study. All rats were housed 2 per cage in a temperature and humidity controlled colony room. Rats were maintained on a reverse 12 h light/12 h dark schedule (lights on at 0700). Food and water were available ad libitum except during habituation and the microdialysis session. All protocols were reviewed and approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee. All experimental procedures were in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
General stereotaxic surgery procedures
While under 2% isoflurane inhalation anesthesia, all rats were implanted ipsilaterally with two guide cannula (Plastics One, Inc., Roanoke, VA, USA) aimed approximately 1.0 mm above their respective target region. A 22 gauge guide cannula targeted the pVTA (AP −5.6 mm, ML +2.1 mm, DV −8.5 mm) while an 18 gauge cannula targeted the AcbSh (AP +1.7 mm, ML +2.3 mm, DV −5.4 mm; Paxinos and Watson, 2005). Both cannula were implanted at a 10° angle from the vertical. Dummy stylets were inserted into the cannula immediately following surgery and remained in place until the start of experimental procedures. Following surgery, all rats were housed singly and provided 7 days to recover prior to the start of testing. During the final 3 days of recovery, all rats underwent daily handling and habituation to the microdialysis chambers. On the final recovery day, loop style microdialysis probes (active length 2.0 mm, Spectra/Por RC, inner diameter 200 µm, molecular weight cut-off: 13,000, Spectrum laboratories, Inc, Rancho Dominguez, CA) were inserted into the AcbSh following handling/habituation.
EMIT System
The electrolytic microinfusion transducer (EMIT) system has been previously used in this laboratory, as well as other laboratories to identify the neuroanatomical substrates that underlie the reinforcing properties of many drugs of abuse (McBride et al., 1999). The EMIT system used in the current study is identical to that described previously (Ding et al., 2009a).
Chemical Agents for Microinjections
ACD (Sigma-Aldrich, St. Louis, MO) was dissolved in artificial cerebrospinal fluid (aCSF) that consisted of 120 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2mM MgSO4, 25mM NaHCO3, 2.5 mM CaCl, and 10 mM d-glucose and the pH was adjusted to 7.4 ± 0.1. To minimize the oxidation of SAL (Sigma-Aldrich, St. Louis, MO), 0.2 ascorbate was added to the aCSF solution described above and the pH of all SAL solutions as well as the aCSF solution were adjusted to 6.5 ± 0.1. All ACD and SAL solutions were protected from light and maintained on ice until transfer into the microinjector reservoir.
General Microinjection/Microdialysis Procedure
Approximately 16 to 18 hrs prior to testing, microdialysis probes were inserted into the AcbSh. The general procedures used for the current experiments have been previously described (Ding et al., 2009a). Briefly, rats were placed in Plexiglass chambers (40 cm X 28 cm X 40 cm) and connected to a Harvard pump using PE20 tubing (inner diameter 0.38 mm; Becton Dickinson & Co., MD). Microdialysis aCSF (140.0 mM NaCl, 3.0 mM KCl, 1.2 mM CaCl2, 2.0 mM Na2HPO47H2O, 1.0 mM MgCl2, pH 7.2 to 7.4) was perfused through the dialysis probes at a flow rate of 1.0 µl/min. Following an initial 90-min washout period, four to five 20-min baseline samples were taken before rats received microinjections of ACD (12, 23, or 90 µM), SAL (0.03, 0.3, 1.0, or 3.0 µM), or aCSF. Each microinjection was administered at a volume of 100 nl over a 5-sec period. Microinjections were given at a rate of 3 microinjections per min with one microinjection every 20 sec (30 microinjections, 3 µl of test solution total per subject) during the first 10 min of sample 5 or 6. An additional five 20-min samples were collected following the microinjections. Lag time from the microdialysis probe outlet was approximately 20 min and was accounted for in all analyses. Samples were collected in microfuge vials which contained 5 µl of perchloric acid (0.1 N), then frozen immediately on dry ice and stored at −70°C.
Sample Analysis
Dialysate samples were analyzed for DA levels using a reversed-phase high pressure liquid chromatography (HPLC) system with electrochemical detection as previously described by Engleman et al., (2006).
Histology
Immediately following microdialysis testing, rats were sacrificed with an overdose of CO2 inhalation. Bromophenol blue (1%; 0.5 µl) was then injected into the pVTA as well as perfused through the dialysis probe in the AcbSh. Brains were rapidly removed and frozen on dry ice and then stored at −70°C. Brains were sectioned (40 µm) using a cryostat microtome. All sections were stained with cresyl violet and examined with a light microscope for verification of placement of both the injector and microdialysis sites using the rat brain atlas of Paxinos and Watson (2005).
Statistical Analysis
Data for the current study were normalized by taking the final 3 baseline microdialysis samples prior to microinjections, averaging them, and presenting the data as a percent of this baseline value. The normalized data were then analyzed using a 4 × 8 and a 5 × 8 (Concentration × Sample) mixed analyses of variance (ANOVA) for the ACD and SAL groups, respectively. When significant effects (p<0.05) were observed using the mixed ANOVA’s, post hoc Tukey’s b or Student’s t-tests were utilized to determine differences between specific drug concentrations and across the time course of sample collection. All analyses were conducted using SPSS® 17.0 for windows.
Results
The effect of ACD microinjections into the pVTA on DA efflux in the AcbSh
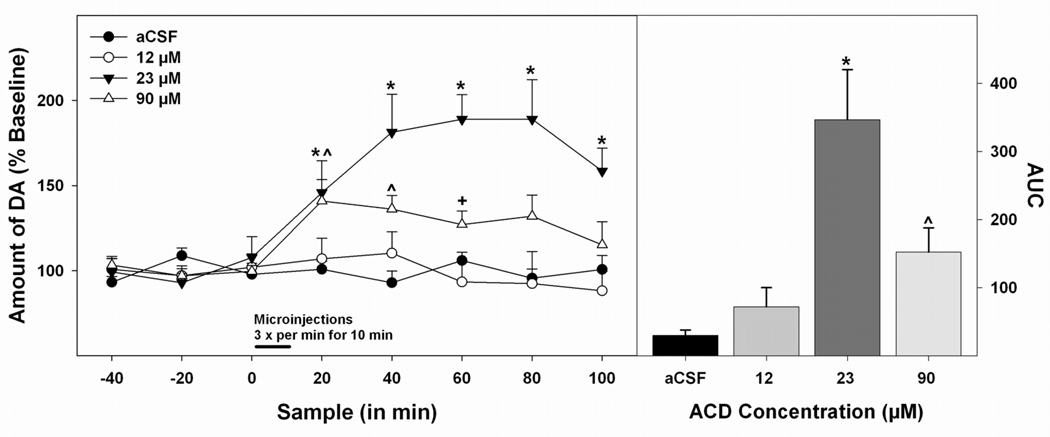
Figure 1 represents the histological placements of the microdialysis cannula in the AcbSh (panel A, left hemisphere) and the injection cannula in the pVTA (panel B, left hemisphere). Approximately 7 rats had cannula placements outside these target brain areas and were excluded from analyses. The data in Figure 2 (left panel) were analyzed with a 4 × 8 (Concentration × Sample) mixed ANOVA which revealed a significant effect of concentration (F3,22 = 10.85; p<0.05), of sample (F7,154 = 8.02; p<0.05) and a significant concentration by sample interaction (F21,154 = 4.36; p<0.05). Further analysis of the main effects via a Tukey’s b post hoc analysis found that microinjections of 23 µM ACD into the pVTA significantly increased DA efflux in the AcbSh compared to all other groups tested (p<0.05). The data in Figure 2 (right panel) represent the results of an area under the curve (AUC) analysis. A series of pairwise comparisons revealed similar findings to that of the ANOVA as the 23 µM ACD group possessed a significantly higher mean AUC than all other groups (p<0.05). Additionally, AUC for the 90 µM ACD group was significantly greater than that for the aCSF group (p<0.05).
Figure 1.
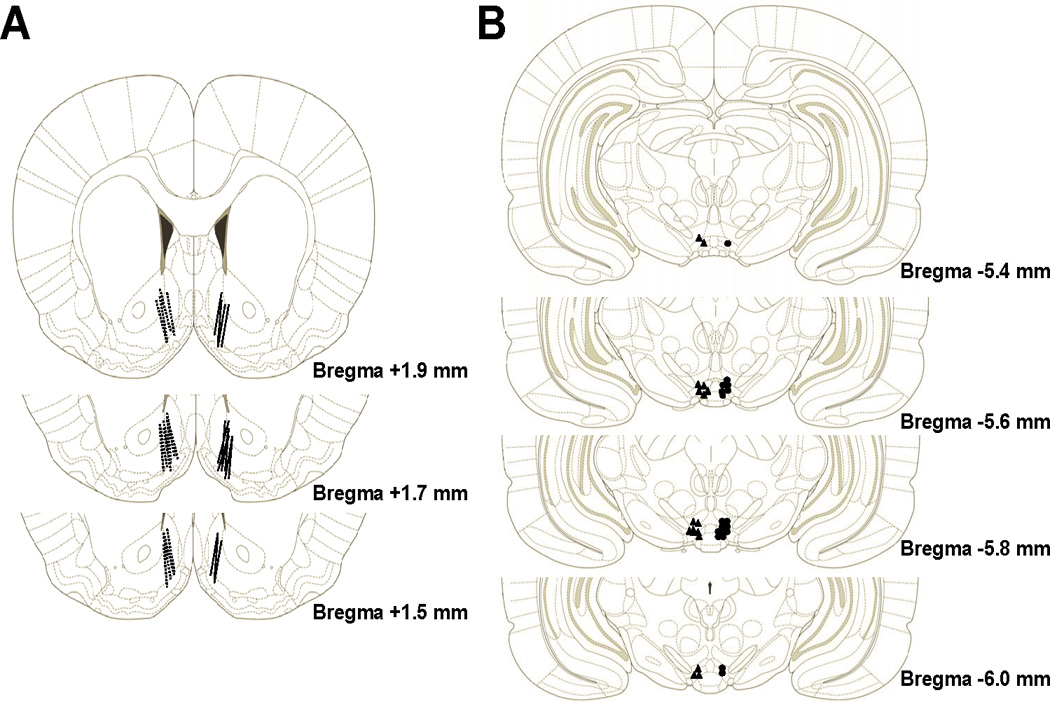
Represents the histological placements of the microdialysis cannula in the AcbSh (panel A) and the microinjection cannula in the posterior VTA (panel B) for Experiments 1 and 2. Placements for Experiment 1 are presented in the left hemisphere while placements for Experiment 2 are presented in the right hemisphere. Animals with cannula outside of these target brain regions were excluded from analyses. Histological plates have been adapted from Paxinos and Watson (2006).
Figure 2.
Dose-response effects of microinjections of ACD into the pVTA on DA efflux in the AcbSh. Microdialysis time-course data (left panel) are presented as means (± SEMs) percent baseline levels. Bar indicates microinjection period, microinjections were given 3 times a min over the first 10 min of sample 3. The area under the curve data is presented as means (± SEMs) area under the curve (right panel). * 23 µM ACD (n = 6) significantly greater (p < 0.05) than all other groups, ^ 90 µM (n = 7) ACD significantly greater (p < 0.05) than aCSF group (n = 6), + 90 µM ACD significantly greater (p < 0.05) than 12 µM ACD (n = 7).
To break down the significant concentration × sample interaction, a series of one-way ANOVA’s were performed, with Tukey’s b post hoc tests when justified, on each post-injection sample to provide a greater resolution as to group differences at each discrete time point. To simplify the presentation of the statistical data, one-way ANOVA’s for all post-injection samples exhibited a significant effect of concentration (F’s > 3.91; p’s<.05). Post hoc analysis for sample 4 (the first post-injection sample) identified that this effect was driven by a significantly higher level of DA exhibited by the 23 µM and 90 µM groups compared to the aCSF and 12 µM groups (p<0.05) and aCSF group (p<0.05), respectively. For sample 5, post hoc analysis indicated that the 23 µM group had a significantly higher DA level than all other groups (p<0.05) and the DA level measured in the 90 µM group significantly exceeded that of the aCSF group (p<0.05). Similar results were observed for sample 6 as the 23 µM group once again exhibited a significantly higher level of DA efflux compared to all of the other groups (p<0.05) and the 90 µM group exhibited a significantly greater DA release than the 12 µM group (p<0.05). For both samples 7 and 8, statistical analysis revealed a significantly higher DA level in the 23 µM group compared to all other groups (p<0.05) with no other group differences present.
The effect of SAL microinjections into the pVTA on DA efflux in the AcbSh
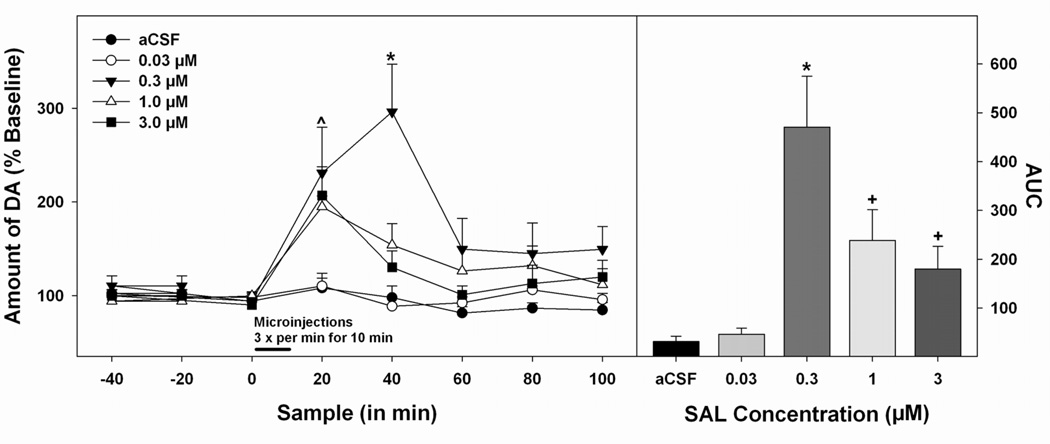
Figure 1 shows the representative histological placements in the AcbSh (panel A, right hemisphere) and pVTA (panel B, right hemisphere). A total of 6 animals had placements outside of the AcbSh or the pVTA and were excluded from analyses. Pulse microinjections of SAL into the pVTA dose-dependently altered DA efflux in the AcbSh. As displayed in Figure 3 (left panel), a 5 × 8 (concentration × sample) mixed ANOVA indicated a significant effect of concentration (F4,31 = 10.318; p<0.05), sample (F7,217 = 14.50; p<0.05) and a significant concentration × sample interaction (F28,217 = 3.74; p<0.05). Post hoc comparisons using the Tukey’s b test revealed that local administration of the 0.3 µM dose of SAL in the pVTA produced a significantly higher level of DA efflux in the AcbSh compared to any other treatment group (p<0.05). Additionally, the 1.0 µM dose of SAL produced a significantly higher amount of DA release than aCSF only (p<0.05). An additional investigation of the data using AUC analysis revealed similar findings (see Figure 3, right panel). Pairwise comparisons found that the mean AUC of the 0.3 µM SAL group significantly exceeded all other groups (p<0.05). The mean AUC for the 1.0 µM and 3.0 µM SAL groups were significantly greater than the aCSF and 0.03 µM SAL groups (p<0.05) but did not significantly differ from one another.
Figure 3.
Dose-response effects of microinjections of SAL into the pVTA on extracellular levels of DA in the AcbSh. Microdialysis time-course data (left panel) are presented as means (± SEMs) percent baseline levels. Bar indicates microinjection period, microinjections were given 3 times a min over the first 10 min of sample 3. Area under the curve (AUC) data (right panel) are presented as means (± SEMs) area under the curve. * 0.3 µM SAL (n = 6) significantly greater (p < 0.05) than all other groups, ^ 3.0 (n = 5), 1.0 (n = 8), and 0.3 µM SAL treatment groups significantly greater (p < 0.05) than 0.03 µM SAL (n = 8) and aCSF (n = 7) groups, + mean area under the curve for 1.0 and 3.0 µM SAL groups significantly greater than 0.03 and aCSF groups (p < 0.05).
In order to tease apart the concentration × sample interaction, separate one-way ANOVA’s with post hoc analyses were completed to compare all five groups at each post-injection sample in an identical fashion to that described for experiment 1. For the first post-injection sample, there was a significant effect of drug concentration (F4,31 = 6.03; p<0.05), as the 0.3 µM, 1.0 µM, and 3.0 µM groups exhibited significantly higher levels of DA compared to the aCSF and 0.03 µM groups (p<0.05). Similarly, on the second post-injection sample, a one-way ANOVA observed a significant effect of concentration (F4,31 = 10.65; p<0.05). However, the post hoc test indicated that this effect was driven by the 0.3 µM SAL group, as it displayed a significantly elevated level of DA compared to all other groups (p<0.05) with no other group differences present. One-way ANOVA’s performed on the final three samples did not yield any significant effects of concentration (F’s < 2.14; p’s>0.63).
Discussion
The present findings support the hypothesis that local administration of ACD or SAL within the pVTA produced an inverted “U-shaped” dose response curve for DA release within the AcbSh. The 23 µM dose of ACD produced the maximal increase in DA efflux to 200% of baseline level. Thus, it appears that ACD acts on DA neurons projecting to AcbSh, in a manner that has been described to underlie the addictive properties of virtually every major drug of abuse, including EtOH (for review see: Willuhn et al., 2010). This finding is in line with accumulating data that implicate the DA reward pathway as a primary mechanism underlying the rewarding properties of ACD (for review see: Deehan et al., 2012). Further, ACD appears to be significantly more potent than EtOH with regard to the stimulation of DA release. Ding et al (2009a) reported that 200 mg% (44 mM) EtOH microinjected into the VTA of EtOH naïve Wistar rats produced the greatest increase in DA efflux (150% of baseline), whereas the optimal dose of ACD (23 µM) was over 1800 fold lower, producing a higher DA efflux (200% of baseline). A similar observation has been reported using the ICSA paradigm, as alcohol-preferring (P) rats exhibited a similar dose response curve, in which 23 µM ACD and 150 mg% EtOH represented the optimal doses that were self-administered into the VTA (Rodd et al., 2005). Moreover, it was demonstrated that the ICSA of both EtOH and ACD were significantly decreased by the co-infusion of the DA D2 receptor agonist quinpirole (Rodd et al., 2005). Thus, the current data offer further support that the rewarding properties of ACD are a function of activation of DA neurons within the pVTA.
The present data also support the hypothesis that microinjections of SAL into the pVTA would increase DA efflux in the AcbSh. The dose-response curve indicated that the optimal dose of SAL (0.3 µM) stimulated extracellular DA levels within the AcbSh to 300% of baseline. Thus, the data indicate that SAL is capable of stimulating DA neuronal activity within the pVTA. This finding compliments previous research showing that the reinforcing properties of SAL in the VTA is dependent on DA signaling (Rodd et al., 2008), and provides further evidence that SAL is highly efficacious at stimulating DA release at low doses. Thus, the current findings, along with past research (Rodd et al., 2008), provide support for the hypothesis that DA release within the AcbSh is a key mediator in the reinforcing properties of SAL.
The current data further elucidate the stimulatory properties of ACD and SAL on pVTA DA neurons. Previous experiments had reported that the reverse microdialysis of 75 µM ACD in the VTA increased DA release in the AcbSh to 150% of baseline (Diana et al., 2008; Melis et al., 2007) whereas a single microinjection of 150 µM SAL into the pVTA increased DA levels within the AcbSh to 130% of baseline (Hipolito et al., 2011). Even though the most effective concentrations in the current study (23 µM ACD and 0.3 µM SAL) were significantly lower (approximately 3 fold and 500 fold lower respectively), differences between the paradigms may be partially responsible. For example, in the case of reverse microdialysis of ACD, probe recoveries of 10–20% could account for the main difference in the effective concentrations. On the other hand, it is difficult to reconcile the 500-fold difference in the effective SAL concentration between the current study and the study of Hipolito et al. (2011), even though different microinjection and microdialysis techniques were used. The 150 uM SAL concentration is much greater than the estimated levels of SAL in brain tissue, and may be producing secondary effects resulting in increased DA release.
It is important to note that the current data represent the first thorough report of the dose-response for both ACD and SAL on DA release at low, pharmacologically relevant, concentrations that have been shown to be rewarding within the pVTA. Furthermore, the optimal dose of ACD, tested in experiment 1, falls within the range of pharmacologically relevant concentrations detected in the brain following EtOH exposure in mice (Isse et al., 2005). Quantitative estimation of brain SAL levels following EtOH exposure has produced variable results; however, recent research has indicated a positive correlation between EtOH consumption and SAL levels in the midbrain (Rojkovicova et al., 2008). Therefore, the current findings offer additional support of a possible role for centrally formed ACD and/or SAL in contributing to the actions of EtOH.
Utilizing a range of concentrations of ACD and SAL, that were similar to concentrations used in the ICSA experiments, the microinjection-microdialysis results also exhibited inverted “U-shaped” dose-response curves similar to those observed for the reinforcing properties of ACD and SAL (Rodd et al., 2005; 2008). Much like the ICSA findings, concentrations of ACD (23 µM) and SAL (0.3 µM) that caused the maximal DA response within the AcbSh fell in the middle of a dose response curve in which higher and lower concentrations were significantly less effective. Intra-pVTA microinjections of ACD and SAL at concentrations higher than the optimum level (23 µM and 0.3 µM, respectively) were less effective at increasing DA release in the AcbSh possibly due to a number of interactions between the higher concentrations of ACD and SAL and several neuronal mechanisms which increase inhibitory and/or decrease excitatory actions on DA neurons. Observations from the current data also coincide with the ICSA data in that the pVTA exhibited a significantly greater sensitivity to SAL compared to ACD. The dose response curve for SAL is far lower (shifted to the left) than that of ACD; the most effective dose of SAL (0.3 µM) is approximately 70-fold lower than the most effective dose of ACD (23 µM). Moreover, SAL caused a greater amount of DA release compared to ACD, as 0.3 µM SAL stimulated a maximal increase in DA release to 300% of baseline, whereas 23 µM ACD stimulated a maximal increase to 200% of baseline, suggesting SAL may be activating more DA neurons in the pVTA.
The data indicated that concentrations that did not support the ICSA of SAL into the pVTA (i.e., 1.0 µM and 3.0 µM) still stimulated DA release in the AcbSh, although the amount of DA was significantly lower compared to 0.3 µM SAL (Figs. 2 & 3). The reduction in DA release observed at the highest concentrations may be a result of SAL having effects on one or more other systems within the pVTA that reduce DA neuronal activity. The observation that higher SAL concentrations did not produce reinforcing effects, as reported by Rodd et al., 2008, could be a combination of neurobiological interactions that result in reduced DA neuronal activity and/or other unknown actions of SAL.
In contrast to the effects observed for SAL, 12 µM ACD was within the range of concentrations self-administered during ICSA testing (Rodd et al., 2005) yet failed to significantly alter DA release (Figs. 2 & 3). One possible reason for this disagreement may be due to the difference in drug exposure between the microinjection/microdialysis and ICSA paradigms. The microinjection/microdialysis paradigm utilized in the current study involved an acute exposure to ACD over a 10-min microinjection period. The ICSA paradigm, on the other hand, involved repeated exposure to ACD over multiple 4-hour sessions run every other day (Rodd et al., 2005; 2008). It is possible that sensitization to the reinforcing properties of ACD may develop during the repeated exposure inherent in the ICSA paradigm, in effect shifting the dose response curve to the left for ACD. A similar argument could be used to explain why a lower dose of SAL (0.03 µM) was self-infused into the pVTA but had little effect on DA release when microinjected into the pVTA. Nonetheless, the current research shows that microinjections of ACD and SAL into the pVTA stimulate DA release in the AcbSh at concentrations that have been found to support the ICSA of both compounds.
There are likely several neurobiological mechanisms that contribute to the observed stimulation of DA release resulting from the local administration of ACD and SAL within the pVTA. Research has implicated the mu opioid receptor (MOR) as a prime candidate for the rewarding aspects of both ACD and SAL as naloxone significantly decreased IV self-administration of ACD (Myers et al., 1984a), whereas naltrexone and β-funaltrexamine (a selective MOR antagonist) microinjected directly into the VTA blocked ACD and SAL induced locomotor activity (Hipolito et al., 2010; Matsuzawa et al., 2000; Sanchez-Catalan et al., 2009). Recently, electrophysiological data indicate SAL indirectly stimulates DA neurons within the pVTA through action at MORs on gamma-aminobutryric acid (GABA) at concentrations tested in the current study (Xie et al., 2012). Thus, it can be speculated that MORs within the mesolimbic system are likely a contributory mechanism underlying the rewarding/reinforcing action of ACD and/or SAL.
In conclusion, various laboratories have suggested that the reinforcing/rewarding properties of both ACD and SAL are mediated, in part, by the DA system. However, a limited amount of the research thus far has administered both ACD and SAL centrally and investigated how DA signaling is altered. The current study represents the first thorough investigation of the stimulatory properties of both ACD and SAL within the pVTA, and the subsequent DA release within the AcbSh. The data from these experiments show that both ACD and SAL are capable of stimulating dopaminergic activity within the reward pathway at low concentrations. Moreover, data from the current research indicate that both ACD and SAL stimulate the DA projection from the VTA to the AcbSh in a manner similar to several drugs of abuse. While the exact extent to which ACD and SAL are involved in the rewarding properties of alcohol remains to be resolved, it will be beneficial for future research to focus on elucidating this relationship which, in turn, may help with the development of novel pharmacotherapies and treatments for individuals suffering from alcohol use disorders.
Acknowledgements
The authors would also like to thank Tylene Pommer and Brandon Dewell for their expert technical assistance.
Research supported by: AA07462, AA014437, AA019366 (The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or NIH.)
Footnotes
None of the authors has a conflict of interest associated with this research.
References
- Amit Z, Brown ZW, Rockman GE. Possible involvement of acetaldehyde, norepinephrine and their tetrahydroisoquinoline derivatives in the regulation of ethanol self-administration. Drug Alcohol Depend. 1977;2:495–500. doi: 10.1016/0376-8716(77)90049-7. [DOI] [PubMed] [Google Scholar]
- Aragon CM, Amit Z. The effect of 3-amino-1,2,4-triazole on voluntary ethanol consumption: evidence for brain catalase involvement in the mechanism of action. Neuropharmacology. 1992;31:709–712. doi: 10.1016/0028-3908(92)90150-n. [DOI] [PubMed] [Google Scholar]
- Aragon CM, Spivak K, Amit Z. Blockade of ethanol induced conditioned taste aversion by 3-amino-1,2,4-triazole: evidence for catalase mediated synthesis of acetaldehyde in rat brain. Life Sci. 1985;37:2077–2084. doi: 10.1016/0024-3205(85)90579-x. [DOI] [PubMed] [Google Scholar]
- Aragon CM, Spivak K, Amit Z. Effects of 3-amino-1,2,4-triazole on ethanol-induced open-field activity: evidence for brain catalase mediation of ethanol's effects. Alcohol Clin Exp Res. 1989;13:104–108. doi: 10.1111/j.1530-0277.1989.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Brown ZW, Amit Z, Smith B. Intraventricular self-administration of acetaldehyde and voluntary consumption of ethanol in rats. Behav Neural Biol. 1980;28:150–155. doi: 10.1016/s0163-1047(80)91487-9. [DOI] [PubMed] [Google Scholar]
- Deehan GA, Jr, Brodie MS, Rodd ZA. What is in that drink: the biological actions of ethanol, acetaldehyde, and salsolinol. In: Sommer WH, Spanagel R, editors. Current Topics in Behavioral Neurosciences: Behavioral Neurobiology of Alcohol Addiction. Berlin, Heidelberg: Springer-Verlag; 2012. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Peana AT, Sirca D, Lintas A, Melis M, Enrico P. Crucial role of acetaldehyde in alcohol activation of the mesolimbic dopamine system. Ann N Y Acad Sci. 2008;1139:307–317. doi: 10.1196/annals.1432.009. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, McBride WJ. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res. 2009a;33:1571–1581. doi: 10.1111/j.1530-0277.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Toalston JE, Oster SM, McBride WJ, Rodd ZA. Involvement of local serotonin-2A but not serotonin-1B receptors in the reinforcing effects of ethanol within the posterior ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 2009b;204:381–390. doi: 10.1007/s00213-009-1468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C, Deitrich RA. A critical evaluation of tetrahydroisoquinoline induced ethanol preference in rats. Pharmacol Biochem Behav. 1980;13:265–281. doi: 10.1016/0091-3057(80)90083-0. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM. Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol. 2006;38:5–12. doi: 10.1016/j.alcohol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Escarabajal D, Miquel M, Aragon CM. A psychopharmacological study of the relationship between brain catalase activity and ethanol-induced locomotor activity in mice. J Stud Alcohol. 2000;61:493–498. doi: 10.15288/jsa.2000.61.493. [DOI] [PubMed] [Google Scholar]
- Font L, Aragon CM, Miquel M. Voluntary ethanol consumption decreases after the inactivation of central acetaldehyde by d-penicillamine. Behav Brain Res. 2006a;171:78–86. doi: 10.1016/j.bbr.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Font L, Aragon CM, Miquel M. Ethanol-induced conditioned place preference, but not aversion, is blocked by treatment with D -penicillamine, an inactivation agent for acetaldehyde. Psychopharmacology (Berl) 2006b;184:56–64. doi: 10.1007/s00213-005-0224-z. [DOI] [PubMed] [Google Scholar]
- Font L, Miquel M, Aragon CM. Prevention of ethanol-induced behavioral stimulation by D-penicillamine: a sequestration agent for acetaldehyde. Alcohol Clin Exp Res. 2005;29:1156–1164. doi: 10.1097/01.alc.0000171945.30494.af. [DOI] [PubMed] [Google Scholar]
- Hipolito L, Marti-Pratz L, Sanchez-Catalan MJ, Polache A, Granero L. Induction of conditioned place preference and dopamine release by salsolinol in posterior VTA of rats: involvement of µ-opioid receptors. Neurochem International. 2011;59:559–562. doi: 10.1016/j.neuint.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Hipolito L, Sanchez-Catalan MJ, Zornoza T, Polache A, Granero L. Locomotor stimulant effects of acute and repeated intrategmental injections of salsolinol in rats: role of mu-opioid receptors. Psychopharmacology (Berl) 2010;209:1–11. doi: 10.1007/s00213-009-1751-9. [DOI] [PubMed] [Google Scholar]
- Isse T, Matsuno K, Oyama T, Kitagawa K, Kawamoto T. Aldehyde dehydrogenase 2 gene targeting mouse lacking enzyme activity shows high acetaldehyde level in blood, brain, and liver after ethanol gavages. Alcohol Clin Exp Res. 2005;29:1959–1964. doi: 10.1097/01.alc.0000187161.07820.21. [DOI] [PubMed] [Google Scholar]
- Jamal M, Ameno K, Uekita I, Kumihashi M, Wang W, Ijiri I. Catalase mediates acetaldehyde formation in the striatum of free-moving rats. Neurotoxicology. 2007;28:1245–1248. doi: 10.1016/j.neuro.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Karahanian E, Quintanilla ME, Tampier L, Rivera-Meza M, Bustamante D, Gonzalez-Lira V, Morales P, Herrera-Marschitz M, Israel Y. Ethanol as a prodrug: brain metabolism of ethanol mediates its reinforcing effects. Alcohol Clin Exp Res. 2011;35:606–612. doi: 10.1111/j.1530-0277.2011.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechling UM, Amit Z. Effects of 3-amino-1,2,4-triazole on brain catalase in the mediation of ethanol consumption in mice. Alcohol. 1994;11:235–239. doi: 10.1016/0741-8329(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M. Involvement of mu-opioid receptor in the salsolinol-associated place preference in rats exposed to conditioned fear stress. Alcohol Clin Exp Res. 2000;24:366–372. [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Melchior C, Collins MA. The route and significance of endogenous synthesis of alkaloids in animals. Crit Rev Toxicol. 1982;9:313–356. doi: 10.3109/10408448209037496. [DOI] [PubMed] [Google Scholar]
- Melis M, Enrico P, Peana AT, Diana M. Acetaldehyde mediates alcohol activation of the mesolimbic dopamine system. Eur J Neurosci. 2007;26:2824–2833. doi: 10.1111/j.1460-9568.2007.05887.x. [DOI] [PubMed] [Google Scholar]
- Myers RD, Melchior CL. Alcohol drinking: abnormal intake caused by tetrahydropapaveroline in brain. Science. 1977;196:554–556. doi: 10.1126/science.557839. [DOI] [PubMed] [Google Scholar]
- Myers WD, Ng KT, Singer G. Effects of naloxone and buprenorphine on intravenous acetaldehyde self-injection in rats. Physiol Behav. 1984a;33:449–455. doi: 10.1016/0031-9384(84)90168-9. [DOI] [PubMed] [Google Scholar]
- Myers W, Ng K, Singer G. Ethanol preference in rats with a prior history of acetaldehyde self-administration. Experientia. 1984b;40:1008–1010. doi: 10.1007/BF01946483. [DOI] [PubMed] [Google Scholar]
- National Institute of Health. Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th ed. New York: Academic Press; 2005. [Google Scholar]
- Peana AT, Enrico P, Assaretti AR, Pulighe E, Muggironi G, Nieddu M, Piga A, Lintas A, Diana M. Key role of ethanol-derived acetaldehyde in the motivational properties induced by intragastric ethanol: a conditioned place preference study in the rat. Alcohol Clin Exp Res. 2008;32:249–258. doi: 10.1111/j.1530-0277.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- Quintanilla ME, Tampier L. Acetaldehyde-reinforcing effects: differences in low-alcohol-drinking (UChA) and high-alcohol-drinking (UChB) rats. Alcohol. 2003;31:63–69. doi: 10.1016/j.alcohol.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Quintanilla ME, Tampier L, Karahanian E, Rivera-Meza M, Herrera-Marschitz M, Israel Y. Reward and relapse: complete gene-induced dissociation in an animal model of alcohol dependence. Alcohol Clin Exp Res. 2012;36:517–522. doi: 10.1111/j.1530-0277.2011.01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, Murphy JM, Goldstein A, Zaffaroni A, Li TK, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology. 2005;30:330–338. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Oster SM, Ding ZM, Toalston JE, Deehan GA, Jr, Bell RL, Li TK, McBride WJ. The reinforcing properties of salsolinol in the ventral tegmental area: evidence for regional heterogeneity and the involvement of serotonin and dopamine. Alcohol Clin Exp Res. 2008;32:230–239. doi: 10.1111/j.1530-0277.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Melendez RI, Zaffaroni A, Goldstein A, McBride WJ, Li TK. The reinforcing effects of acetaldehyde in the posterior ventral tegmental area of alcohol-preferring rats. Pharmacol Biochem Behav. 2002;72:55–64. doi: 10.1016/s0091-3057(01)00733-x. [DOI] [PubMed] [Google Scholar]
- Rojkovicova T, Mechref Y, Starkey JA, Wu G, Bell RL, McBride WJ, Novotny MV. Quantitative chrial analysis of salsolinol in different brain regions of rats genetically predisposed to alcoholism. J Chromatog. 2008;863:206–214. doi: 10.1016/j.jchromb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Sanchez-Catalan MJ, Hipolito L, Zornoza T, Polache A, Granero L. Motor stimulant effects of ethanol and acetaldehyde injected into the posterior ventral tegmental area of rats: role of opioid receptors. Psychopharmacology (Berl) 2009;204:641–653. doi: 10.1007/s00213-009-1495-6. [DOI] [PubMed] [Google Scholar]
- Smith BR, Amit Z, Splawinsky J. Conditioned place preference induced by intraventricular infusions of acetaldehyde. Alcohol. 1984;1:193–195. doi: 10.1016/0741-8329(84)90097-1. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampier L, Quintanilla ME. Effect of acetaldehyde on acute tolerance and ethanol consumption in drinker and nondrinker rats. J Stud Alcohol. 2002;63:257–262. doi: 10.15288/jsa.2002.63.257. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Hipolito L, Zuo W, Polache A, Granero L, Kmjevic K, Ye J-J. Salsolinol stimulates dopamine neurons in slices of posterior ventral tegmental area indirectly by activating µ-opioid receptors. J Pharmacol Exp Ther. 2012;341:43–50. doi: 10.1124/jpet.111.186833. [DOI] [PMC free article] [PubMed] [Google Scholar]