Abstract
SCOPE
Women seeking alternatives to hormone replacement therapy for menopausal symptoms often try botanical dietary supplements containing extracts of hops (Humulus lupulus L.). Hops contain 8-prenylnaringenin (8-PN), a potent phytoestrogen, the related flavanones 6-prenylnaringenin (6-PN) and isoxanthohumol (IX), and the prenylated chalcone xanthohumol (XN).
METHODS AND RESULTS
After chemically and biologically standardizing an extract of spent hops to these marker compounds, an escalating dose study was carried out in menopausal women to evaluate safety and pharmacokinetics. 8-PN, 6-PN, IX, and XN, sex hormones, and prothrombin time (PT/INR) were determined in blood samples and/or 24-h urine samples. There was no effect on sex hormones or blood clotting. The maximum serum concentrations of the prenylated phenols were dose-dependent and were reached from 2 to 7 h, indicating slow absorption. The marker compounds formed glucuronides that were found in serum and urine. Secondary peaks at 5 h in the serum concentration-time curves indicated enterohepatic recirculation. The serum concentration-time curves indicated demethylation of IX to form 8-PN and cyclization of XN to IX. Slow absorption and enterohepatic recirculation contributed to half-lives exceeding 20 h.
CONCLUSION
This human study indicated long half-lives of the estrogenic and proestrogenic prenylated phenols in hops but no acute toxicity.
INTRODUCTION
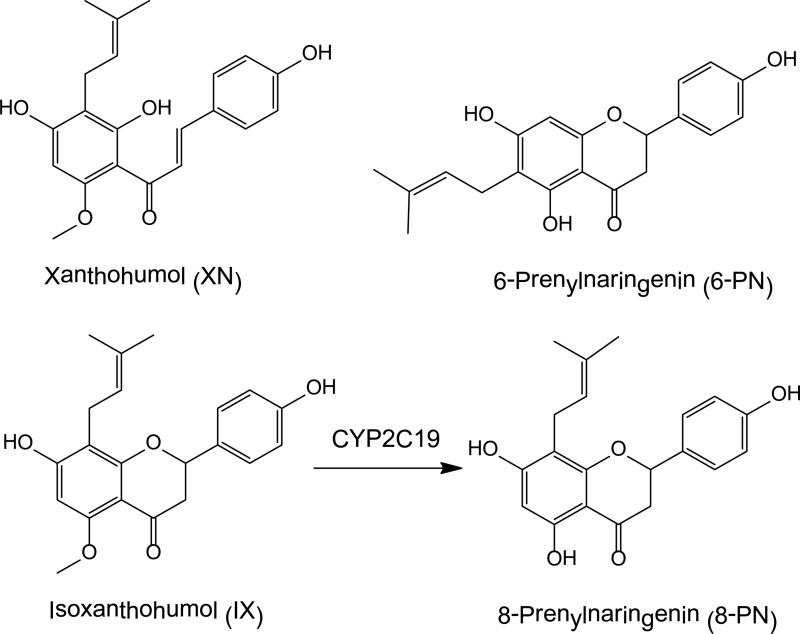
Used in the brewing of beer, the female inflorescenses (cones) of hops (Humulus lupulus L.) are under investigation for their estrogenic and chemopreventive properties and are being used by women as dietary supplements and alternatives to conventional hormone replacement therapy for the management of menopausal hot flashes [1]. Among the bioactive compounds of hops, the prenylated phenols including the chalcone xanthohumol (XN) and the flavanones isoxanthohumol (IX), 6-prenylnaringenin (6-PN) and 8-prenylnaringenin (8-PN) have received the most attention (Figure 1). Representing the most abundant of these compounds and comprising up to 1% of the dry weight of hop cones [2,3], XN, exhibits anti-proliferative activity against breast, colon and ovarian cancer cell lines and is a potent inducer of chemoprevention enzymes regulated by the antioxidant response element [4,5]. In contrast, the estrogenic prenylated flavanones are minor constituents of hops and occur at 10-100 fold lower concentrations than does XN [2].
Figure 1.
Chemical structures of prenylated hop phenols with estrogenic and/or proestrogenic activities.
The estrogenicity of flavanone 8-PN, one of the most potent phytoestrogens [6], has been confirmed in numerous in vitro and animal studies [7,8]. Although a weaker estrogen than 8-PN, IX can be metabolized to 8-PN through enzymatic O-demethylation by cytochrome P450 1A2 (CYP1A2) [9] or by microbes in the intestine [10]. In addition, XN can be converted to IX through acid-catalyzed cyclization with a half-life of 37 min in the stomach [11].
Using a rat model, Overk, et al., [7] measured plasma and tissue levels of 8-PN, IX and their major metabolites after intraperitoneal injection of pure compounds, whereas Rad, et al., [12] reported the pharmacokinetics of 8-PN after a single oral dose of up to 750 mg pure 8-PN to post menopausal women. In both cases, the pharmacokinetics of 8-PN was characterized by pronounced enterohepatic recirculation and tight dose linearity. Bolca, et al., [13] studied the in vivo conversion of IX into 8-PN and disposition of XN, IX and 8-PN in breast tissue of 21 healthy women electing breast reduction surgery following oral administration of capsules containing a commercially available hop dietary supplement standardized to 0.100 mg 8-PN. Legette, et al., reported pharmacokinetics studies of XN in rats [14] and humans [15] following oral administration of pure XN. In both cases, XN had long elimination half-lives (32 h in rats and 18-20 h in humans) and showed extensive glucuronidation. In the present investigation, the pharmacokinetics of not only XN or only 8-PN but XN, 8-PN, IX, and 6-PN were investigated in women following oral administration of a hop extract that had been standardized both chemically and biologically. The initial dosages of the hop extract used in the present escalating dose investigation were similar to those currently used in commercial products [13].
MATERIALS AND METHODS
Materials
An extract from spent hops, which is residual hop cone material remaining after supercritical fluid carbon dioxide extraction of bitter acids and essential oils for the brewing industry [16], were prepared using good manufacturing practice at Hopsteiner (New York, NY, USA). The extract was standardized biologically for estrogenic activity using the estrogen responsive alkaline phosphatase (AP) enzyme in the Ishikawa cell line as described previously [17]. The product was also standardized chemically for XN, IX, 6-PN, and 8-PN (Figure 1) content using high performance liquid chromatography-tandem mass spectrometry (LC/MS-MS) as previously described [17]. The spent hop extract contained the following levels of prenylated phenols (g/100 g dry weight of the extract): 0.42 ± 0.02 of 8-PN; 2.18 ± 0.0 of 6-PN; 1.35 ± 0.08 of IX; and 35.78 ± 0.31 of XN. The hop extract, a pale yellow powder, was encapsulated for clinical investigation in size 3, opaque green gelatin capsules (59.5 mg extract per capsule), so that each contained 0.25 mg 8-PN, 1.30 mg 6-PN, 0.80 mg IX, and 21.3 mg XN.
8-PN, 6-PN, IX, XN, and 8-pentylprenylnaringenin (internal standard) were isolated or prepared within the UIC/NIH Center for Botanical Dietary Supplements Research (Chicago, IL) [17]. All solvents were HPLC grade and purchased from Thermo Fisher (Fair Lawn, NJ). Purified water was prepared by using a Millipore Milli-Q purification system (Millipore, Billerica, MA, USA). β-Glucuronidase and sulfatase from Helix pomatia were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were ACS reagent grade.
Clinical Design
The clinical protocol was approved by the Institutional Review Boards of both the University of Illinois at Chicago and Northwestern University. Five healthy post-menopausal women (age 55 to 68 years; Supplemental Table 1) were recruited for the study and were provided informed consent. Subjects on hormone replacement therapy halted medication for at least 2 months prior to the study. No beer consumption was allowed up to 1 month before or during the study. One capsule containing the hop extract (low dose) was administered to each subject once daily for 5 days. The subjects were followed for 7 more days to observe any delayed effects. After a 1-month washout, the study was repeated with the same subjects using a dosage of 2 capsules/day (medium dose). After another month washout, the study was repeated for a third time using the same subjects at a dosage of 4 capsules/day (high dose). Blood samples were obtained hourly from each subject for the first 24 h, and then once a day for the next 4 days. A 24-h urine collection was obtained during the first day post-dose. Blood samples were assayed for estradiol, follicle stimulating hormone, luteinizing hormone, and prothrombin time (PT/INR) at 0 h and 12 h as well as on day 4 and day 7. Serum and urine samples were frozen at −80 °C until analysis for hop prenylated phenols.
Quantitative analysis of hop prenylated phenols
XN, IX, 8-PN, and 6-PN were measured in human serum using a validated ultrahigh-pressure liquid chromatography-tandem mass spectrometry (UHPLCMS/MS) method that was reported previously [18]. Urine samples were measured using a variation of this method with the following changes. After thawing at room temperature, 1 mL urine was mixed with 0.5 mL of 100 mM sodium acetate buffer (pH 5.0) containing 8-pentylprenylnaringenin as internal standard (20 ng/mL), hydrolyzed for 1 h at 37 °C using 15 units of sulfatase and 400 units of β-glucuronidase and then extracted twice with 4.5 mL of methyl-t-butyl ether in a glass tube. To assess the extent of conjugation, some urine samples were not hydrolyzed prior to extraction. Calibration standards and quality control samples were prepared by mixing 100 µL of each standard or QC solution with 0.9 mL blank urine and 0.5 mL of internal standard (20 ng/mL) in 100 mM sodium acetate (pH 5.0) followed by liquid-liquid extraction. After centrifugation for 5 min at 4000 x g at 4 °C, the organic layers were removed, combined and evaporated to dryness under a stream of nitrogen. Each residue was reconstituted in 100 μL of 70% aqueous methanol. The concentrations of XN, IX, 8-PN, and 6-PN in the urine extracts were then measured using UHPLC/MS-MS as on a Shimadzu LCMS-8030 triple quadrupole mass spectrometer equipped with a Nexera UHPLC system and a Shim-pack XR-ODS III (2.0 x 50 mm, 1.6 μm) C18 column as described for serum analysis [18].
Pharmacokinetics
The mean plasma concentration over time profile for each hop prenylated phenol was generated using Graphpad Prism (Version 5.0, San Diego, CA). The pharmacokinetics parameters were derived using WinNonlin 6.2 (Pharsight, Mountain View, CA) for each dosage group and expressed as mean ± S.D. A noncompartmental analysis was used to calculate the kinetic parameters including area under curve (AUC), the peak serum concentration (Cmax), time to reach peak concentration (Tmax), the apparent volume of distribution (Vd/F), the elimination half-life (T1/2), and the apparent clearance (CL/F). The AUC from time 0 to the last measureable time point defined by the lower limit of quantitation (defined as a signal-to-noise level of 10:1) was calculated using the log-linear trapezoidal rule. The half-life was calculated using the terminal elimination constant λz, which was derived from linear regression analysis of the serum concentrations. The half-life equation was determined using the following equation: T1/2 = 0.693/λz.
RESULTS AND DISCUSSION
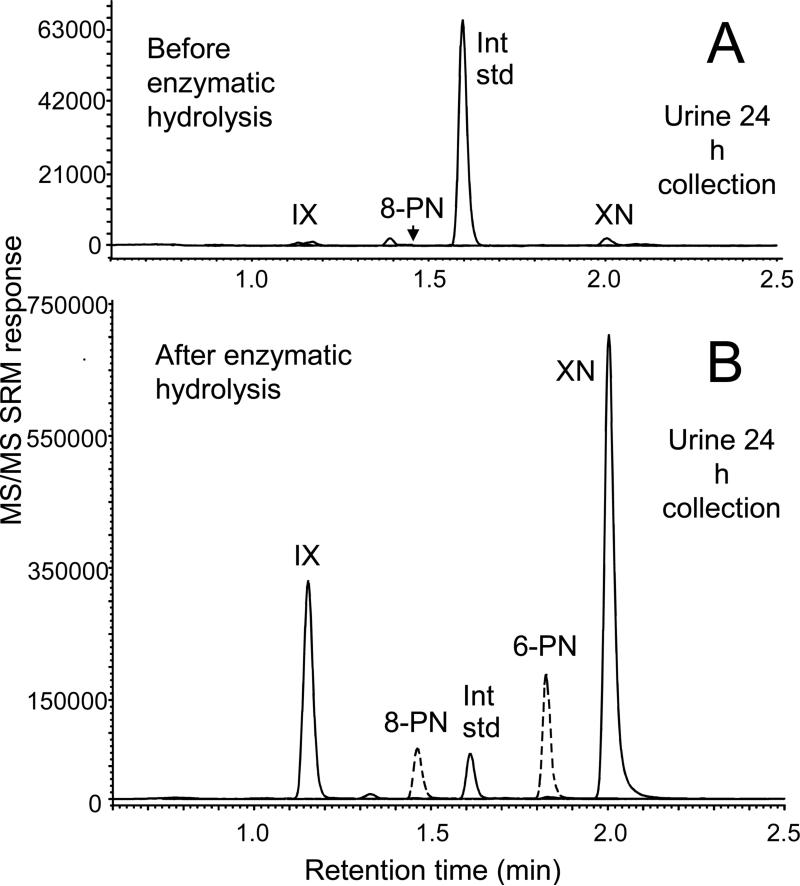
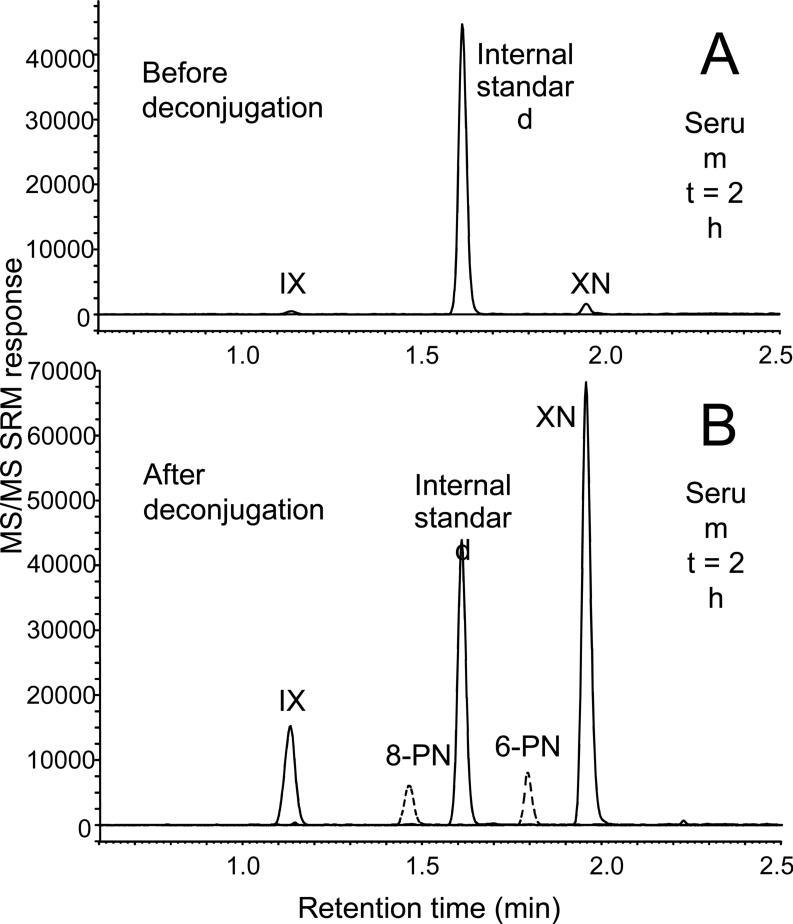
At time zero, no prenylated hop phenols were detected using UHPLC-MS/MS in any of the serum or urine samples (Figures 2 and 3), indicating that all subjects were successful in avoiding sources of hops and hop products. Traces of all unconjugated prenylated phenols except 6-PN were detected in the 24-h urine collections (Figure 2), while only IX and XN were detected in their unconjugated form in serum and only at early time points of medium and high dose subjects (Figure 3). After enzymatic deconjugation, intense signals for all 4 hop compounds were detected in serum (Figure 2) and in the 24-h urine collections (Table 1 and Figure 3).
Figure 2.
Negative ion electrospray UHPLC-MS/MS with selected reaction monitoring (SRM) chromatograms of prenylated hop phenols in human urine from a single subject obtained A) before enzymatic deconjugation; and B) after deconjugation using β-glucuronidase and sulfatase. Note the large increase in the levels of prenyl phenols after enzymatic deconjugation, indicating that conjugates (especially glucuronides) were the major circulating forms of these compounds.
Figure 3.
Negative ion electrospray UHPLC-MS-MS SRM chromatograms of prenylated hop phenols in human serum from the same subject as in Figure 2, A) before enzymatic deconjugation; and B) after deconjugation using β-glucuronidase and sulfatase.
Table 1.
Urinary excretion (total and % of dose) of XN, IX, 8-PN, and 6-PN during the first 24 h after administration of single doses of a standardized extract of spent hops.
| Dosage | XN | IX | 8-PN | 6-PN |
|---|---|---|---|---|
| Low | 23.9 ± 17.9a | 29.8 ± 32.3 | 5.1 ± 4.4 | 26.2 ± 24.1 |
| 0.040±0.030% | 1.318±1.428% | 0.693±0.597% | 0.685±0.631% | |
| Medium | 37.0 ± 26.3 | 89.5 ± 54.5 | 21.3 ± 21.6 | 49.8 ± 39.8 |
| 0.031±0.022% | 1.98±1.205% | 1.449±1.467% | 0.651±0.521% | |
| High | 138 ± 113 | 172 ± 67.4 | 29.4 ± 22.9 | 150 ± 104 |
| 0.058±0.047% | 1.902±0.746% | 1.001±0.781% | 0.982±0.684% |
Data are expressed as Mean ± S.D. (nmol) (N = 5)
Possible effects of the spent hop extract on sex hormone levels and blood clotting were measured in blood samples obtained at baseline, 1 h, 12 h, day 4, and day 7 after administration of the standardized hop extract. There were no significant changes in prothrombin time (Supplemental Table 1). No changes were observed for the sex hormones estradiol, follicle stimulating hormone or luteinizing hormone (Supplemental Table 2). In addition, no acute toxicity from the hop extract was observed in any of the subjects.
Over 98% of the hop prenylated phenols in urine were in the form of glucuronides, and as the dosage of the hop extract increased, the urinary excretion of each compound increased proportionally (Table 1). However, urinary excretion of each compound accounted for less than 2% of the dose, which indicated that alternative routes of elimination such as biliary secretion might be more prominent. This is consistent with Rad, et al., [12] who reported that 21-24% of 8-PN administered as single oral doses to women was recovered in feces within 48 h, compared with only 4.6% to 8.1% of the doses being excreted in urine during this time. Similarly, after oral administration of XN to rats, 89% was recovered in the feces in the first 48 h [19].
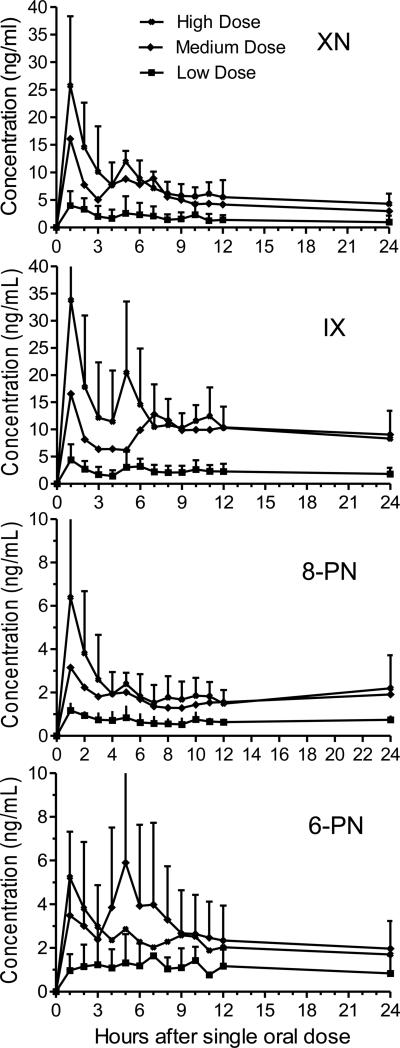
The mean serum concentration over time profiles for each prenylated hop phenol after enzymatic deconjugation are shown in Figure 4. The pharmacokinetic parameters for each compound were calculated using non-compartmental analysis, and are summarized in Table 2. Non-compartmental analysis was used since it provided the best fit for initial estimation of pharmacokinetic parameters while, for example, a two-compartment model did not fit all of the curves due to the secondary peaks or the lag time in absorption. These data indicate that, after oral administration of the hop extract, prenylated hop phenols were absorbed slowly into the bloodstream, followed by slow elimination during the next 24 h. No elimination data are available beyond 24 h after the first dose, as second doses were administered on day 2 as part of the safety studies.
Figure 4.
Serum concentration-time curves of the 4 major prenylated hop phenols following oral administration of single doses of an extract of spent hops to 5 women. The curves represent total content of each compound (free + aglycon following enzymatic deconjugation) and were obtained by averaging concentrations from all 5 subjects. Secondary peaks occurring approximately 5-6 h post-dose suggest enterohepatic recirculation.
Table 2.
Pharmacokinetic parameters for prenylated hop phenols following oral administration of a standardized hop extract at each of the three dosages.
| Xanthohumol (XN) | ||||
|---|---|---|---|---|
| Unit | Low (21.3 mg) | Medium (42.6 mg) | High (85.2 mg) | |
| AUC0-24 | h × ng/mL | 37.1 ± 31.9 | 126 ± 40 | 172 ± 45 |
| AUC0-inf | h × ng/mL | 73.2 ± 60.1 | 167 ± 62 | 323 ± 170 |
| T1/2 | h | 18.3 ± 5.3 | 9.5 ± 2.2 | 20.7 ± 12.7 |
| Tmax | h | 3.8 ± 4.4 | 3.6 ± 2.6 | 1.8 ± 1.8 |
| Cmax | ng/mL | 4.4 ± 3.0 | 22.2 ± 12.1 | 27.6 ± 8.9 |
| Vd/F | L | 17300 ± 21000 | 3770 ± 1390 | 7880 ± 1810 |
| CL/F | L/h | 657 ± 670 | 278 ± 87 | 329±163 |
| Isoxanthohumol (IX) | ||||
|---|---|---|---|---|
| Unit | Low (0.80 mg) | Medium (1.60 mg) | High (3.20 mg) | |
| AUC0-24 | H × ng/mL | 61 ± 26 | 229 ± 243 | 282 ± 115 |
| AUC0-inf | H × ng/mL | 149 ± 86 | 418 ± 446 | 546 ± 301 |
| T1/2 | h | 27.5 ± 5.7 | 24.8 ± 24.3 | 19.9 ± 8.7 |
| Tmax | h | 4.2 ± 4.2 | 3.2 ± 3.2 | 4.0 ± 4.8 |
| Cmax | ng/mL | 5.7 ± 2.7 | 19.1 ± 15.5 | 37.6 ± 17.6 |
| Vd/F | L | 262 ± 135 | 99 ± 56 | 182 ± 53 |
| CL/F | L/h | 7.1 ± 4.2 | 5.7 ± 4.9 | 7.3 ± 3.5 |
| 8-Prenylnaringenin (8-PN) | ||||
|---|---|---|---|---|
| Unit | Low (0.25 mg) | Medium (0.50 mg) | High (1.00 mg) | |
| AUC0-24 | H × ng/mL | 16.7 ± 5.1 | 41.0 ± 26.4 | 50.4 ± 26.7 |
| AUC0-inf | H × ng/mL | 24.3 ± 8.9 | 69.2 ± 38.9 | 88.6 ± 52.6 |
| T1/2 | h | > 24 | > 24 | > 24 |
| Tmax | h | 5.3 ± 4.5 | 7.0 ± 9.7 | 2.8 ± 4.0 |
| Cmax | ng/mL | 1.4 ± 0.3 | 3.9 ± 2.6 | 6.7 ± 3.8 |
| Vd/F | L | 118 ±75 | 186 ± 186 | 271 ± 216 |
| CL/F | L/h | 11.1 ± 3.4 | 10.4 ± 8.1 | 13.7 ± 5.5 |
| 6-Prenylnaringenin (6-PN) | ||||
|---|---|---|---|---|
| Unit | Low (1.30 mg) | Medium (2.60 mg) | High (5.20 mg) | |
| AUC0-24 | h × ng/mL | 26.4 ± 18.6 | 64.6 ± 48.3 | 54.5 ± 35.3 |
| AUC0-inf | h × ng/mL | 69.2 ± 60.0 | 126 ± 75 | 103 ± 43 |
| T1/2 | h | 23.5 ± 6.9 | 24.1 ± 10.7 | 21.4 ± 6.4 |
| Tmax | h | 7.5 ± 3.5 | 5.6 ± 2.8 | 1.8 ± 1.9 |
| Cmax | ng/mL | 1.6 ± 0.9 | 7.3 ± 6.2 | 5.8 ± 3.2 |
| Vd/F | L | 887 ± 581 | 1000 ± 755 | 1770 ± 759 |
| CL/F | L/h | 30.0 ± 26.1 | 28.7 ± 18.1 | 55.4 ± 15.4 |
Data are expressed as Mean ± S.D. (N = 5)
The times required to reach the maximum concentrations (Tmax) of 8-PN, 6-PN, IX, and XN in serum were long, ranging from 1.8 h in the high dose group to 7.5 h in the low dose group, and indicate slow absorption (Table 2). Considering that XN has been shown to concentrate in human colon cancer epithelial Caco-2 cells in a saturable manner [20, 21], the colon epithelium might serve as a reservoir for slow release of these prenylated phenols into the circulation. At the highest dosages, uptake and storage of these compounds in the colon epithelium might become saturated, thereby explaining the shorter time to serum Tmax at high doses.
The maximum concentrations (Cmax) of 8-PN and 6-PN were dose-dependent and ranged from 1.4 ng/mL to 6.7 ng/mL for 8-PN, and from 1.6 ng/mL to 7.3 ng/mL for 6-PN, for the lowest and highest doses, respectively (Table 2). Similar dose-dependent Cmax results were observed for XN and IX, with values ranging from 4.4 ng/mL (low dose) to 27.6 ng/mL (high dose) for XN, and from 5.7 ng/mL to 37.6 ng/mL for the lowest and highest doses of IX, respectively (Table 2). At the medium dose, Cmax values were higher than expected for one subject, which resulted in higher mean values of prenylated hop phenols and larger standard deviations for this dosage.
Although the amount of IX in the hop extract was 27-fold lower than that of XN, the Cmax values for IX were almost identical to those of XN at all doses (Table 2). Furthermore, the total amount of IX excreted in urine (including its Phase 2 metabolism conjugates) was significantly larger than that of urinary XN. As XN is a chalcone (Figure 1) that can cyclize to form the flavonoid IX in the intestine [10] or in the presence of gastric acid in the stomach [11], conversion probably occurred to a major extent in the gastrointestinal system during absorption. It should be noted that conversion of XN to IX in serum during storage and preparation prior to analysis has been investigated and determined to be insignificant [18].
The half-life of XN after oral administration was approximately 19 h for all dosages with a range from 18.3 h at the low dose of 21.3 mg to 20.7 h at the highest dose of 85.2 mg (Table 2). For IX, the half-life decreased from 27.5 h at the low dose of 0.8 mg to 19.9 h at the high dose of 3.2 mg. The half-life of 6-PN was similar across all dosages and ranged from 21 h to 24 h (Table 2). These half-life values are essentially the same as those reported by Leggett, et al., [15] who found that the half-life of XN for both men and women ranged from 21 to 30 h for single doses of 60 or 180 mg.
As the serum concentration of 8-PN was increasing from 12 h post-dose to 24 h post-dose (measured after deconjugation, Figure 4), the slope of the elimination phase and therefore the half-life could not be determined accurately. Therefore, we estimate the half-life of 8-PN to be over 24 h. The accumulation of 8-PN beginning at 12 h post-dose indicated that 8-PN is actively being formed in vivo, probably by O-demethylation of IX by human CYP1A2 [9] or through microbial metabolism in the gut during enterohepatic recirculation [10, 14, 22].
The area under the concentration-time curves (AUC0-24) for each hop extract dosage (measured after deconjugation, Figure 4) was determined using a log-linear trapezoidal rule from time zero to the last measureable time point. The AUC0-24 ratios of 8-PN after normalization by dose were 1:1.2:0.8 which is in agreement with Rad, et al., [12] who reported a linear relationship between AUC and dosage of pure 8-PN up to 750 mg. The mean value of the AUC0-24 (n=5) for 8-PN for our high dose group (containing 1 mg 8-PN) was 50.4 h.ng/mL (Table 2). Assuming that the AUC for 8-PN administered in a hop extract would remain linear with dose as described by Rad, et al., for administration of 8-PN alone, then the AUC0-24 of a theoretical dose of 50 mg 8-PN in the hop extract would theoretically be 2520 h.ng/mL. This value is significantly higher than that of 453 h.ng/mL, which was reported by Rad, et al., [12] for a single oral dose of 50 mg 8-PN. Therefore, the hop extract must have provided an indirect source of 8-PN in vivo, which was probably through the metabolic conversion (O-demethylation) of IX to 8-PN. In vivo conversion of XN to IX and then 8-PN was also observed by Dietz, et al, [23], who detected 8-PN and 8-PN glucuronide in liver and mammary glands of rats after subcutaneous injection of XN only.
One out of the 5 subjects appeared to have higher exposure to 8-PN than the others at the medium and high doses. For example, the ratio of total serum concentrations (measured after enzymatic deconjugation) of 8-PN and IX (8-PN/IX) for this subject after receiving the high dose of the hop extract was 0.35 compared with a mean value of only 0.14 for the other 4 subjects. A high ratio of serum 8-PN/IX was also observed at the medium dose for this subject (0.65 compared with a mean value of 0.17) but not at the low dose. In terms of urinary excretion, the ratio of total 8-PN/IX ratio was ~3 fold higher in this subject at the medium and high dosages compared with the other 4 subjects. In agreement with the serum data, the difference was not pronounced in the low dose group which was in agreement with serum data.
These results are consistent with inter-individual differences in IX conversion to 8-PN, which was proposed previously by our group [9] to be expected due to polymorphic CYP1A2 metabolism of IX, and then demonstrated by Bolca, et al., [24] to be also a result of variable conversion by intestinal microbiota. As phenotyping of CYP1A2 was not carried out and gut microbiota species were not determined for this subject, the major contributing factor in this case is uncertain. Some variants of human CYP1A2 are expressed at just 30-60% and show activities of only 1-3% of wild type levels [25]. Moreover, CYP1A2 can be induced or inhibited by various environmental factors, such as prescription drugs [26], alcohol consumption and smoking [27]. Individual variations in the composition of gut microbiota also strongly affect the conversion of IX into 8-PN [10, 22]. In an intervention study of 50 healthy women receiving oral hop dietary supplements, ~15% of the subjects were determined to be strong producers of 8-PN based on urinary excretion and gut microbiota bioactivation capacity [24]. Therefore, enzymatic polymorphisms as well as environmental factors such as microbiota variations can directly impact individual rates of conversion of IX to 8-PN.
For 6-PN, the AUC0-24 ratios after normalization by dose were 1: 1.2: 0.5 (based on data in Table 2). The lack of dose linearity at the highest dosage resulted primarily from the AUC0-24 for a single subject. The AUC0-24 ratios of XN after normalization by dose were 1: 1.7: 1.2, and the AUC0-24 normalized to dose for IX was 1: 1.8: 1.2. Excluding a single subject from these calculations, the AUC0-24 ratio for IX, for example, becomes 1:1:1, which confirms dose linearity.
The apparent volumes of distribution, Vd/F, of XN were high, with values of 17300 L, 3770 L, and 7880 L for the low, medium and high doses of hops, respectively. For comparison, the Vd/F values for IX, 6-PN and 8-PN were much lower and ranged from 99 to 262 L for IX, 887 to 1770 L for 6-PN and 118 to 271 L for 8-PN. High Vd/F values for XN have also been reported in rats [14]. The high Vd/F values for XN indicate extensive tissue distribution and are consistent with the rapid uptake and concentration of XN by intestinal epithelial cells reported by Pang, et al., [20]. Binding to cytosolic proteins might explain a high concentration of XN in intestinal cells. The apparent clearance, CL/F, of XN was also high and ranged from 657 L/h at the low dose of 21.3 mg to 278 and 329 for the medium and high doses of 42.6 mg and 85.2 mg XN, respectively. These apparent clearance values are identical to those reported by Leggett, et al., [15] for the administration of single doses of 60 mg or 180 mg of purified XN to men and women.
By using MALDI mass spectrometric imaging, the tissue distributions of XN and XN-glucuronide were assessed previously by our group in rats [28]. Following daily dosing of 100 mg/kg XN to rats by oral gavage for 5 days, XN was found to be widely distributed but showed highest concentrations in the small intestine, colon and the liver. The primary metabolite of XN, XN-glucuronide, was found primarily along the epithelial layers of these tissues. Taking these results together, high Vd/F values for XN in the present pharmacokinetics study are probably due to high tissue distribution and binding to cytosolic proteins.
All of these prenylated phenols are known to be extensively conjugated with glucuronic acid and, to a smaller extent, with sulfate [14, 29]. Although XN has shown activity as a chemoprevention agent [4, 5] and 8-PN has been demonstrated to be a potent phytoestrogen [7, 8], the activities of the Phase 2 conjugates of these compounds have not yet been determined. In the present study, the Cmax of total 8-PN (derived primarily from 8-PN glucuronides, as no free 8-PN was detected in any serum specimens) was 6.7 ng/mL (19.7 nM) after oral administration of 1 mg of 8-PN. In an Ishikawa cell-based bioassay of estrogenicity, the EC50 value of 8-PN was determined to be 13 nM [30]. Therefore, the concentrations of conjugated 8-PN in human serum are comparable to the effective estrogenic levels of unconjugated 8-PN.
In the serum concentration-time curves (Figure 4), all 4 of the hop prenylated phenols showed a secondary peak at approximately 5 h, which was probably due to enterohepatic recirculation in which each compound is absorbed, conjugated through Phase 2 metabolism, excreted in the bile, deconjugated by gut microbiota, and then reabsorbed. Rad, et al., [12] reported a secondary peak at 5-8 h post dose due to enterohepatic recirculation of 8-PN in women, and Legette, et al., [15] reported a secondary peak at 4 h due to enterohepatic recirculation of XN in both men and women. Enterohepatic recirculation enhances exposure to the unconjugated as well as conjugated forms of each of the hop prenylated phenols, while prolonging their half-lives to over 20 h.
CONCLUDING REMARKS
Beginning with a botanically authenticated extract of spent hops that was chemically standardized to the 4 key estrogenic and proestrogenic prenylated phenols as well as biologically standardized [17] for estrogenicity to ensure reproducibility, the aim of this study was to investigate the pharmacokinetics of all four of these prenylated phenols following oral administration to menopausal women. Previous studies have addressed the administration of only one of these compounds at a time, and simultaneous standardization to more than one marker or active compound is also not commonly performed. Serum concentrations were dose-dependent with a relatively long interval to reach Cmax values. The predominant forms of all of these compounds in serum were glucuronides, which underwent enterohepatic recirculation that helped enhance the half-lives of all of the compounds to at least 20 h. The elevated levels of IX in serum and urine relative to the chalcone XN indicated in vivo cyclization of the chalcone to the flavonoid, IX, and the half-life and total amounts of the most estrogenic of the prenylated phenols, 8-PN, were enhanced in serum and urine by in vivo conversion of IX to 8-PN. This study confirms that short-term consumption of a chemically and biologically standardized preparation of spent hops is safe for women and that once daily dosing might be appropriate.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by NIH grant P50 AT00155 from the Office of Dietary Supplements and the National Center for Complementary and Alternative Medicine. The authors thank Shimadzu Scientific Instruments for providing the UHPLC-MS/MS instrumentation used during this study.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
References
- 1.Chadwick LR, Pauli GF, Farnsworth NR. The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties. Phytomedicine. 2006;13:119131. doi: 10.1016/j.phymed.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens JF, Page JE. Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry. 2004;65:1317–1330. doi: 10.1016/j.phytochem.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 3.Stevens JF, Ivancic M, Hsu V, Deinzer ML. Prenylflavonoids from Humulus lupulus. Phytochemistry. 1997;44:1575–1585. [Google Scholar]
- 4.Miranda CL, Stevens JF, Helmrich A, Henderson MC, Rodriguez RJ, et al. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem. Toxicol. 1999;37:271–285. doi: 10.1016/s0278-6915(99)00019-8. [DOI] [PubMed] [Google Scholar]
- 5.Dietz BM, Kang YH, Liu G, Eggler AL, Yao P, et al. Xanthohumol isolated from Humulus lupulus Inhibits menadione-induced DNA damage through induction of quinone reductase. Chem Res. Toxicol. 2005;18:1296–1305. doi: 10.1021/tx050058x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milligan SR, Kalita JC, Heyerick A, Rong H, De Cooman L, et al. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J. Clin. Endocrin. Metab. 1999;84:2249–2252. doi: 10.1210/jcem.84.6.5887. [DOI] [PubMed] [Google Scholar]
- 7.Overk CR, Guo J, Chadwick LR, Lantvit DD, Minassi A, et al. In vivo estrogenic comparisons of Trifolium pratense (red clover) Humulus lupulus (hops), and the pure compounds isoxanthohumol and 8-prenylnaringenin. Chem.-Biol. Interact. 2008;176:30–39. doi: 10.1016/j.cbi.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer O, Humpel M, Fritzemeier KH, Bohlmann R, Schleuning WD. 8-Prenyl naringenin is a potent ERalpha selective phytoestrogen present in hops and beer. J. Steroid Biochem. Mol. Biol. 2003;84:359–360. doi: 10.1016/s0960-0760(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 9.Guo J, Nikolic D, Chadwick LR, Pauli GF, van Breemen RB. Identification of human hepatic cytochrome P450 enzymes involved in the metabolism of 8-prenylnaringenin and isoxanthohumol from hops (Humulus lupulus L.). Drug Metab. Dispos. 2006;34:1152–1159. doi: 10.1124/dmd.105.008250. [DOI] [PubMed] [Google Scholar]
- 10.Possemiers S, Heyerick A, Robbens V, De Keukeleire D, Verstraete W. Activation of proestrogens from hops (Humulus lupulus L.) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J. Agric. Food Chem. 2005;53:6281–6288. doi: 10.1021/jf0509714. [DOI] [PubMed] [Google Scholar]
- 11.Nikolic D, Li Y, Chadwick LR, Pauli GF, van Breemen RB. Metabolism of xanthohumol and isoxanthohumol, prenylated flavonoids from hops (Humulus lupulus L.), by human liver microsomes. J. Mass Spectrom. 2005;40:289–299. doi: 10.1002/jms.753. [DOI] [PubMed] [Google Scholar]
- 12.Rad M, Humpel M, Schaefer O, Schoemaker RC, Schleuning WD, et al. Pharmacokinetics and systemic endocrine effects of the phyto-oestrogen 8-prenylnaringenin after single oral doses to postmenopausal women. Br. J. Clin. Pharmacol. 2006;62:288–296. doi: 10.1111/j.1365-2125.2006.02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolca S, Wyns C, Possemiers S, Depypere H, De Keukeleire D, et al. Cosupplementation of isoflavones, prenylflavonoids, and lignans alters human exposure to phytoestrogen-derived 17beta-estradiol equivalents. J. Nutr. 2009;139:2293–2300. doi: 10.3945/jn.109.113639. [DOI] [PubMed] [Google Scholar]
- 14.Legette L, Ma L, Reed RL, Miranda CL, Christensen JM, et al. Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Mol. Nutr. Food Res. 2012;56:466–74. doi: 10.1002/mnfr.201100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legette L, Karnpracha C, Reed RL, Choi J, Bobe G, et al. Human pharmacokinetics of xanthohumol, an antihyperglycemic flavonoid from hops. Mol. Nutr. Food Res. 2014;58:248–55. doi: 10.1002/mnfr.201300333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neve RA. Hops. Chapman and Hall; New York: 1991. [Google Scholar]
- 17.Krause E, Yuan Y, Hajirahimkhan A, Dong H, Dietz BM, et al. Biological and chemical standardization of a hop (Humulus lupulus) botanical dietary supplement. Biomed. Chromatogr. 2014 doi: 10.1002/bmc.3177. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan Y, Qiu X, Nikolić D, Dahl JH, van Breemen RB. Method development and validation for ultra-high-pressure LC/MS/MS determination of hop prenylflavonoids in human serum. J. AOAC Int. 2012;95:1744–1749. doi: 10.5740/jaoacint.11-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nookandeh A, Frank N, Steiner F, Ellinger R, Schneider B, et al. Xanthohumol metabolites in faeces of rats. Phytochemistry. 2004;65:561–570. doi: 10.1016/j.phytochem.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Pang Y, Nikolic D, Zhu D, Chadwick LR, Pauli GF, et al. Binding of the hop (Humulus lupulus L.) chalcone xanthohumol to cytosolic proteins in Caco-2 intestinal epithelial cells. Mol. Nutr. Food Res. 2007;51:872–879. doi: 10.1002/mnfr.200600252. [DOI] [PubMed] [Google Scholar]
- 21.Wolff H, Motyl M, Hellerbrand C, Heilmann J, Kraus B. Xanthohumol uptake and intracellular kinetics in hepatocytes, hepatic stellate cells and intestinal cells. J. Agric. Food Chem. 2011;59:12893–12901. doi: 10.1021/jf203689z. [DOI] [PubMed] [Google Scholar]
- 22.Possemiers S, Bolca S, Grootaert C, Heyerick A, Decroos K, et al. The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine. J. Nutr. 2006;136:1862–1867. doi: 10.1093/jn/136.7.1862. [DOI] [PubMed] [Google Scholar]
- 23.Dietz BM, Hagos GK, Eskra JN, Wijewickrama GT, Anderson JR, et al. Differential regulation of detoxification enzymes in hepatic and mammary tissue by hops (Humulus lupulus) in vitro and in vivo. Mol. Nutr. Food Res. 2013;57:1055–1066. doi: 10.1002/mnfr.201200534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolca S, Possemiers S, Maervoet V, Huybrechts I, Heyerick A, et al. Microbial and dietary factors associated with the 8-prenylnaringenin producer phenotype: a dietary intervention trial with fifty healthy post-menopausal Caucasian women. Br. J. Nutr. 2007;98:950–959. doi: 10.1017/S0007114507749243. [DOI] [PubMed] [Google Scholar]
- 25.Saito Y, Hanioka N, Maekawa K, Isobe T, Tsuneto Y, et al. Functional analysis of three CYP1A2 variants found in a Japanese population. Drug Metab. Dispos. 2005;33:1905–1910. doi: 10.1124/dmd.105.005819. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Ramamoorthy Y, Kilicarslan T, Nolte H, Tyndale RF, et al. Inhibition of cytochromes P450 by antifungal imidazole derivatives. Drug Metab. Dispos. 2002;30:314–318. doi: 10.1124/dmd.30.3.314. [DOI] [PubMed] [Google Scholar]
- 27.Zevin S, Benowitz N. Drug interactions with tobacco smoking: an update. Clin. Pharmacokin. 1999;36:425–438. doi: 10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]
- 28.Nikolic D, van Breemen RB. Analytical methods for quantitation of prenylated flavonoids from hops. Curr. Anal. Chem. 2013;9:71–85. [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolic D, Li Y, Chadwick LR, van Breemen RB. In vitro studies of intestinal permeability and hepatic and intestinal metabolism of 8-prenylnaringenin, a potent phytoestrogen from hops (Humulus lupulus L.). Pharm. Res. 2006;23:864–872. doi: 10.1007/s11095-006-9902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overk CR, Yao P, Chadwick LR, Nikolic D, Sun Y, et al. Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense). J. Agric. Food Chem. 2005;53:6246–53. doi: 10.1021/jf050448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.