Abstract
This review summarizes recent epidemiology of Gram-negative infections in selected countries from Latin American and Caribbean adult intensive care units (ICUs). A systematic search of the biomedical literature (PubMed) was performed to identify articles published over the last decade. Where appropriate, data also were collected from the reference list of published articles, health departments of specific countries, and registries. Independent cohort data from all countries (Argentina, Brazil, Chile, Colombia, Cuba, Mexico, Trinidad and Tobago, and Venezuela) signified a high rate of ICU infections (prevalence: Argentina, 24%; Brazil, 57%). Gram-negative pathogens, predominantly Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli, accounted for >50% of ICU infections, which were often complicated by the presence of multidrug-resistant strains and clonal outbreaks. Empirical use of antimicrobial agents was identified as a strong risk factor for resistance development and excessive mortality. Infection control strategies utilizing hygiene measures and antimicrobial stewardship programs reduced the rate of device-associated infections. To mitigate the poor health outcomes associated with infections by multidrug-resistant Gram-negative bacteria, urgent focus must be placed on infection control strategies and local surveillance programs.
1. Introduction
Recognition that critically ill patients receive greater medical attention and have better health outcomes when they are grouped together in one patient care center gave rise to the intensive care unit (ICU) in 1952 [1]. An unintended consequence of housing critically ill patients together in ICUs is increased risk for infection [2–5], the epidemiology of which has been studied more extensively in North America, Europe, and Australasia [2–10] than in Latin America [11].
Between 3% and 12% of hospitalized patients in developed countries acquire a health care-associated infection (HAI) [12]. Of all HAIs, at least one-quarter occur in ICUs [13, 14]. In the extended prevalence of infection in intensive care (EPIC II) study, infection was independently associated with an increased risk of hospital death; the ICU mortality rate of infected patients was more than twice that of noninfected patients (25% versus 11%, resp.; P < 0.001), as was the hospital mortality rate (33% versus 15%, resp.; P < 0.001) [5].
Gram-negative bacteria represent the most common nosocomial isolates, primarily Pseudomonas aeruginosa, Escherichia coli, Klebsiella spp., and Acinetobacter spp. [5]. Infection by Gram-negative pathogens is complicated by emergence and global spread of strains expressing numerous mechanisms for antimicrobial resistance. The probability of encountering such a pathogen is far higher in the ICU than in other patient care areas [15].
This review describes the epidemiology of Gram-negative infections in Latin American and Caribbean adult ICUs by country over the last 10 years.
2. Materials and Methods
A systematic search of the biomedical literature was conducted. MEDLINE (via PubMed) was searched, limited by the dates of June 6, 2002, to March 9, 2014, for articles using the following terms and Boolean logic: (“intensive care unit” OR “ICU” OR “hospital” OR “nosocomial”) AND (“Gram-negative infection” OR “Gram-negative pathogen” OR “bacilli”) AND (“Latin America” OR “South America” OR “Central America” OR “Mexico” OR “Guatemala” OR “Honduras” OR “Nicaragua” OR “Costa Rica” OR “El Salvador” OR “Belize” OR “Panama” OR “Colombia” OR “Venezuela” OR “Guyana” OR “Suriname” OR “French Guiana” OR “Brazil” OR “Ecuador” OR “Peru” OR “Bolivia” OR “Paraguay” OR “Uruguay” OR “Chile” OR “Argentina”). In addition, the same search strategy was conducted this time featuring 27 countries and overseas territories of the Caribbean. No delimiters were applied to the search strategy. The subject matter of all citations yielded from the search was screened by the authors for relevance. We were particularly interested in observational studies (retrospective and prospective) that reported information on the frequency, morbidity, and mortality of ICU infections by Gram-negative bacteria, the phenotypic and genotypic characteristics of these bacteria, and risk factors for acquiring such infections. Except for studies reporting on clonality, only those reporting information on >50 patients were summarized. In addition, we used data from http://www.provenra.com.ve/ which publishes clinical microbiologic findings specific to Venezuela.
3. Results
3.1. Literature Search Results
Twenty-five observational studies were identified and selected for review. The features and properties of these studies are summarized in the Supplementary Table (see Supplementary Material available online at http://dx.doi.org/10.1155/2014/480463). Most studies pertained to ICUs in Brazil (n = 11), followed by Argentina (n = 5), Colombia (n = 3), Chile (n = 2), Cuba (n = 1), Mexico (n = 1), Trinidad and Tobago (n = 1), and Venezuela (n = 1).
3.2. ICU Infection Frequency
Table 1 shows that a range of different study designs were utilized to estimate the epidemiology of ICU infections in Argentina and Brazil. The prevalence of HAIs in adult ICUs was 24% in a pooled analysis of 2 multicenter, observational, cross-sectional studies conducted within the framework of Argentina's National Surveillance of Hospital Infections Program in 2004 and 2005 [16]. The most frequently occurring infection was pneumonia (43%), followed by primary bloodstream infection (21%) and urinary tract infection (13%). The incidence of ventilator-associated pneumonia (VAP) microbiologically confirmed by significant growth (>104 colony forming units/mL in bronchoalveolar lavage culture) was 15% in a study performed in ICUs of 6 hospitals in the metropolitan area of Buenos Aires during 1999 to 2001 [17].
Table 1.
Infection rates in ICUs of Argentinian and Brazilian hospitals.
| Reference | Study design | Location | Year of study | Number of patients | Rate of infection |
|---|---|---|---|---|---|
| Lossa et al. 2008 [16] | Cross-sectional, multicenter | Argentina nationwide | 2004 and 2005 | 356 | (i) Pooled prevalence, 24% (95% CI, 20%–29%) (a) Pneumonia, 43% (b) BSI, 21% (c) UTI, 13% |
|
| |||||
| Luna et al. 2003 [17] | Prospective, multicenter | Buenos Aires, Argentina | 1999–2001 | 472 | (i) VAP incidence, 63/472 (13%) |
|
| |||||
| Toufen Junior et al. 2003 [18] | 1-day point prevalence | São Paulo, Brazil | 2000 | 126 | (i) Overall prevalence, 72/126 (57%) (a) CAI, 15/72 (21%) (b) Non-ICU nosocomial infection, 24/72 (33%) (c) ICU-acquired infection, 22/72 (31%) (d) Undefined, 11/72 (15%) |
|
| |||||
| de Queiroz Guimarães and Rocco 2006 [19] | Prospective observational | Rio de Janeiro, Brazil | 1999–2001 | 278 | (i) VAP prevalence, 38% (36 cases/1000 ventilator days) |
|
| |||||
| Lima et al. 2007 [20] | Prospective observational | São Paulo, Brazil | 2006 | 71 | (i) Prevalence, 47/71 (66%) |
|
| |||||
| da Rocha et al. 2008 [21] | Prospective observational | Uberlândia, Brazil | 2005-2006 | 275 | (i) VAP prevalence, 31% (25 cases/1000 ventilator days) |
|
| |||||
| Rodrigues et al. 2009 [22] | Prospective observational | Rio de Janeiro, Brazil | 2005–2007 | 233 | (i) VAP prevalence, 27% (17 cases/1000 ventilator days) |
|
| |||||
| de Oliveira et al. 2010 [23] | Prospective observational | Minas Gerais, Brazil | 2005–2008 | 2300 | (i) CAI, 437/2300 (19%) (a) 284 (12%) patients colonized by resistant microorganisms during ICU hospitalization, 61% of whom developed an infection (ii) Nosocomial infection, 311/2300 (14%) (a) 84/311 (27%) owing to resistant pathogens |
ICU: intensive care unit, CI: confidence interval, BSI: bloodstream infection, UTI: urinary tract infection, VAP: ventilator-acquired pneumonia, and CAI: community-acquired infection.
In Brazil, a study conducted in 19 ICUs at the Hospital das Clínicas, University of São Paulo, São Paulo, and which had a similar design to EPIC II [5] reported an overall infection rate of 57% [18]. This finding is comparable with the 60% point prevalence estimated in EPIC II for Central/South America [5]. Thirty-one percent of the Hospital das Clínicas infections were acquired in the ICU, yielding an ICU HAI point prevalence of 17% [18]. The ICU HAI rate was slightly lower in a recent (2005–2008) prospective observational study conducted in Minas Gerais (14%) [23]. In prospective observational studies, the prevalence of VAP in Brazil ranged from 27% to 66% [19–22].
3.3. Frequency of Gram-Negative Bacterial Infections
Figure 1 shows that Gram-negative bacteria were the predominant infectious agents in Latin American and Caribbean ICUs, although polymicrobial infections were not unusual [16, 17, 22, 33–36]. Acinetobacter spp., Klebsiella spp., and P. aeruginosa were the 3 most frequently encountered pathogens in isolates collected throughout the continent, and Acinetobacter spp. particularly so in VAP isolates [16]. Time-trend data pertaining to 741 mechanically ventilated patients with suspected nosocomial pneumonia were available between 2007 and 2010 for the 3 ICUs of the Hermanos Ameijeiras Hospital, Havana, Cuba [35]. The most prevalent bacterial infections were Acinetobacter spp. (26%), Pseudomonas spp. (18%), and Klebsiella spp. (9%), which represented a 1% decrease in the Pseudomonas spp. prevalence rate and a 4% and 11% increase in prevalence of Acinetobacter spp. and Klebsiella spp., respectively [35]. The distribution of infectious agents in the ICU of San Fernando, Trinidad, was different to that in ICUs of other countries in that Citrobacter spp. (21%) and Enterobacter spp. (17%) represented the second and third most frequently isolated pathogens after P. aeruginosa (35%). When these data were stratified by anatomical collection site, P. aeruginosa, K. pneumoniae, Citrobacter spp., and Enterobacter spp. were the predominant isolates from sputum, while from urine it was P. aeruginosa and K. pneumoniae and from blood it was Citrobacter spp.
Figure 1.
Frequency of Gram-negative bacteria (all n/N) among bacteriologically documented infections in (a) Latin American and Caribbean ICUs and (b) Brazilian ICUs specifically [16–22, 28, 33–36, 42, 66]. VAP: ventilator-associated pneumonia, CAUTI: catheter-associated urinary tract infection, CLABSI: central line-associated bloodstream infection, HAI: hospital-acquired infection, BSI: bloodstream infection, and ICU: intensive care unit.
3.4. Susceptibility Data
Variable but clinically significantly high rates of multidrug resistance were observed throughout Latin America. Of the small number of isolates collected by Argentina's National Surveillance of Hospital Infections Program in 2004 and 2005 that underwent phenotypic profiling, most strains of Acinetobacter spp. and Klebsiella spp. were resistant to ceftazidime, ciprofloxacin, amikacin, and gentamicin (Figure 2). Furthermore, their susceptibility to the carbapenems, meropenem and imipenem, was compromised [16]. Retrospective data collected from the ICUs of 3 hospitals in Buenos Aires demonstrated that of 61 episodes of VAP caused by Acinetobacter spp. or P. aeruginosa only 30 isolates were carbapenem susceptible and 31 were colistin-only susceptible [25].
Figure 2.
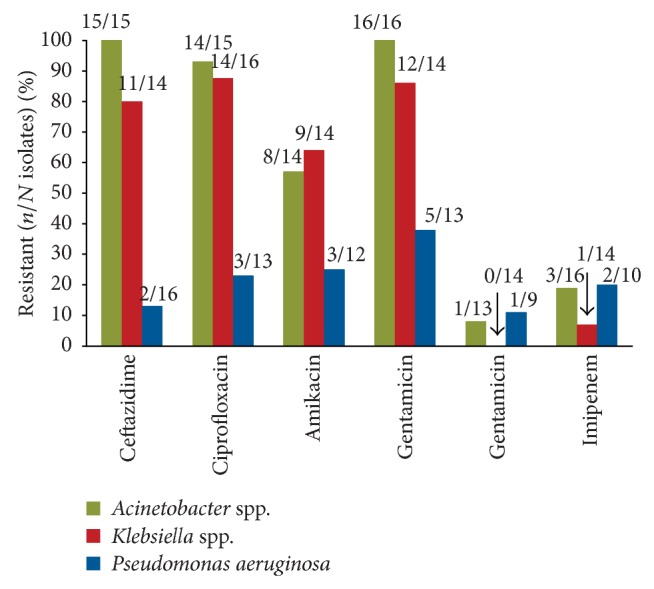
Resistant Gram-negative bacilli. Resistance profile of Gram-negative bacilli isolated from adult intensive care patients who participated in Argentina's National Surveillance of Hospital Infections Program in 2004 and 2005 [16].
In Brazil, findings from a 10-year prospective study conducted between 1999 and 2008 in a 716-bed (including 89 beds in ICUs) tertiary university hospital (São José do Rio Preto, SP, Brazil) showed that 6314 of 9416 multidrug-resistant Gram-negative bacteria isolated from hospitalized patients were from patients in the ICU (P < 0.001) [37]. In rank order, the 3 most common multidrug-resistant Gram-negative bacteria were A. baumannii, P. aeruginosa, and K. pneumoniae. A major finding from the study was the marked increase in prevalence of multidrug-resistant Gram-negative bacteria from study commencement (332 isolates in 1999) to termination (1221 isolates in 2008). The assertion probably holds true for the ICU given the disproportionate number of multidrug-resistant Gram-negative bacteria detected in this patient care area [37].
More detailed antimicrobial susceptibility data comes from the Brazilian Surveillance and Control of Pathogens of Epidemiological Importance (SCOPE) study, which collected 2447 isolates from patients with bloodstream infections (half of whom were in an ICU) [33]. Cephalosporins, aminoglycosides, fluoroquinolones, and carbapenems were not active against >50% of Acinetobacter spp. isolates tested, in which the β-lactamase OXA-23 (bla OXA-23) gene was detected in 85 of 112 (76%) isolates tested. More than one-third of the P. aeruginosa isolates were resistant to commonly used antimicrobial agents, including imipenem and meropenem. The bla IMP gene was detected in 6 (10%) isolates and the bla SPM gene was detected in 24 (41%) isolates out of 59 carbapenem-resistant P. aeruginosa isolates. Similarly, high proportions of Klebsiella spp. were resistant to ampicillin/sulbactam, piperacillin/tazobactam, ceftazidime, and cefepime (54%, 34%, 54%, and 50%, resp.). Resistance to imipenem and meropenem was observed in 0.3% and 1.3% of the isolates, respectively. Of the 94 extended spectrum β-lactamase- (ESBL-) positive isolates tested, bla TEM, bla CTX, and bla SHV genes were present in 84 (89%), 86 (91%), and 68 (72%) strains, respectively. Carbapenem resistance was associated with harboring of the bla KPC gene (K. pneumoniae carbapenemase (KPC)) [33].
Resistance rates among the 14 cultures positive for P. aeruginosa at the Hospital das Clínicas, São Paulo, Brazil, were 50% for both ceftazidime and gentamicin, 42% for both ciprofloxacin and amikacin, and 30% for imipenem [18]. Similarly, in 2 separate ICUs in Rio de Janeiro hospitals, multidrug-resistant organisms were identified in 43% of culture-positive patients with VAP in 1 ICU [19], while half of all A. baumannii strains collected from patients with VAP were resistant to carbapenems in the other [22].
In Chile, low levels of antimicrobial susceptibility were found for A. baumannii and Klebsiella pneumoniae isolates while P. aeruginosa susceptibility ranged from 48% (for ciprofloxacin) to 73% (for amikacin; Figure 3) [34]. In a separate Chilean study conducted in 2007, susceptibility data describing 454 A. baumannii ICU isolates collected by an independent network showed that the percentage of isolates susceptible to imipenem and meropenem was 62% and 57%, respectively; this was far lower than in other patient care areas (83% and 84%, resp.). Similarly, lower levels of P. aeruginosa susceptibility (N = 716 isolates) to both antimicrobials occurred in ICUs (54% and 58%, resp.) than in other patient-care areas (78% and 77%, resp.) [38].
Figure 3.

Gram-negative organisms (Chilean ICUs). The proportion of susceptible A. baumannii (n = 159), P. aeruginosa (n = 173), and K. pneumoniae (n = 135) isolates collected from all 31 hospitals [34]. CAUTI: catheter-associated urinary tract infection, VAP: ventilator-associated pneumonia, CLABSI: central line-associated bloodstream infection, and ICU: intensive care unit.
In Colombia, temporal WHONET 5.4 (World Health Organization Collaborating Centre for Surveillance of Antimicrobial Resistance, Boston, MA, USA) antimicrobial resistance data for Gram-negative bacilli isolated from 14 ICUs from 2006 until 2008 belonging to the Colombian Nosocomial Resistance Study Group was available [39]. Antimicrobial resistance frequencies of K. pneumoniae and E. cloacae to third-generation cephalosporins remained steady over the 3-year study period (range, 20–41%), while resistance of E. coli to third-generation cephalosporins showed a decreasing trend (ceftazidime, 6% to 2%; ceftriaxone, 8% to 7%; both P ≤ 0.001). Rodríguez et al. [40] reported simultaneous increased percentages of K. pneumoniae resistance toimipenem (1.3% to 4.0%), ciprofloxacin (10% to 14%), and cefotaxime (28% to 31%) during 2007 to 2009; increases also were noted in ceftazidime-resistant strains of E. coli (8% to 10%) and imipenem-resistant strains of A. baumannii (56% to 63%), with reduced percentages of ciprofloxacin-resistant E. coli (28% to 26%), ceftazidime-resistant P. aeruginosa (31% to 24%), and ciprofloxacin-resistant P. aeruginosa (28% to 24%; P < 0.01).
Of 155 bacterial strains isolated from 119 nosocomial infections in Mexico, 89 were nonfermenting Gram-negative rods resistant to all commonly used drug classes except ticarcillin/clavulanic acid (45%) and imipenem (16%) [24]. The 43 Enterobacteriaceae were slightly more susceptible, with 52% of isolates resistant to cefotaxime, 21% to cefpirome, 47% to aztreonam, 40% to amikacin, 19% to ciprofloxacin, and 2% to imipenem [24].
In Venezuela, blood cultures of patients in the ICU during 2011 indicated that P. aeruginosa (18%), A. baumannii (16%), K. pneumoniae (13%), and E. coli (11%) were the most common Gram-negative organisms isolated (Figure 1) [36]. High levels of resistance of P. aeruginosa and K. pneumoniae to some antimicrobialshave been documented (Figure 4) [36]. All isolates of A. baumannii remained susceptible to colistin while most P. aeruginosa isolates were susceptible to cefepime (80%). Resistance to the aminoglycosides, amikacin and gentamicin, and ciprofloxacin was high among A. baumannii and P. aeruginosa [36].
Figure 4.

Gram-negative organisms (Venezuelan intensive care units). Susceptibility of Gram-negative bacilli isolated from adult intensive care patients who participated in the Venezuelan Surveillance Program on Antimicrobial Resistance [16].
Susceptibility data were available for two Caribbean countries [41, 42]. In Cuba, Acinetobacter spp. showed high levels of resistance to commonly used antimicrobial drug classes; 2% of isolates were resistant to colistin. Pseudomonas spp. and Klebsiella spp. were highly resistant to ampicillin/sulbactam and had moderate or low resistance to other agents [41]. Data from the same hospital revealed that tigecycline and colistin were the only antibiotics fully effective against A. baumannii strains isolated in 2011 patients with VAP; only colistin was fully effective against P. aeruginosa strains [41]. In Trinidad by contrast, isolates were relatively antimicrobial susceptible although these data are now >10 years old [42]. Of the 10 antimicrobial agents tested, the highest rate of resistance was for ampicillin (88%) and the lowest rates were for imipenem (6%), ciprofloxacin (6%), and piperacillin-tazobactam (12%). The most common isolate P. aeruginosa was susceptible to >82% of the antimicrobials [42].
3.5. Mortality and Risk Factors
Inappropriate and tardy antimicrobial therapies have been associated with resistance and, to a lesser extent, higher mortality in patients in Latin American ICUs [17, 24–28, 43]. Table 2 also shows that a previous ICU stay and a previous episode of VAP predispose to infections by multidrug-resistant Gram-negative bacteria [25–28].
Table 2.
Multivariate analysis of risk factors for crude mortality and acquisition of drug-resistant Gram-negative infections in Latin American ICUs.
| Outcome | Risk factor | OR (95% CI) | P value | Reference |
|---|---|---|---|---|
| Crude mortality | Inadequate antibiotic treatment | 70.5 | <0.00001 | Zaidi et al. 2002 [24] |
| Development of VAP | 7.7 | 0.004 | ||
|
| ||||
| Colistin-susceptible VAP | Overall ICU stay, 40 days | 31.6 (31.5–495.9) | 0.014 | |
| Duration of prior antimicrobial therapy >10 days | 13.2 (2.2–78.7) | 0.005 | Rios et al. 2007 [25] | |
| Previous episode of VAP | 6.0 (1.0–35.7) | 0.047 | ||
|
| ||||
| Imipenem-resistant P. aeruginosa | Previous ICU stay | 3.54 (1.3–9.7) | 0.03 | Furtado et al. 2009 [26] |
|
| ||||
| HAP by imipenem-resistant P. aeruginosa | Piperacillin/tazobactam | 14.31 (1.0–200.2) | 0.04 | Furtado et al. 2010 [27] |
| Third-generation cephalosporin | 7.45 (1.8–30.9) | 0.006 | ||
|
| ||||
| Antimicrobial resistance to Acinetobacter spp. and P. aeruginosa | Prior exposure to antimicrobial agents | NR | <0.05 | Weyland et al. 2011 [28] |
ICU: intensive care unit, OR: odds ratio, CI: confidence interval, VAP: ventilator-associated pneumonia, HAP: hospital-acquired pneumonia, and NR: not reported.
In Argentina, the VAP mortality rate due to any pathogen was higher among 46 patients who had received prior antibiotics than among 17 patients who had not received antibiotics (59% versus 29%, P = 0.075) [17]. Prior antimicrobial treatment in this study was defined as current use of antimicrobials or previous use of antimicrobials for >24 hours during the 10 days before the diagnosis of VAP [17]. Findings of a later study by the same investigators showed that the overall mortality rate in VAP was 29% in the 24 patients who received therapy that provided coverage against all isolated pathogens at VAP onset (i.e., appropriate therapy) compared with a rate of 64% in the 52 patients who received either inadequate therapy or delayed initiation of appropriate therapy (P = 0.007) [43]. In another Argentinian study, prior antimicrobial therapy for >10 days and a previous episode of VAP remained significantly associated with colistin-only susceptible VAP; 41% of VAP by colistin-only susceptible strains, but none of those by carbapenem-susceptible strains had received prior carbapenem therapy [25].
In Mexico, a multivariate analysis of prospective, nested, case-control data collected from 4 ICUs in Mexico from 1995 to 1996 indicated that inadequate antibiotic treatment and development of VAP were major risk factors for the 59% crude mortality rate (Table 2) [24]. The term inadequate antibiotic treatment was not limited to initial empirical antibiotic therapy but included failure to administer proper antibiotics according to in vitro susceptibility results [24]. Although antibiotic resistance in Gram-negative rods was not an independent risk factor for mortality in this study, there was a strong association between antibiotic resistance and inadequate treatment [24].
The phenotype of A. baumannii was a risk factor for death on day 30 of hospitalization in a Colombian study, in which HAIs by multidrug-resistant strains were associated with significantly greater 30-day mortality than drug-sensitive strains (42% versus 9%, resp.; P = 0.0074) [44]. This difference was maintained when the patients' risk factors were evaluated by multivariate analysis.
Mortality associated with Gram-negative infection approximated 33% in San Fernando, Trinidad, with all fatalities occurring in patients with pneumonia and bloodstream infections [42]. No formal analysis was conducted in this study to detect any association between mortality and antimicrobial resistance rates; however, resistance to the most commonly used antimicrobials was high and correlated with consumption [42].
3.6. Clonal Outbreaks
A review of the molecular epidemiology of clones in the Latin American hospital setting has been reviewed elsewhere [45, 46]. Our literature findings indicated that outbreaks of infections within ICUs are often owing to a small number of clones (Table 3). A. baumannii pulsed-field gel electrophoresis clone I has been widespread in several Buenos Aires hospitals since 1981, and carbapenem-resistant pulsed-field gel electrophoresis clone IV became prevalent in the 1990s [47]. Multidrug-resistant epidemic and endemic clones of Acinetobacter spp. and P. aeruginosa emerged in Brazilian ICUs during the late 1990s [48, 49]. In the Meropenem Yearly Susceptibility Test Information Collection (MYSTIC) program conducted in 5 ICUs in São Paulo and Brasilia during 2002, 36 multidrug-resistant P. aeruginosa isolates were clustered into 5 genotypes [29]. Two carbapenem-resistant P. aeruginosa outbreaks were reported in the ICUs of Brazilian teaching hospitals; 1 in 2001 (Hospital Universitário São Francisco, São Paulo, Brazil) and the other between 2003 and 2005 (Minas Gerais, Brazil) [30, 31]. As detected in the general hospital setting [45, 46], a nosocomial outbreak of KPC-producing K. pneumoniae featured a Buenos Aires ICU with a high capacity for dissemination and a high mortality rate [32].
Table 3.
Molecular epidemiology of Gram-negative clones in intensive care units of Latin America.
| Reference | Location, year | Clonal type | Antimicrobial susceptibility | Mechanism of antimicrobial resistance | Notes |
|---|---|---|---|---|---|
| Figueiredo-Mendes et al. 2005 [29] | São Paulo, Brazil (4 centers) and Brasília, Brazil (1 center), 2002 | 36 P. aeruginosa isolates (clones A, B, C, D, and G) | Multidrug resistant; carbapenem MIC, ≥32 µg/mL | MBL production (except for clone D1 and D2) | Interhospital spread probably owing to transfers of infected patients, share of health care workers, and exchange of medical equipment among institutions |
|
| |||||
| Gales et al. 2004 [30] | Hospital Universitário São Francisco, São Paulo, Brazil, 2001 | 5 carbapenem-resistant P. aeruginosa strains from 4 patients (clone B and C1) | Clone B susceptible to all classes except carbapenems; clone C1 susceptible to polymyxin B only | NR | Dissemination may have been owing to cross-contamination. Clone C1 had a similar PGFE pattern to clone C2 (previously isolated from Hospital São Paulo, São Paulo, Brazil) |
|
| |||||
| Cezário et al. 2009 [31] | University Hospital in Minas Gerais, Brazil, 2003–2005 | 36 multidrug P. aeruginosa isolates (clones A, B, C, and D) | Multidrug resistant | MBL-producing strains were positive for bla SPM-1 | Strong temporal/spatial relationship indicated cross-contamination |
|
| |||||
| Córdova et al. 2012 [32] | Hospital Dr. Cosme Argerich, Buenos Aires, Argentina, 2009-2010 | 6 patients infected with KPC-producing K. pneumoniae ST258 | Susceptible to tigecycline and colistin only | KPC | Attributable mortality, 26%; ST258 had a high capacity for dissemination |
MIC: minimum inhibitory concentration, MBL: metallo-β-lactamase, NR: not reported, PGFE: pulsed gel field electrophoresis, bla SPM-1: β-lactamase SPM-1 gene, and KPC: K. pneumoniae carbapenemase.
4. Discussion
Based on the published information garnered, which was heterogeneous with respect to study collection dates and methodology, the overall prevalence rate of infections in Brazilian ICUs ranged from 31% to 66%, with pneumonia/VAP prevalence tending to be the most frequent type of infection. Data from Argentinian and Brazilian ICUs indicated that the prevalence of infections acquired in ICUs was 17% to 24%. Infections in ICUs throughout the Latin American and Caribbean countries evaluated reflect a preponderance of Gram-negative pathogens and the dissemination of multidrug-resistant strains, including carbapenem-resistant strains of Acinetobacter spp., P. aeruginosa, and K. pneumoniae. Genes encoding ESBLs, KPC, and metallo-β-lactamase among some of these organisms can be considered a major public health problem. Risk factors for Gram-negative infections were similar to those found in other countries and included previous or inappropriate empiric antimicrobial therapy. In three ICUs, inadequate antibiotic treatment of Gram-negative infections was a risk factor for mortality. It should be noted, however, that the only information available on inadequate antimicrobial treatment is related to previous therapy, current empirical therapy, or a current change to empirical therapy. No information was available regarding the effect of deescalating antimicrobial therapy on resistance and mortality, which would have better characterized the associations. Shortening the duration of antimicrobial therapy may be possible for those VAP patients exhibiting a good outcome based on serial measurements of clinical pulmonary infection score [17], and deescalation is now recognized as one component of an optimal care strategy in patients with sepsis [50].
Mortality stemming from device-associated Gram-negative infections in Latin American and Caribbean ICUs underscores the need for the development, implementation, and reinforcement of infection control strategies. We recommend use of a sequential checklist to remind health care personnel of simple measures to reduce the opportunity for nosocomial infection. Use of such a checklist was associated with a sustained reduction in central line-associated bloodstream infections over an initial 18-month period [51], and the benefits persisted at 36 months after implementation [52]. Similar checklists in the form of the so-called “prevention bundles” in the United States have aided the prevention of ICU VAP [52, 53] and led to the development of a unique checklist for the prevention of surgical infections. The World Health Organization has adopted this safe surgery checklist and has recommended its application worldwide [54, 55].
Limitations of results include lack of data available for most countries in Latin America, as well as bias in data reported from different centers or networks. For instance, most data were obtained from a few tertiary centers in each country, which likely report elevated resistance rate relative to their national averages. Methodology of data reporting, collection, and analysis also may differ among laboratories, countries, and surveillance networks.
The high rates and continent-wide dissemination of multidrug-resistant Gram-negative bacteria means that empiric prescribing of antimicrobials (without cultures) cannot be recommended. Furthermore, findings from our literature review showed a clear and direct association between antimicrobial use and resistance development in Argentina, Brazil, Cuba, Mexico, and Trinidad. Thus, diagnostic and treatment decisions should be made based on the local susceptibility patterns within each institution, framed within regional, national, and global surveillance programs. Phenotypic methods are useful in identifying bacterial isolates at the genus and species level and provide the clinician with an antimicrobial resistance profile to guide therapy [56]. More discriminating phenotypic techniques such as 3-dimensional testing are required, given the exponential increase in Gram-negative bacteria harboring ESBLs, KPCs, and metallo-β-lactamase. It is important to note that clinical microbiologic laboratories routinely fail to identify pathogens harboring multiple β-lactamases, inducible AmpC-encoded β-lactamases, and KPCs [57–59].
In practice, critically ill patients receiving appropriate antimicrobial therapy (i.e., empirical therapy modified due to clinical response or pathogen identification) have better health outcomes than those receiving inappropriate therapy (i.e., unmodified empirical treatment) [60]. Similarly, Luna et al. [61] studied 283 patients that were mechanically ventilated for ≥48 hours to determine if biweekly routine endotracheal aspirate (ETA) cultures were more effective for managing VAP than the American Thoracic Society/Infectious Diseases Society of America guidelines [62]. Unless the sample was available ≤2 days of the onset of VAP, the guidelines-based approach was more accurate than the ETA-based approach for prescribing initial empiric antibiotics. Fewer days of antimicrobial therapy resulted when ETA cultures were considered.
Finally, we recommend use of antimicrobial stewardship programs with instructions on limiting inappropriate antimicrobial use, administering the correct dose via the correct route of administration, and the optimum duration of therapy [63]. We also encourage antimicrobial recycling and restricting certain antimicrobial agents for specific indications [64]. When properly implemented, the programs result in significant changes in the prevalence of bacterial resistance with attendant reductions in morbidity, mortality, and costs [63–65]. Despite the financial and logistic difficulties associated with implementing the components of antimicrobial stewardship programs, we recommend the pursuit of guidelines for antimicrobial use and formulary restrictions with appropriate review and feedback clauses in Latin America and the Caribbean [64].
5. Conclusion
There are high infection rates by Gram-negative bacteria in Latin American and Caribbean ICUs aggravated by spread of multidrug-resistant bacterial strains and clonal outbreaks. High rates of morbidity and mortality will prevail unless modifiable risk factors are better controlled.
Supplementary Material
The features and properties of the 25 observational studies conducted in Latin American and Caribbean ICUs that were identified and selected for our review are included in a supplementary table. Most studies pertained to ICUs in Brazil (n = 11), followed by Argentina (n = 5), Colombia (n = 3), Chile (n = 2), Cuba (n = 1), Mexico (n = 1), Trinidad and Tobago (n = 1), and Venezuela (n = 1).
Acknowledgments
C. M. Luna, E. Rodríguez-Noriega, L. Bavestrello, and M. Guzmán-Blanco conceived the study, identified reference sources, and helped draft and review all stages of the paper. All members of the Latin America Working Group on Bacterial Resistance reviewed the paper. Medical writing support was provided by Malcolm Darkes and Charlotte Kenreigh of Engage Scientific Solutions and funded by Pfizer Inc. Latin America Working Group on Bacterial Resistance members are Carlos Alvarez (Hospital Universitario San Ignacio and Pontificia Universidad Javeriana, Bogotá, Colombia); Luis Bavestrello (Clinica Reñaca, Viña Del Mar, Chile); Eitan Berezin (Santa Casa de São Paulo School of Medicine, São Paulo, Brazil); Eduardo Gotuzzo (Universidad Peruana Cayetano Heredia, Lima, Perú); Manuel Guzmán-Blanco (Hospital Privado Centro Médico de Caracas, y Hospital Vargas de Caracas, Caracas, Venezuela); Jaime A. Labarca (Pontificia Universidad Católica de Chile, Santiago, Chile); Carlos M. Luna (Hospital de Clínicas José de San Martin Hospital, Universidad de Buenos Aires, Buenos Aires, Argentina); Carlos Mejía (Hospital Roosevelt, Guatemala City, Guatemala); Simone Nouer (Hospital Universitario Clementino Fraga Filho, Rio de Janeiro, Brazil); Eduardo Rodríguez-Noriega (Hospital Civil de Guadalajara Fray Antonio Alcalde, Guadalajara, Mexico); Mauro José Costa Salles (Hospital Irmandade da Santa Casa de Misericórdia de São Paulo, São Paulo, Brazil); Carlos Seas (Universidad Peruana Cayetano Heredia, Lima, Perú); Fortino Solórzano Santos (Hospital de Pediatría Centro Médico Nacional Siglo XXI, Mexico City, Mexico); Maria Virginia Villegas (International Center for Medical Research and Training (CIDEIM), Cali, Colombia); Jeannete Zurita (Hospital Vozandes and Pontificia Universidad Católica del Ecuador, Quito, Ecuador).
Conflict of Interests
C. M. Luna has received an honorarium for giving talks for Merck Sharp and Dohme and as a member of an advisory board for AstraZeneca, Cerexa, and Pfizer Inc. E. Rodríguez-Noriega is a member of an advisory board for Pfizer Inc., has served as a consultant for Johnson & Johnson, Novartis, Pfizer Inc., and Wyeth, and has received research grants from Cerexa, Johnson & Johnson, Pfizer Inc., Schering-Plough, and Wyeth. L. Bavestrello is a member of an advisory board for Pfizer Inc. M. Guzmán-Blanco is a member of an advisory board for Merck and Pfizer Inc., respectively, and has received research grants from GlaxoSmithKline, Merck, and Pfizer Inc.
References
- 1.Ibsen B. The anaesthetist's viewpoint on the treatment of respiratory complications in poliomyelitis during the epidemic in Copenhagen, 1952. Proceedings of the Royal Society of Medicine. 1954;47(1):72–74. [PMC free article] [PubMed] [Google Scholar]
- 2.Brun-Buisson C., Doyon F., Carlet J., Dellamonica P., Gouin F., Lepoutre A., Mercier J.-C., Offenstadt G., Regnier B. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults: a multicenter prospective study in intensive care units. Journal of the American Medical Association. 1995;274(12):968–974. doi: 10.1001/jama.1995.03530120060042. [DOI] [PubMed] [Google Scholar]
- 3.Jarvis W. R., Edwards J. R., Culver D. H., et al. Nosocomial infection rates in adult and pediatric intensive care units in the United States. The American Journal of Medicine. 1991;91(3, supplement 2):S185–S191. doi: 10.1016/0002-9343(91)90367-7. [DOI] [PubMed] [Google Scholar]
- 4.Richards M. J., Edwards J. R., Culver D. H., Gaynes R. P. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infection Control and Hospital Epidemiology. 2000;21(8):510–515. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 5.Vincent J. L., Rello J., Marshall J., Silva E., Anzueto A., Martin C. D., Moreno R., Lipman J., Gomersall C., Sakr Y., Reinhart K. International study of the prevalence and outcomes of infection in intensive care units. Journal of the American Medical Association. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 6.Brun-Buisson C., Meshaka P., Pinton P., Vallet B. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Medicine. 2004;30(4):580–588. doi: 10.1007/s00134-003-2121-4. [DOI] [PubMed] [Google Scholar]
- 7.Finfer S., Bellomo R., Lipman J., et al. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Medicine. 2004;30(4):589–596. doi: 10.1007/s00134-004-2157-0. [DOI] [PubMed] [Google Scholar]
- 8.Kohlenberg A., Schwab F., Geffers C., Behnke M., Rüden H., Gastmeier P. Time-trends for Gram-negative and multidrug-resistant Gram-positive bacteria associated with nosocomial infections in German intensive care units between 2000 and 2005. Clinical Microbiology and Infection. 2008;14(1):93–96. doi: 10.1111/j.1469-0691.2007.01879.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin G. S., Mannino D. M., Eaton S., Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. The New England Journal of Medicine. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 10.Padkin A., Goldfrad C., Brady A. R., Young D., Black N., Rowan K. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Critical Care Medicine. 2003;31(9):2332–2338. doi: 10.1097/01.CCM.0000085141.75513.2B. [DOI] [PubMed] [Google Scholar]
- 11.Silva E., Pedro M. A., Sogayar A. C. B., et al. Brazilian sepsis epidemiological study (BASES study) Critical Care. 2004;8(4):R251–R260. doi: 10.1186/cc2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Report on the Burden of Endemic Health Care-Associated Infection Worldwide. World Health Organization; 2011. http://whqlibdoc.who.int/publications/2011/9789241501507_eng.pdf. [Google Scholar]
- 13.Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Hidron A. I., Edwards J. R., Patel J., Horan T. C., Sievert D. M., Pollock D. A., Fridkin S. K. Antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infection Control and Hospital Epidemiology. 2008;29(11):996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 15.Tognim M. C. B., Andrade S. S., Silbert S., Gales A. C., Jones R. N., Sader H. S. Resistance trends of Acinetobacter spp. in Latin America and characterization of international dissemination of multi-drug resistant strains: five-year report of the SENTRY Antimicrobial Surveillance Program. International Journal of Infectious Diseases. 2004;8(5):284–291. doi: 10.1016/j.ijid.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Lossa G. R., Lerena R. G., Fernández L. E., Vairetti J., Díaz C., Arcidiácono D., Peralta N. Prevalence of hospital infections in adult intensive care units in Argentina. Revista Panamericana de Salud Publica. 2008;24(5):324–330. doi: 10.1590/s1020-49892008001100004. [DOI] [PubMed] [Google Scholar]
- 17.Luna C. M., Blanzaco D., Niederman M. S., et al. Resolution of ventilator-associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Critical Care Medicine. 2003;31(3):676–682. doi: 10.1097/01.CCM.0000055380.86458.1E. [DOI] [PubMed] [Google Scholar]
- 18.Toufen Junior C., Hovnanian A. L. D., Franca S. A., Carvalho C. R. R. Prevalence rates of infection in intensive care units of a tertiary teaching hospital. Revista do Hospital das Clínicas. 2003;58(5):254–259. doi: 10.1590/s0041-87812003000500004. [DOI] [PubMed] [Google Scholar]
- 19.de Queiroz Guimarães M., Rocco J. R. Prevalence of ventilator-associated pneumonia in a university hospital and prognosis for the patients affected. Jornal Brasileiro de Pneumologia. 2006;32(4):339–346. [PubMed] [Google Scholar]
- 20.Lima M. E., de Andrade D., Haas V. J. Prospective assessment of the occurrence of infection in critical patients in an intensive care unit. Revista Brasileira de Terapia Intensiva. 2007;19:342–347. [PubMed] [Google Scholar]
- 21.da Rocha L. D. A., Vilela C. A. P., Cezário R. C., Almeida A. B., Gontijo Filho P. Ventilator-associated pneumonia in an adult clinical-surgical intensive care unit of a Brazilian University Hospital: incidence, risk factors, etiology, and antibiotic resistance. Brazilian Journal of Infectious Diseases. 2008;12(1):80–85. doi: 10.1590/s1413-86702008000100017. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues P. M. D. A., do Carmo Neto E., Santos L. R. D. C., Knibel M. F. Ventilator-associated pneumonia: epidemiology and impact on the clinical evolution of ICU patients. Jornal Brasileiro de Pneumologia. 2009;35(11):1084–1091. doi: 10.1590/S1806-37132009001100005. [DOI] [PubMed] [Google Scholar]
- 23.de Oliveira A. C., Silva R. S., Díaz M. E. P., Iquiapaza R. A. Bacterial resistance and mortality in an intensive care unit. Revista Latino-Americana de Enfermagem. 2010;18(6):1152–1160. doi: 10.1590/S0104-11692010000600016. [DOI] [PubMed] [Google Scholar]
- 24.Zaidi M., Sifuentes-Osornio J., Rolón A. L., Vázquez G., Rosado R., Sánchez M., Calva J. J., De León-Rosales S. P. Inadequate therapy and antibiotic resistance. Risk factors for mortality in the intensive care unit. Archives of Medical Research. 2002;33(3):290–294. doi: 10.1016/S0188-4409(01)00380-0. [DOI] [PubMed] [Google Scholar]
- 25.Rios F. G., Luna C. M., Maskin B., Saenz Valiente A., Lloria M., Gando S., Sosa C., Baquero S., Llerena C., Petrati C., Apezteguia C. Ventilator-associated pneumonia due to colistin susceptible-only microorganisms. European Respiratory Journal. 2007;30(2):307–313. doi: 10.1183/09031936.00156906. [DOI] [PubMed] [Google Scholar]
- 26.Furtado G. H. C., Bergamasco M. D., Menezes F. G., Marques D., Silva A., Perdiz L. B., Wey S. B., Medeiros E. A. S. Imipenem-resistant Pseudomonas aeruginosa infection at a medical-surgical intensive care unit: Risk factors and mortality. Journal of Critical Care. 2009;24(4):625.e9–625.e14. doi: 10.1016/j.jcrc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Furtado G. H., Gales A. C., Perdiz L. B., Santos A. F., Wey S. B., Medeiros E. A. Risk factors for hospital-acquired pneumonia caused by imipenem-resistant Pseudomonas aeruginosa in an intensive care unit. Anaesthesia and Intensive Care. 2010;38(6):994–1001. doi: 10.1177/0310057X1003800605. [DOI] [PubMed] [Google Scholar]
- 28.Weyland B., Perazzi B., Garcia S., Rodríguez C., Vay C., Famiglietti A. Bacterial etiology of nosocomial pneumonia and antimicrobial resistance in patients with and without antimicrobial treatment. Revista Argentina de Microbiologia. 2011;43(1):18–23. doi: 10.1590/S0325-75412011000100004. [DOI] [PubMed] [Google Scholar]
- 29.Figueiredo-Mendes C. M., Sinto S., Mello-Sampaio J. L., et al. Pseudomonas aeruginosa clonal dissemination in Brazilian intensive care units. Enfermedades Infecciosas y Microbiología Clínica. 2005;23(7):402–405. doi: 10.1157/13078798. [DOI] [PubMed] [Google Scholar]
- 30.Gales A. C., Torres P. L., Vilarinho D. S. O., Melo R. S., Silva C. F. L., Cereda R. F. Carbapenem-resistant Pseudomonas aeruginosa outbreak in an intensive care unit of a teaching hospital. The Brazilian Journal of Infectious Diseases. 2004;8(4):267–271. doi: 10.1590/s1413-86702004000400001. [DOI] [PubMed] [Google Scholar]
- 31.Cezário R. C., Duarte De Morais L., Ferreira J. C., Costa-Pinto R. M., Da Costa Darini A. L., Gontijo-Filho P. P. Nosocomial outbreak by imipenem-resistant metallo-β-lactamase-producing pseudomonas aeruginosa in an adult intensive care unit in a Brazilian teaching hospital. Enfermedades Infecciosas y Microbiologia Clinica. 2009;27(5):269–274. doi: 10.1016/j.eimc.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Córdova E., Lespada M. I., Gómez N., Pasterán F., Oviedo V., Rodríguez-Ismael C. Clinical and epidemiological study of an outbreak of KPC-producing Klebsiella pneumoniae infection in Buenos Aires, Argentina. Enfermedades Infecciosas y Microbiología Clínica. 2012;30(7):376–379. doi: 10.1016/j.eimc.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Marra A. R., Camargo L. F. A., Pignatari A. C. C., Sukiennik T., Behar P. R. P., Medeiros E. A. S., Ribeiro J., Girão E., Correa L., Guerra C., Brites C., Pereira C. A. P., Carneiro I., Reis M., De Souza M. A., Tranchesi R., Barata C. U., Edmond M. B., Andrade S., Machado A. M., Bispo P., Wey S. B., Colombo A. L., Martino M. D. V., Molina R., Puga L. S., Dantas G. M., Carlesse F., Moura A. Nosocomial bloodstream infections in Brazilian hospitals: analysis of 2,563 cases from a prospective nationwide surveillance study. Journal of Clinical Microbiology. 2011;49(5):1866–1871. doi: 10.1128/JCM.00376-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bustamante R. R., Solar V. E. Informe de vigilancia epidemiológica de infecciones intrahospitalarias. Ministry of Health (Chile); 2007. http://web.minsal.cl/portal/url/item/73627aa2edca0374e04001011f01734d.pdf. [Google Scholar]
- 35.Medell M., Martínez A., Valdés R. Characterization and sensitivity to antibiotics of bacteria isolated from the lower respiratory tract of ventilated patients hospitalized in intensive care units. Brazilian Journal of Infectious Diseases. 2012;16(1):45–51. doi: 10.1016/S1413-8670(12)70273-8. [DOI] [PubMed] [Google Scholar]
- 36.Venezuela P. Programa venezolano de vigilancia de la resistencia bacteriana a los antimicrobianos. 2013, http://www.provenra.org/
- 37.Rubio F. G., Oliveira V. D. C., Rangel R. M. C., Nogueira M. C. L., Almeida M. T. G. Trends in bacterial resistance in a tertiary university hospital over one decade. Brazilian Journal of Infectious Diseases. 2013;17(4):480–482. doi: 10.1016/j.bjid.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva F. O., Cifuentes M., Pinto E. C. Results of antimicrobial susceptibility surveillance in Chile: consolidating a network. Revista Chilena de Infectología. 2011;28(1):19–27. [PubMed] [Google Scholar]
- 39.Briceño D. F., Correa A., Valencia C., et al. Antimicrobial resistance of Gram negative bacilli isolated from terciary-care hospitals in Colombia. Biomedica. 2010;30(3):371–381. doi: 10.7705/biomedica.v30i3.271. [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez A. P. V., Ortega M. H. D., Garzón L. I. B., Vargas S. M. R., Iguarán D. E. H., Botero M. V. V., Restrepo C. G. R., Castro A. L. L. Trends of bacterial resistance phenotypes in high-complexity public and private hospitals in Colombia. Revista Panamericana de Salud Publica. 2011;30(6):627–633. doi: 10.1590/S1020-49892011001200022. [DOI] [PubMed] [Google Scholar]
- 41.Medell M., Hart M., Marrero O., Espinosa F., Montes de Oca Z., Valdés R. Clinical and microbiological characterization of pneumonia in mechanically ventilated patients. Brazilian Journal of Infectious Diseases. 2012;16(5):442–447. doi: 10.1016/j.bjid.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Orrett F. A. Resistance patterns among selective Gram-negative bacilli from an intensive care unit in Trinidad, West Indies. Saudi Medical Journal. 2004;25(4):478–483. [PubMed] [Google Scholar]
- 43.Luna C. M., Aruj P., Niederman M. S., Garzón J., Violi D., Prignoni A., Ríos F., Baquero S., Ganado S., Apezteguía C., Desmery P., Matarucco W., Pálizas F., Menga G. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. European Respiratory Journal. 2006;27(1):158–164. doi: 10.1183/09031936.06.00049105. [DOI] [PubMed] [Google Scholar]
- 44.Lemos E. V., De La Hoz Restrepo F., Alvis N., Quevedo E., Cañon O., León Y. Acinetobacter baumanniirelated mortality in intensive care units in Colombia. Revista Panamericana de Salud Publica. 2011;30(4):287–294. [PubMed] [Google Scholar]
- 45.Labarca J. A., Salles M. J., Seas C., Guzmán-Blanco M. Carbapenem resistance in Pseudomonas aeruginosa and Acinetobacter baumannii in the nosocomial setting in Latin America. Critical Reviews in Microbiology. 2014 doi: 10.3109/1040841X.2014.940494. [DOI] [PubMed] [Google Scholar]
- 46.Guzmán-Blanco M., Labarca J. A., Villegas M. V., Gotuzzo E. Extended spectrum β-lactamase producers among nosocomial Enterobacteriaceae in Latin America. The Brazilian Journal of Infectious Diseases. 2014;18(4):421–433. doi: 10.1016/j.bjid.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbolla R. E., Centrón D., Maimone S., Rospide F., Salgueira C., Altclas J., Catalano M. Molecular epidemiology of Acinetobacter baumannii spread in an adult intensive care unit under an endemic setting. The American Journal of Infection Control. 2008;36(6):444–452. doi: 10.1016/j.ajic.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Gales A. C., Jones R. N., Forward K. R., Liñares J., Sader H. S., Verhoef J. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997–1999) Clinical Infectious Diseases. 2001;32(10):S104–S113. doi: 10.1086/320183. [DOI] [PubMed] [Google Scholar]
- 49.Gales A. C., Jones R. N., Turnidge J., Rennie R., Ramphal R. Characterization of Pseudomonas aeruginosa isolates: occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY antimicrobial surveillance program, 1997–1999. Clinical Infectious Diseases. 2001;32(10) supplement 2:S146–S155. doi: 10.1086/320186. [DOI] [PubMed] [Google Scholar]
- 50.Dellinger R. P., Levy M. M., Rhodes A., et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical Care Medicine. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 51.Pronovost P., Needham D., Berenholtz S., Sinopoli D., Chu H., Cosgrove S., Sexton B., Hyzy R., Welsh R., Roth G., Bander J., Kepros J., Goeschel C. An intervention to decrease catheter-related bloodstream infections in the ICU. The New England Journal of Medicine. 2006;355(26):2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 52.Pronovost P. J., Goeschel C. A., Colantuoni E., et al. Sustaining reductions in catheter related bloodstream infections in Michigan intensive care units: observational study. British Medical Journal. 2010;340, article c309 doi: 10.1136/bmj.c309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Resar R., Pronovost P., Haraden C., Simmonds T., Rainey T., Nolan T. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. The Joint Commission Journal on Quality and Patient Safety. 2005;31(5):243–248. doi: 10.1016/s1553-7250(05)31031-2. [DOI] [PubMed] [Google Scholar]
- 54.Haynes A. B., Weiser T. G., Berry W. R., et al. A surgical safety checklist to reduce morbidity and mortality in a global population. The New England Journal of Medicine. 2009;360(5):491–499. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization . WHO Guidelines for Safe Surgery 2009. WHO; 2009. http://whqlibdoc.who.int/publications/2009/9789241598552_eng.pdf. [PubMed] [Google Scholar]
- 56.O'Brien T. F., Stelling J. Integrated multilevel surveillance of the world's infecting microbes and their resistance to antimicrobial agents. Clinical Microbiology Reviews. 2011;24(2):281–295. doi: 10.1128/CMR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacoby G. A. AmpC β-lactamases. Clinical Microbiology Reviews. 2009;22(1):161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nordmann P., Cuzon G., Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. The Lancet Infectious Diseases. 2009;9(4):228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 59.Villegas M. V., Lolans K., Correa A., Suarez C. J., Lopez J. A., Vallejo M., Quinn J. P. First detection of the plasmid-mediated class a carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrobial Agents and Chemotherapy. 2006;50(8):2880–2882. doi: 10.1128/AAC.00186-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alvarez-Lerma F., Martinez Pellus A., Alvarez Sánchez B., Pérez Ortiz E., Jorda R., Barcenilla F., Maravi E., Galvan B., Palomar M., Serra J., Bermejo B., Mateu A., Quintana E., Sanchez Palacios M., Giral R., González V., López Mesa J., Melgarejo J. A., Martinez J., Insausti J., Olaechea P., Chánovas M., Gilabert A., Junquera C., Vallés J., Palacios F., Calvo R., Mesalles E., Nava J., Santos A., Armengol S., Marzo D. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. Intensive Care Medicine. 1996;22(5):387–394. doi: 10.1007/BF01712153. [DOI] [PubMed] [Google Scholar]
- 61.Luna C. M., Sarquis S., Niederman M. S., et al. Is a strategy based on routine endotracheal cultures the best way to prescribe antibiotics in ventilator-associated pneumonia? Chest. 2013;144(1):63–71. doi: 10.1378/chest.12-1477. [DOI] [PubMed] [Google Scholar]
- 62.American Thoracic Society, Infectious Diseases Society of America Guidelines for the management of a dults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. American Journal of Respiratory and Critical Care Medicine. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 63.Dellit T. H., Owens R. C., McGowan J. E., Jr., et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clinical Infectious Diseases. 2007;44(2):159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 64.MacDougall C., Polk R. E. Antimicrobial stewardship programs in health care systems. Clinical Microbiology Reviews. 2005;18(4):638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fishman N. Antimicrobial stewardship. The American Journal of Medicine. 2006;119(6) supplement 1:S53–S61. doi: 10.1016/j.amjmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Salgado F. X. C., Gonçalves J. C., de Souza C. M., da Silva N. B., Sanchez T. E. G., de Oliveira Karnikowski M. G. Cost of antimicrobial treatment in patients infected with multidrug-resistant organisms in the intensive care unit. Medicina. 2011;71(6):531–535. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The features and properties of the 25 observational studies conducted in Latin American and Caribbean ICUs that were identified and selected for our review are included in a supplementary table. Most studies pertained to ICUs in Brazil (n = 11), followed by Argentina (n = 5), Colombia (n = 3), Chile (n = 2), Cuba (n = 1), Mexico (n = 1), Trinidad and Tobago (n = 1), and Venezuela (n = 1).



