Abstract
The bifunctional chelator and radiometal have been shown to have a direct effect on the pharmacokinetics of somatostatin receptor (SSTR)-targeted imaging agents. We evaluated three Y3-TATE analogues conjugated to NOTA-based chelators for radiolabeling with 64Cu and 68Ga for small-animal positron emission tomographic/computed tomographic (PET/CT) imaging. Two commercially available NOTA analogues, p-SCN-Bn-NOTA and NODAGA, were evaluated. The p-SCN-Bn-NOTA analogues were conjugated to Y3-TATE through β-Ala and PEG8 linkages. The NODAGA chelator was directly conjugated to Y3-TATE. The analogues labeled with 64Cu or 68Ga were analyzed in vitro for binding affinity and internalization and in vivo by PET/CT imaging, biodistribution, and Cerenkov imaging (68Ga analogues). We evaluated the effects of the radiometals, chelators, and linkers on the performance of the SSTR subtype 2-–targeted imaging agents and also compared them to a previously reported agent, 64Cu-CB-TE2A-Y3-TATE. We found that the method of conjugation, particularly the length of the linkage between the chelator and the peptide, significantly impacted tumor and nontarget tissue uptake and clearance. Among the 64Cu- and 68Ga-labeled NOTA analogues, NODAGA-Y3-TATE had the most optimal in vivo behavior and was comparable to 64Cu-CB-TE2A-Y3-TATE. An advantage of NODAGA-Y3-TATE is that it allows labeling with 64Cu and 68Ga, providing a versatile PET probe for imaging SSTr subtype 2–positive tumors.
Somatostatin receptors are overexpressed on a variety of human neuroendocrine tumors and have become an important target for molecular imaging. There are five receptor subtypes; somatostatin receptor subtype 2 (SSTR2) is found in a variety of malignancies and has become the target for molecular imaging radiolabeled somatostatin analogues.1–9 Previous research has demonstrated that somatostatin analogues can be labeled directly with 18F and 124I or modified with bifunctional chelators, allowing the incorporation of radiometals.10–12
The radiometals 64Cu and 68Ga have desirable characteristics for use in positron emission tomographic (PET) imaging. 64Cu (T1/2 = 12.7 hours; β+ [17.6%] 653 keV; β− [38.4%] 579 keV) is ideal for tracers with slower accumulation within the target site and clearance from nontargeted tissues and is also a promising radiometal for radiotherapy due to β− emission.13 Gallium-68 (T1/2 = 67.7 minutes; β+ [87.7%] 1,899 keV) has become a more widely used radiometal for PET imaging due to the convenience of its production from a 68Ge/68Ga generator.14 In addition, the high-energy positron emitted by 68Ga has potential for Cerenkov luminescence imaging, which can be monitored using simpler and less expensive whole-animal optical imaging equipment.15,16
The choice of bifunctional chelator and radiometal has been shown to have a direct effect on the pharmacokinetics of SSTR-targeted imaging agents. The chelator NOTA and its analogues form stable complexes with both 64Cu and 68Ga.17–19 The NOTA analogues NODAGA and p-SCN-Bn-NOTA have three carboxylates available for radiometal complexation after conjugation to peptides and proteins. Lin and colleagues demonstrated that 68Ga-[Tyr3]-octreotide modified with a NOTA analogue having three carboxylates demonstrated greater accumulation in SSTR-positive xeno-graft with superior pharmacokinetics than analogues with more carboxylates.20 In addition, this analogue demonstrated pharmacokinetics akin to the DOTA analogue but with superior clearance from the liver.20 Fani and colleagues radiolabeled NODAGA-LM3, a modified somatostatin antagonist, with 64Cu and 68Ga and evaluated the effects of the radiometal on their in vivo performance.21 The accumulation in the SSTR-positive xenograft was similar for both agents, but there were significant differences in the clearance from the kidneys and pancreas.
Here we compare commercially available chelators, p-SCN-Bn-NOTA and NODAGA, conjugated to the SSTR2-targeted somatostatin agonist Y3-TATE and radiolabeled with 64Cu and 68Ga. The rationale for comparing NOTA analogues is to determine the effect of radiometal and linkages of NOTA analogues on the in vivo performance of the agonist, Y3-TATE, as this analogue has been investigated in human studies.22–26 The NODAGA chelator was directly conjugated to Y3-TATE, whereas the p-SCN-Bn-NOTA chelators were conjugated to Y3-TATE through β-Ala and PEG8 linkages. The in vitro and in vivo results were compared to evaluate the effects of the radiometal, chelators, and linker. The NOTA conjugates were compared to 64Cu-CB-TE2A-Y3-TATE, which previously showed high SSTR2-positive tumor uptake with clearance through nontarget tissues.27,28
Materials and Methods
General
64Cu was purchased from Washington University School of Medicine and University of Wisconsin School of Medicine and Public Health. 68Ga (Eckert & Ziegler Isotope Products, Berlin, Germany) was eluted directly to a Modular-Lab (Eckert & Ziegler Isotope Products), concentrated on a Strata-X-C column from Phenomenex (Torrance, CA), and the 68Ga-eluate was collected by desorbing it with 0.8 mL of 0.01 M HCl/98% acetone solution. HCT116 cells were provided by Dr. Bert Vogelstein at Johns Hopkins University and were transfected with SStr2 as previously described.29 S-2-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA) was purchased from Macrocyclics (Dallas, TX), and 2,2′-(7-(1-carboxy-4-((2,5-dioxopyrrolidin-1-yl)oxy)-4-oxobutyl)-1,4,7-triazonane-1,4-diyl)diacetic acid (NODAGA-NHS ester) was purchased from CheMatech (Dijon, France). All other chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA).
Synthesis of Y3-TATE Analogues
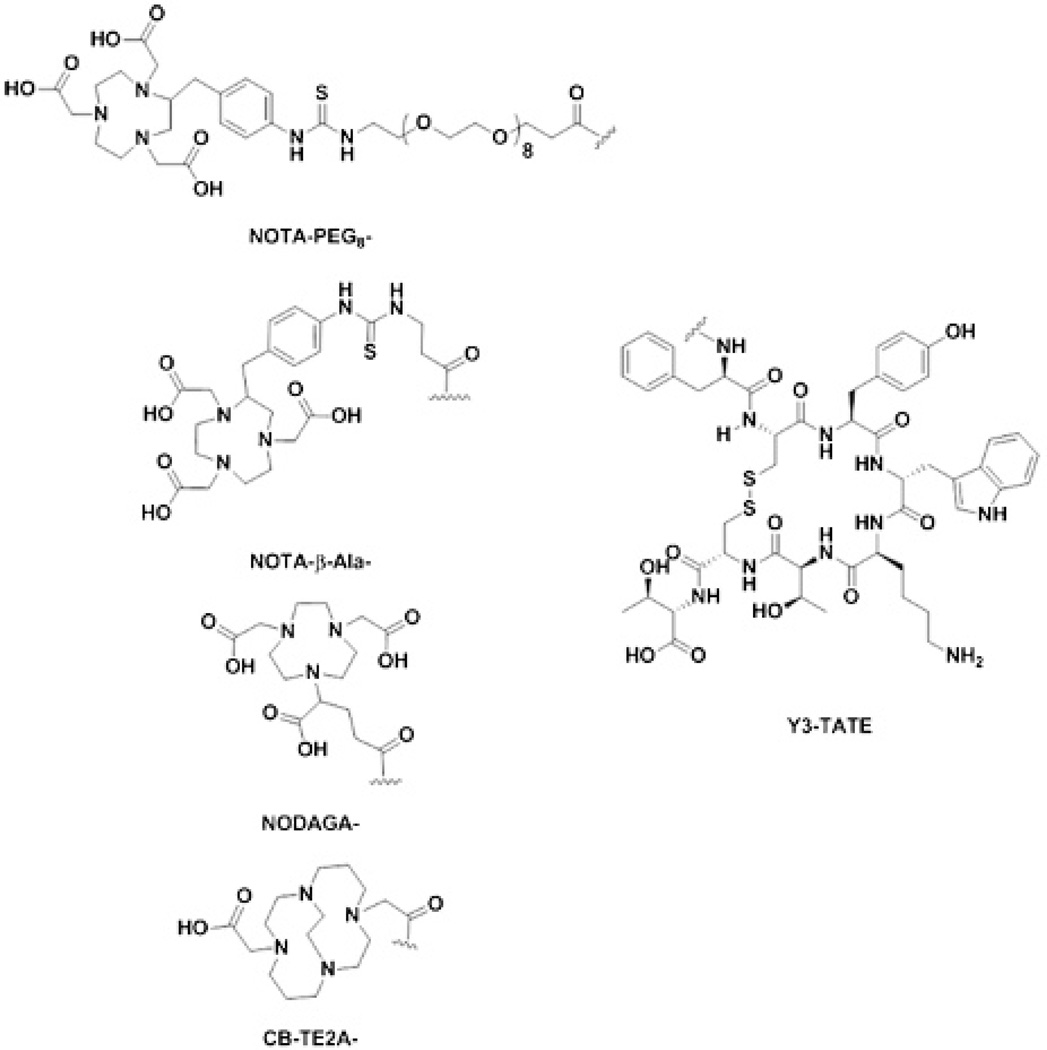
4,11-Bis(carboxymethyl)-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane (CB-TE2A) conjugated to Y3-TATE was synthesized as previously reported.29 The general protocol for peptide synthesis of the conjugates used for this study has been previously described.28,30 Briefly, the peptides were prepared on a solid support by standard Fmoc procedures using a preloaded Fmoc-Thr(Boc)-Wang resin. Fmoc deprotection was achieved by washing the resin with 20% piperidine DMF (five times) for 2 minutes, followed by washing the resin three times with DMF. The carboxyl group was activated using 2-(1H-benzotriazole-1-yl)-1,1,1,3-tetramethyl-uronium hexafluorophosphate (HBTU) and di-isopropyl-ethylamine (DIPEA) to couple the subsequent Fmoc-protected amino acids. Cyclization of the peptide was accomplished by treating the resin with Tl(TFA)3 in dimethylformamide (DMF). Following cyclization, the Fmoc was removed from D-Phe to expose the free amine. The free amine was treated under three conditions to provide the Y3-TATE analogues for this study. NODAGA-Y3-TATE was prepared by treating the resin with NODAGA-NHS (2 Eq) and N,N′-diisopropylethylamine (DIPEA) (5 Eq) in DMF overnight. NOTA-PEG8-Y3-TATE (NOTA-PEG8) was prepared by first treating the resin with Fmoc-PEG8-CH2CH2-COOH following standard Fmoc procedures. Following the deprotection, the resulting free amine was treated with p-SCN-Bn-NOTA (1.5 Eq) and DIPEA (6 Eq) dissolved in DMF and reacted overnight. NOTA-β-Ala-Y3-TATE (NOTA-β-Ala) was prepared under the same methods as NOTA-PEG8, with Fmoc-β-Ala used as the linker instead of Fmoc-PEG8-CH2CH2-COOH. The resins were washed, the individual peptides were cleaved, and side chain–protecting groups were removed using a trifluoroacetic acid (TFA) solution (90% TFA, 5% water, 5% triisopropylsilane). The cleaved peptides were precipitated out of solution using ice-cold diethyl ether and washed twice with diethyl ether. The peptides were dissolved in 10% acetic acid and purified using preparatory high-performance liquid chromatography (HPLC), where solvent A is 0.1% TFA in water and solvent B is 0.1% TFA in acetonitrile. The HPLC purification was carried out on a Phenomenex Jupiter 5u C18 300 Å semipreparative column (250 × 10 mm, 5 microns) starting at 82% A held for 2 minutes, then a linear gradient to 65% A over 10 minutes, followed by a linear gradient to 50% over 3 minutes and a linear gradient to 10% A over 1 minute. This was held for an additional minute followed by a linear gradient to 82% A over 1 minute and held for 4 minutes. The solvent was removed in vacuo to yield pure Y3-TATE analogues. The Y3-TATE analogues (Figure 1) were characterized on a Waters e2695/ LCT Premier XE LCMS: NODAGA-Y3-TATE (C64H87N13O19S2), [M] calculated 1405.5683, found 1405.4971; NOTA-PEG8-Y3-TATE (C88H127N15O27S3), [MH]+ calculated 1922.8266, found 1923.0698; NOTA-β-Ala-Y3-TATE (C72H95N15O19S3), [M] calculated 1569.6091, found 1570.3997.
Figure 1.
Structures of NOTA- and CB-TE2A-Y3-TATE analogues.
Synthesis of Cold Standards of Cu- and Ga-Y3-TATE Analogues
Cold copper labeling was achieved by reacting 300 µg of each of the NOTA-Y3-TATE analogues, 300 µL 10% acetic acid, and 2 mg of copper acetate for 30 minutes at 95°C. Cold gallium labeling was achieved under the same conditions using gallium trichloride. The reaction solution was purified using preparatory HPLC, where solvent A is 0.1% TFA in water and solvent B is 0.1% TFA in acetonitrile. The HPLC purification was carried out on a Phenomenex Jupiter 5u C18 300 Å semipreparative column (250 × 10 mm, 5 microns) starting at 97% A, a linear gradient to 3% A over 10 minutes, and then held for an additional 7 minutes. This was followed by a linear gradient to 97% over 1 minute and held for an additional 4 minutes. The desired peak was collected and solvent was removed in vacuo to yield pure Cu- and Ga-NOTA-Y3-TATE analogues. The Cu- and Ga-NOTA-Y3-TATE analogues were characterized on a Waters (Milford, MA) e2695/LCT Premier XE LCMS: Cu-NODAGA-Y3-TATE (C64H87CuN13O19S2), [M] calculated 1467.103, found 1467.272; Ga-NODAGA-Y3-TATE (C64H87GaN13O19S2), [M] calculated 1473.280, found 1473.163; Cu-NOTA-PEG8-Y3-TATE (C88H127CuN15O27S3), [M] calculated 1983.751, found 1983.592;Ga-NOTA-PEG8-Y3-TATE (C88H127GaN15O27S3), [M] calculated 1989.928, found 1989.298; Cu-NOTA-β-Ala-Y3-TATE (C72H95CuN15 O19S3), [M] calculated 1631.331, found 1631.794; Ga-NOTA-β-Ala-Y3-TATE (C72H95GaN15O19S3), [MH]+ calculated 1636.519, found 1636.989.
Radiolabeling
64Cu radiolabeling was achieved by reacting 2 to 2.5 µg of each of the Y3-TATE analogues, 200 µL 0.4 M NH4OAc (initial pH 7.0), and ≈ 37 MBq of 64CuCl2 in 0.1 N hydrochloric acid for 30 minutes at 95°C. The coordination of 68Ga was achieved under similar conditions. Two micrograms of each Y3-TATE analogue, 200 µL 0.4 M NH4OAc (initial pH 7.0) and ≈ 37 MBq of 68GaCl3 in 0.1 N hydrochloric acid/98% acetone solution were reacted for 30 minutes at 95°C in an open vial. 64Cu-CB-TE2A-Y3-TATE was prepared as previously described.28 Quality control of the radiolabeled peptides was performed on a Waters 2489/1525 HPLC to determine radiolabeling yield.
Receptor Binding Assays
Membrane preparations of HCT116-SSTr2 cells were used for binding assays, and assays were performed on Perkin-Elmer Unifilter (Waltham, MA) 96-well, GF/B filtration plates using previously described methods, with some modifications.28,29,31 Membranes were diluted in binding buffer (50 mM Tris-hydrochloride [pH 7.4]; 5 mM MgCl2·6 H2O; 0.1% bovine serum albumin; and 0.5 mg of aprotinin, 200 mg of bacitracin, 10 mg of leupeptin, and 10 mg of pepstatin A per milliliter), and 15 µg of membrane protein was used per well. Increasing concentrations of 64Cu-labeled Y3-TATE analogues were added to membranes to measure total binding, and nonspecific binding was determined by conducting the assay in the presence of an excess of Y3-TATE. After incubation of the membranes at room temperature for 2 hours, the medium was removed and the membranes were washed twice with 200 µL of binding buffer. OptiPhase Super-Mix (50 µL; PerkinElmer, Waltham, MA) was added to each well, and bound activity was measured with a liquid scintillation and luminescence counter (2450 Microbeta2, PerkinElmer). All dissociation constant (Kd) values were estimated from nonlinear curve fitting of bound peptide versus the sum of the concentrations of 64Cu-Y3-TATE analogues and Y3-TATE using Prism software (GraphPad, La Jolla, CA).
Competitive Binding Assay
Receptor binding affinities (Ki) of cold Cu- and Ga-labeled NOTA-Y3-TATE analogues were calculated from half-maximal inhibitory concentration (IC50) values determined by a competitive binding assay using 64Cu-NODAGA-Y3-TATE. Assays were performed on Unifilter 96-well, GF/B filtration. Plates were prepared by adding the following, as ordered, to each well: binding buffer, varying concentrations of cold Cu- or Ga-NOTA-Y3-TATE analogues (0–1,000 nM), 64Cu-NODAGA-Y3-TATE (final concentration 0.5 nM), and 15 µg of membrane protein. Membranes and binding buffer were prepared as stated above in the receptor binding assay. The plates were allowed to incubate for 3 hours at room temperature (incubation time was four times the Koff of 64Cu-NODAGA-Y3-TATE; data not shown). The cells were then washed twice with phosphate-buffered saline, OptiPhase Super-Mix (50 µL; PerkinElmer) was added to each well, and bound activity was measured with a liquid scintillation and luminescence counter (2450 Microbeta2). The IC50 values were calculated by fitting the quadruplicate data with nonlinear regression using GraphPad Prism software. The Ki values were calculated by using the Cheng-Prusoff equation.32
Internalization Studies
Internalization studies were performed as previously described.27 Briefly, HCT116-SSTr2 cells were cultured in McCoy’s 5A medium supplemented with 10% fetal bovine serum, 1% pencillin-streptomycin-glutamine, and Zeocin (1 µg/mL); cells were incubated at 37°C in a humidified 5% CO2 atmosphere. Before each assay, aliquots of prepared 2 × 107 cells/mL were placed in a 12-well plate and incubated overnight. The wells were prepared as previously described,27 and 64Cu-labeled Y3-TATE analogues (6 ng/10 µL) were added to blocked and unblocked wells (n = 3). Blocked wells were pretreated with 2 µg/10 µL of Y3-TATE and washed, and new growth medium was added. The cells were allowed to incubate for 10 minutes at 37°C. Following incubation, the medium was collected in separate fractions; the surface bound and the lysed cells were counted on a Packard Cobra II automated gamma counter (Packard Instrument Company, Downers Grove, IL). The total protein concentration in the cell lysate was determined using the BCA Protein Assay (Pierce Biotechnology, Rockford, IL). Internalized and surface-bound fractions were expressed as fmol/mg of protein.
Biodistribution
All animal studies were conducted under protocols approved by the University of Pittsburgh and Washington University Institutional Animal Care and Use Committees (IACUC). Biodistribution experiments were conducted as previously described with some modifications.31 Briefly, healthy NCr nude female mice (6–8 weeks, Taconic Labs, Hudson, NY) bearing HCT116-SSTR2-positive tumors were injected with 64Cu- and 68Ga-Y3-TATE analogues (0.74–1.85 MBq) via the tail vein. Animals were sacrificed at selected time points following the injection, and organs of interest were removed, weighed, and counted on a WIZARD2 gamma counter (PerkinElmer). In addition, blocking studies were performed for 64Cu analogues at 4 hours where the mice were injected with 50 µg of Y3-TATE 30 minutes prior to injection of the radiotracer, except CB-TE2A-Y3-TATE, which was coinjected with 100 µg of Y3-TATE at 4 hours. The percent injected dose per gram (%ID/g) was calculated by comparison to a weighed, diluted standard.
PET/CT Imaging
Imaging studies were performed using NCr nude female mice (6–8 weeks, Taconic Labs) bearing HCT116-SSTR2 tumors either in the shoulder or flank. Mice were injected with the 64Cu- and 68Ga-labeled Y3-TATE analogues (3.7–8.4 MBq) via the tail vein. After probe injection, imaging was performed at the following time points: 68Ga probes = 1 hour, 64Cu probes = 1 and 4 hours. For tail vein injection and throughout imaging, the mice were anesthetized with 2 to 3% isoflurane under oxygen at a flow rate of 2 L/min. PET/CT imaging was performed on an Inveon small-animal PET/CT scanner (Siemens Molecular Imaging, Knoxville, TN) with the following parameters: 600-second PET acquisition time, two-dimensional (2D) ordered subset expectation maximization (OSEM) (standard uptake value [SUV] calculations) and three-dimensional (3D) OSEM (PET/CT images) reconstruction algorithms, CT-based attenuation correction, voxel size 0.7 mm3; CT exposure settings: 80 kV, 500 mA, 120 ms exposure time, 220° rotation with 120 steps, low magnification, bin 4, voxel size 0.8 mm3; CT reconstruction: Shepp-Logan reconstruction filter, bilinear interpolation, downsample factor 2, voxel size: x = 412.9 µm, y = 412.9 µm, z = 533.33 µm. 64Cu-CB-TE2A-Y3-TATE was imaged as previously described at 2 hours postinjection.27,33,34 All PET images were manually coregistered to the CT, analyzed, and prepared using the Inveon Research Workplace software (Siemens Molecular Imaging). PET images were exported as maximum intensity projections, and final images were prepared using ImageJ software (National Institutes of Health [NIH], Bethesda, MD) and Adobe Photoshop CS5.
Cerenkov Luminescence Imaging
Following PET/CT imaging, mice injected with 68Ga-based probes were placed into an IVIS Lumina XR optical imaging station (PerkinElmer) to evaluate Cerenkov luminescence in the tumors. Images were acquired using the following parameters: no light acquisition filter, acquisition time: 300 seconds, binning: 8 × 8, F/stop: 1, field of view: 10 cm × 10 cm. Animals were anesthetized with 2% isoflurane under oxygen with a flow rate of 2 L/min throughout imaging and were heated by a 37°C platform throughout imaging. Machine control was performed using Living Image software version 3.1 (PerkinElmer). The 16-bit TIFF output images were opened using ImageJ software, and outlier hotspots due to cosmic radiation were removed using the “remove outliers” tool. Images were then background subtracted using the rolling ball algorithm (radius = 500 pixels). Free-hand regions of interest (ROI) were drawn around the tumor and the leg opposite the tumor as a muscle reference, and mean pixel intensities were measured within each ROI. Tumor to muscle ratios were then calculated. Image labels were added using Adobe Photoshop CS5.
Statistical Analysis
Prism version 5 software was used to determine p values and statistical significance. An unpaired t-test was used to compare biodistribution values presented in this article.
Results
Synthesis and Radiolabeling
Y3-TATE was prepared on resin as previously described.28,30 The terminal D-Phe was deprotected on the resin to expose the free amine for incorporation of the NOTA chelators and linkers. The free amine was modified to provide the chelator-Y3-TATE analogues in the following yields: NODAGA-Y3-TATE (1.9%), NOTA-β-Ala-Y3-TATE (2.1%), and NOTA-PEG8-Y3-TATE (3.4%). These analogues were radiolabeled in high yield and purity with 64Cu (≥ 95%) and 68Ga (≥ 99%). The specific activities ranged between 42.2 and 150.6 MBq/µmol (Table 1).
Table 1.
Specific Activities (GBq/µmol) of NOTA Analogues at End of Bombardment (64Cu) and End of Elution (68Ga)
| 64Cu | 68Ga | |
|---|---|---|
| NODAGA-Y3-TATE | 91.2–110.0 | 42.2–44.9 |
| NOTA-β-Ala-Y3-TATE | 115.8–150.6 | 56.5–68.7 |
| NOTA-PEG8-Y3-TATE | 84.0–125.9 | 81.0–102.1 |
Receptor Binding Assays
A saturation binding assay was performed using HCT116-SSTR2 membranes.31 The dissociation constant (Kd) for 64Cu-NODAGA-Y3-TATE (0.5 ± 0.1 nM) was similar to the previously reported values for 64Cu-CB-TE2A-Y3-TATE (Table 2)29; however, the Bmax was greater for the CB-TE2A analogue compared to the NODAGA analogue (4200 ± 200 vs 2048 ± 79.9 fmol/mg, respectively). Linker modifications to p-SCN-Bn-NOTA significantly decreased the affinity of the radiotracers (β-Ala analogue 1.8 ± 0.7 nM; PEG8 analogue: 2.3 ± 0.9 nM; p ≤ .05); in addition, increasing the size of the linker resulted in lower Bmax values. The use of the β-Ala linker demonstrated a slightly lower Bmax (1660 ± 190 fmol/mg) to the linker free NODAGA analogue, whereas the use of the PEG8 linker greatly reduced the Bmax (980 ± 130 fmol/mg); p ≤ .05).
Table 2.
Dissociation Constant, Bmax and Binding Affinity of NOTA and CB-TE2A Analogs
| Copper-64 |
Copper* |
Gallium* |
||||
|---|---|---|---|---|---|---|
| Kd (nM) | Bmax (fmol/mg) | Ki (nM) | 95% CI | Ki (nM) | 95% CI | |
| NODAGA-Y3-TATE | 0.5 ± 0.1 | 2050 ± 80 | 0.6 | 0.3–1.3 | 0.6 | 0.2–2.4 |
| NOTA-β-Ala-Y3-TATE | 1.8 ± 0.7 | 1660 ± 186 | 1.5 | 0.1–30 | 4.0 | 0.1–195 |
| NOTA-PEG8-Y3-TATE | 2.3 ± 0.9 | 984 ± 126 | 2.5 | 0.1–53 | 16 | 3.1–87 |
| CB-TE2A-Y3-TATE† | 0.5 ± 0.1 | 4200 ± 200 | N/A | |||
N/A = not available.
Ki assays were preformed once with the cold standards of the NOTA-Y3-TATE analogues.
Previously reported.29
Competitive Binding Assays
The IC50 values were determined in a competitive binding assay using 64Cu-NODAGA-Y3-TATE as the radioligand and HCT116-SSTR2 membranes (see Table 2). The competitive binding assays were performed once, and the Ki value was calculated from the IC50 using the Cheng-Prusoff equation. Cu- and Ga-NODAGA-Y3-TATE presented the lowest Ki values for the NOTA analogues; both analogues had a Ki of 0.6 nM. As seen with the dissociation constants, the addition of linkers demonstrated an increase in the Ki values. For the β-Ala linker, the cold Cu analogue had a Ki of 1.5 nM, which was slightly lower than the Ga analogue, with a Ki of 4.0 nM. The difference between the Cu- and Ga-NOTA analogues was the greatest for NOTA-PEG8-Y3-TATE: Cu-NOTA-PEG8-Y3-TATE (Ki = 16 nM) and Ga-NOTA-PEG8-Y3-TATE (Ki = 2.5 nM).
Internalization Studies
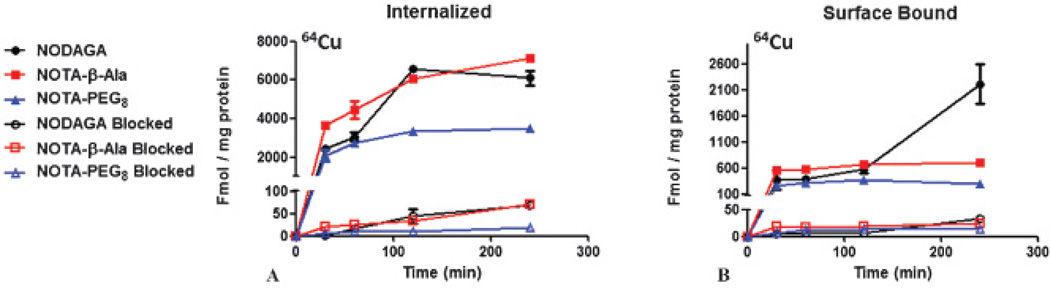
Internalization studies were performed with HCT116-SSTR2 cells (Figure 2). 64Cu-NOTA-PEG8-Y3-TATE demonstrated rapid internalization within 30 minutes, slightly increasing over 4 hours (3460 ± 130 fmol/mg). Unlike 64Cu-NOTA-PEG8-Y3-TATE, the internalization of the β-Ala and NODAGA analogues continued to significantly increase over the 4-hour window, 64Cu-NOTA-β-Ala-Y3-TATE by 49% and 64Cu-NODAGA-Y3-TATE by 59% from the initial 15-minute time point. The addition of the blocking agent at each time point reduced the uptake of all 64Cu-labeled NOTA-Y3-TATE analogues, indicating that the internalization was receptor mediated.
Figure 2.
Internalization studies performed with 64Cu-labeled NOTA-Y3-TATE analogues using HCT116-SSTR2-positive colorectal carcinoma cells (n = 3). Blocking was achieved with cold Y3-TATE (n = 3). (A) Internalized and (B) surface-bound 64Cu-NOTA-Y3-TATE analogues in HCT116-SSTR2-positive cells with and without blocking.
Biodistribution
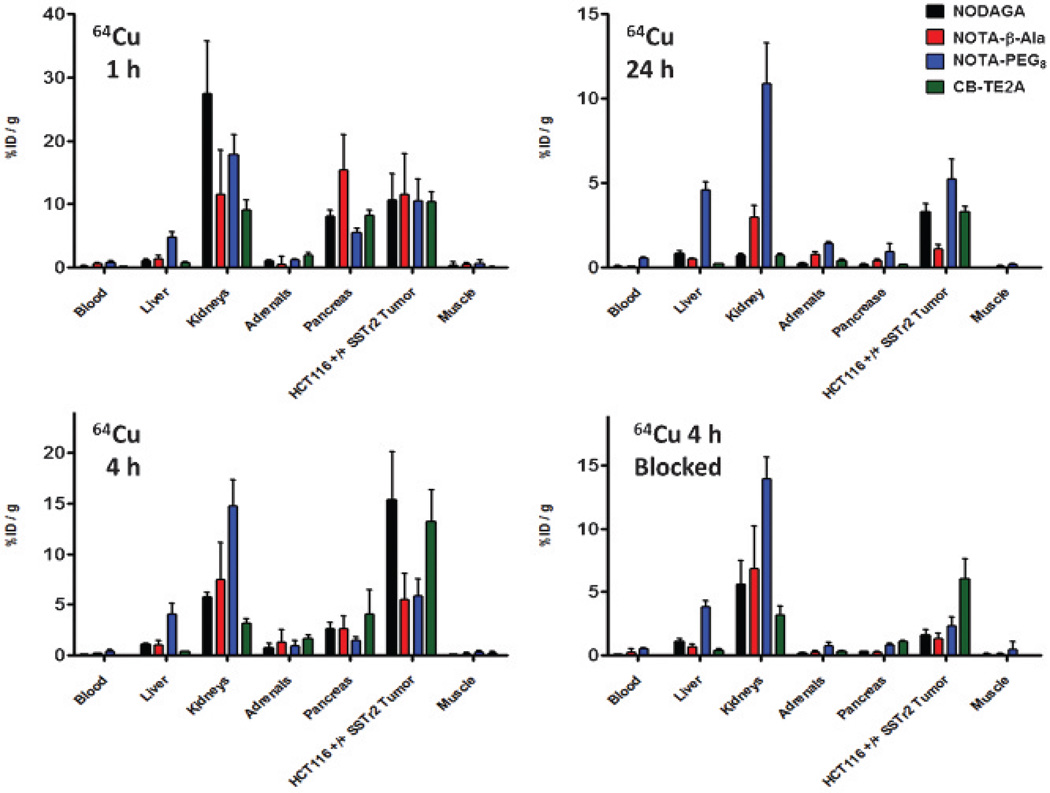
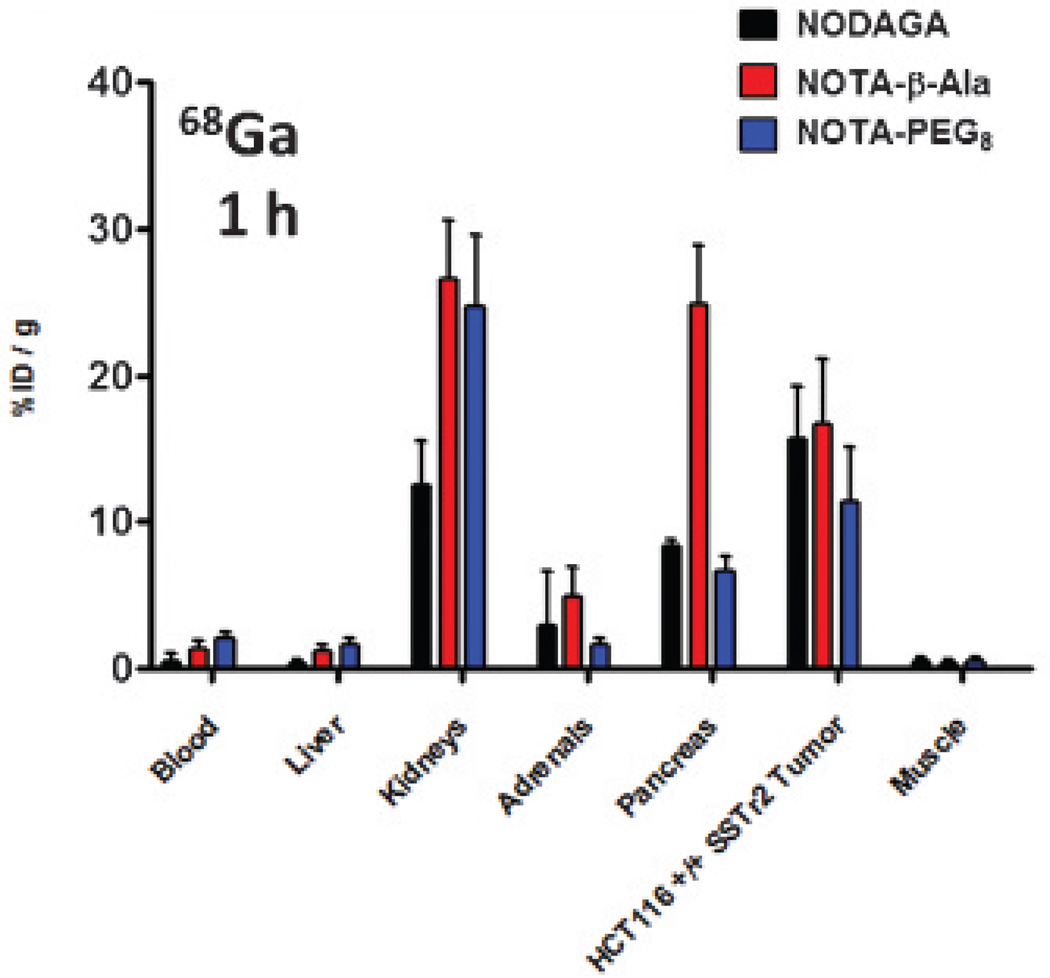
Biodistribution studies were carried out in NCr nude female mice (6–8 weeks) bearing HCT116-SSTR2 tumors. At 1 hour, all 64Cu and 68Ga analogues had high uptake in the kidneys, pancreas, and SSTR2-positive tumors (Figure 3 and Figure 4). At 1 hour, the 64Cu- and 68Ga-labeled compounds showed significant differences in kidney uptake. The Ga-labeled NOTA analogues demonstrated higher kidney uptake compared to the Cu-labeled analogues, with the exception of the NODAGA-Y3-TATE. The uptake in the pancreas for all analogues decreased to background by 24 hours. Compared to the 64Cu- and 68Ga-labeled NODAGA and PEG8 analogues, the 64Cu- and 68Ga-labeled NOTA-β-Ala-Y3-TATE analogues exhibited significantly higher uptake in the pancreas at 1 hour (p values ≤ .05), which decreased by 4 hours, presenting uptake similar to that of the other analogues. Although the 68Ga-NOTA analogues had greater %ID/g values at 1 hour compared to the analogous 64Cu compounds, the differences were not statistically significant (p values ≥ .1).
Figure 3.
Biodistribution of 64Cu-NOTA and CB-TE2A-Y3-TATE analogues at 1 hour, 4 hours, 4 hours blocked, and 24 hours in NCr nude mice bearing HCT116-SSTR2 tumors (n = 4 for each group).
Figure 4.
Biodistribution of 68Ga-NOTA-Y3-TATE analogues at 1 hour in NCr nude mice bearing HCT116-SSTR2-positive tumors (n = 4 for each group).
At 4 hours, 64Cu-NODAGA-Y3-TATE and 64Cu-CB-TE2A-Y3-TATE demonstrated significantly higher uptake in the tumor (15 ± 4.8% and 13 ± 3.1 %ID/g) than both the PEG8 and β-Ala analogues (5.5 ± 1.7 and 5.8 ± 2.6 %ID/g; p < .01). At 24 hours, 64Cu-NOTA-PEG8-Y3-TATE (5.2 ± 1.2 %ID/g; p < .03) presented with the highest tumor uptake, but uptake in the kidneys and liver was greater than in the CB-TE2A, NODAGA, and β-Ala analogues. 64Cu-NODAGA-Y3-TATE and 64Cu-CB-TE2A-Y3-TATE at 24 hours had the highest tumor uptake (3.3 ± 0.5 %ID/g; 3.3 ± 0.3 %ID/g), with significant clearance from nontargeted organs. At 24 hours, 64Cu-NOTA-β-Ala-Y3-TATE showed the highest uptake in the kidneys (3.0 ± 0.3 %ID/g), followed by the tumor (1.1 ± 0.3 %ID/g).
Of all compounds evaluated, 64Cu-NODAGA-Y3-TATE demonstrated superior tumor to blood/muscle ratios for all time points; however, at 1 hour, the tumor to blood/muscle ratio of 64Cu-NODAGA-Y3-TATE and the tumor to muscle ratios of all the 64Cu-labeled NOTA analogues at 4 hours were not statistically significant (p ≥ .07) compared to 64Cu-CB-TE2A-Y3-TATE (Table 3). For the NOTA analogues, 68Ga- and 64Cu-labeled NODAGA-Y3-TATE provided the highest tumor to blood/muscle ratios. The tumor to muscle and tumor to blood ratios for the 64Cu-labeled analogues were highest at 4 hours with the exception of NOTA-PEG8-Y3-TATE, where the tumor to muscle/blood ratios were similar at 1 and 4 hours (see Table 3). 64Cu-CB-TE2A-Y3-TATE demonstrated the highest tumor to muscle/blood ratios of all the analogues at 1 hour (74 ± 25 and 120 ± 60, respectively). Of the NOTA analogues at 1 hour, 64Cu-NODAGA-Y3-TATE demonstrated the highest tumor to muscle/blood (73 ± 78 and 58 ± 26, respectively); however, it should be noted that the differences in tumor to muscle ratios are not statistically significant.
Table 3.
Biodistribution Tumor to Blood/Muscle Ratios of NOTA and CB-TE2A Y3-TATE Analogues in HCT116 SSTR2-Positive Tumor-Bearing Mice at 1, 4, and 24 Hours (n = 4 for each group)
|
68Ga, 1 h |
64Cu, 1 h |
64Cu, 4 h |
64Cu, 24 h |
|||||
|---|---|---|---|---|---|---|---|---|
| Tumor to | Blood | Muscle | Blood | Muscle | Blood | Muscle | Blood | Muscle |
| NODAGA-Y3-TATE | 42 ± 34 | 54 ± 43 | 58 ± 26* | 73 ± 78* | 90 ± 39 | 195 ± 57* | 36 ± 2 | 100 ± 15 |
| NOTA-β-Ala-Y3-TATE | 14 ± 6† | 53 ± 29† | 18 ± 3 | 27 ± 16 | 30 ± 9 | 58 ± 39* | 13 ± 3 | 28 ± 15 |
| NOTA-PEG8-Y3-TATE | 5.8 ± 2† | 28 ± 18† | 13 ± 4 | 32 ± 23 | 14 ± 1 | 31 ± 20* | 10 ± 3 | 29 ± 11 |
| CB-TE2A-Y3-TATE | N/A | N/A | 74 ± 25 | 120 ± 60 | 251 ± 58 | 220 ± 173 | 122 ± 18 | 366 ± 225 |
N/A = not available.
Not statistically significant (p values ≥ .07) compared to 64Cu “gold standard,” 64Cu-CB-TE2A-Y3-TATE.
Not statistically significant (p values ≥ .07) compared to 68Ga-NODAGA-Y3-TATE.
Blocking with unlabeled Y3-TATE demonstrated a reduction in SSTR2-targeted agents for all compounds. Preblocking caused a 90% decrease in tumor uptake of 64Cu–NODAGA-Y3-TATE (1.8 ± 0.5 %ID/g), a 76% decrease in 64Cu-NOTA-β-Ala-Y3-TATE (1.3 ± 0.4 %ID/g), and a 61% decrease in 64Cu-NOTA-PEG8-Y3-TATE (2.3 ± 0.8 %ID/g). 64Cu-CB-TE2A-Y3-TATE (6.1 ± 1.6 %ID/g) was coinjected with Y3-TATE but still demonstrated reduced uptake in the tumor by 54%. The decrease in tumor uptake when preinjected or coinjected with Y3-TATE indicates selective binding of SSTR2 for all analogues.
PET/CT Imaging
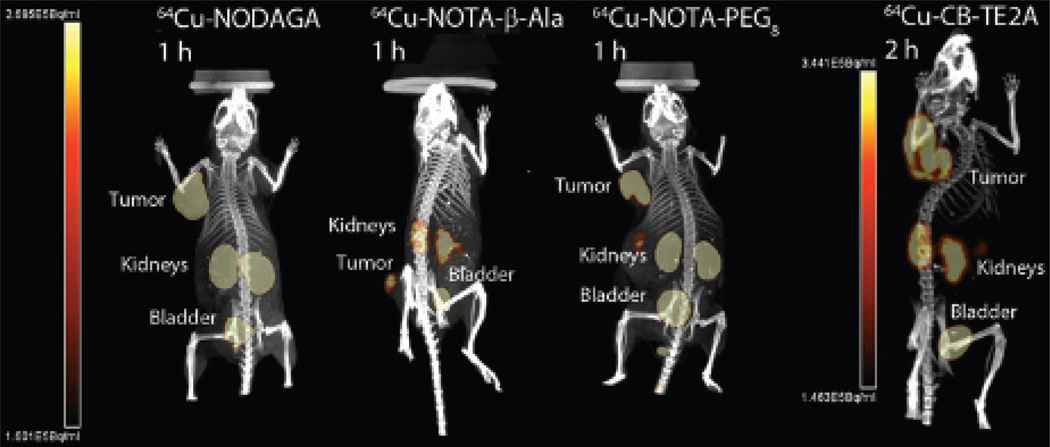
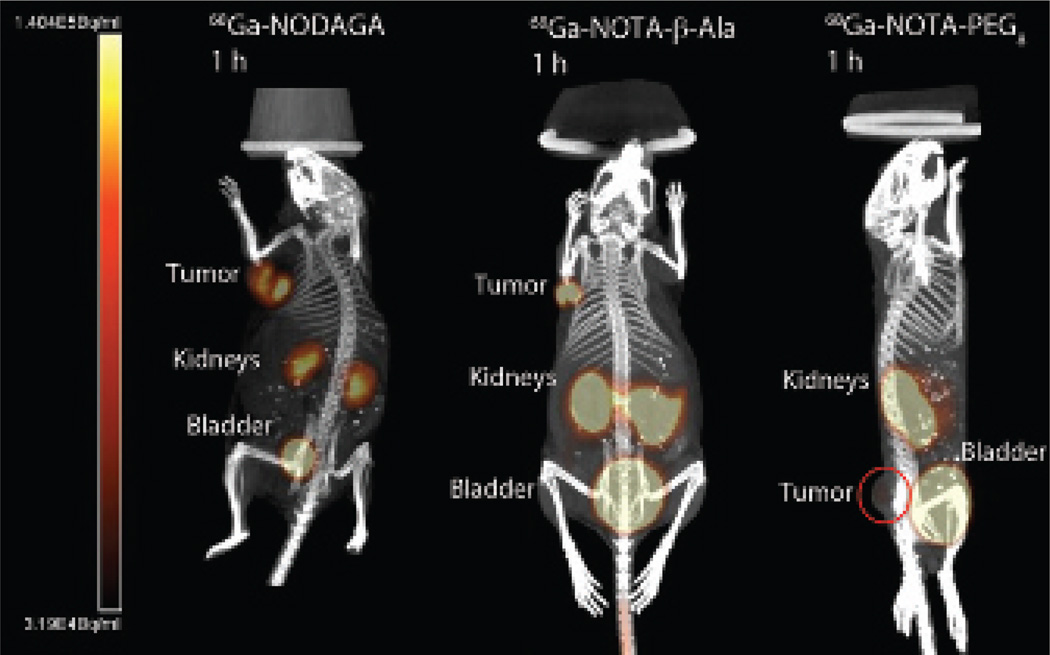
The PET/CT images for all the 64Cu and 68Ga Y3-TATE analogues evaluated in this study (n = 2) had high contrast for the tumor along with no-target uptake in kidneys and bladder (Figure 5 and Figure 6), except 68Ga-NOTA-PEG8-Y3-TATE (n = 1), which had low contrast to nontarget uptake. The nontarget uptake in the kidneys and bladder is due to clearance of the PET agents. In comparing the 64Cu- or 68Ga-labeled analogues, it should be noted that there were no significant differences in the SUV and tumor to muscle ratios. The SUVs ranged between 0.48 and 4.0, with 64Cu-NOTA-β-Ala-Y3-TATE demonstrating the highest SUV at 1 hour (4.0 ± 0.6), and this agent and 64Cu-NODAGA-Y3-TATE had the highest SUVs at 4 hours (3.1 ± 0.5 and 3.1 ± 0.9) (Table 4). The 64Cu-labeled β-Ala and PEG8 NOTA-Y3-TATE analogues demonstrated lower SUVs at 1 hour compared to the NODAGA conjugate. 64Cu-NODAGA-Y3-TATE demonstrated the second highest SUV (3.1 ± 1.3) at 1 hour, whereas 64Cu-labeled NOTA-β-Ala-Y3-TATE (4.0 ± 0.6) had the highest, followed by the NODAGA, NOTA-PEG8 (2.6 ± 0.6), and CB-TE2A (2.2 ± 0.6) analogues. At 1 hour, the tumor to muscle trend was as follows: 64Cu-NODAGA-Y3-TATE (324 ± 438) demonstrated the highest, followed by 64Cu-CB-TE2A-Y3-TATE (34 ± 16), 64Cu-NOTA-β-Ala-Y3-TATE (13 ± 5.0), and 64Cu-PEG8-Y3-TATE (8.9 ± 1.6). At 4 hours, the trend reversed for the NOTA analogues, with 64Cu-PEG8-Y3-TATE (38 ± 3.4) having the highest and 64Cu-NODAGA-Y3-TATE (26 ± 2.1) the lowest.
Figure 5.
PET/CT images of 64Cu-NOTA-Y3-TATE analogues 1 hour postinjection: NODAGA (6.1 MBq), NOTA-β-Ala (2.4 MBq), NOTA-PEG8 (4.2 MBq). PET/CT image of 64Cu-CB-TE2A-Y3-TATE 2 hours postinjection (7.2 MBq). PET/CT images were performed with NCr nude mice bearing HCT116-SSTR2 tumors. Images are maximum intensity projections and rotated to achieve the best presentation of the tumor.
Figure 6.
PET/CT images of 68Ga-NOTA-Y3-TATE analogues 1 hour post-injection: NODAGA (5.2 MBq), NOTA-β-Ala (4.6 MBq), NOTA-PEG8 (6.8 MBq). PET/CT images were performed with NCr nude mice bearing HCT116-SSTR2 tumors. Images are maximum intensity projections and rotated to achieve the best presentation of the tumor; in the 68Ga-NOTA-PEG8-Y3-TATE image, the tumor is circled due to high activity in the bladder.
Table 4.
PET/CT Data of 68Ga and 64Cu NOTA-Y3-TATE and CB-TE2A-Y3-TATE Analogues in HCT116 SSTR2-Positive Tumor-Bearing Mice (n = 2 per group unless otherwise noted)
|
68Ga 1 h |
64Cu 1 h |
64Cu 4 h |
||||
|---|---|---|---|---|---|---|
| SUV | T:M | SUV | T:M | SUV | T:M | |
| NODAGA-Y3-TATE | 2.0 ± 0.1 | 18 ± 4.4 | 3.1 ± 1.3 | 324 ± 438 | 3.1 ± 0.8 | 26 ± 2.1 |
| NOTA-β-Ala-Y3-TATE | 0.81 ± 1.0 | 8.5 ± 7.2 | 4.0 ± 0.6 | 13 ± 4.9 | 3.1 ± 0.5 | 29 ± 34 |
| NOTA-PEG8-Y3-TATE* | 0.48 | 15 | 2.6 ± 0.6 | 8.9 ± 1.6 | 1.9 ± 0.4 | 38 ± 3.4 |
| CB-TE2A-Y3-TATE† | N/A | N/A | 2.2 ± 0.6† | 34 ± 16† | N/A | N/A |
N/A = not available; PET/CT = positron emission tomography/computed tomography; SUV = standardized uptake value; T:M = tumor to muscle ratio.
68Ga image analysis, n = 1.
PET/CT performed at 2 hours.
68Ga-NODAGA-Y3-TATE demonstrated the highest SUV of the 68Ga-labeled analogues (2 ± 0.14). 68Ga-NODAGA-Y3-TATE and 68Ga-NOTA-PEG8-Y3-TATE (n = 1) had similar tumor to muscle ratios compared to 68Ga-NOTA-β-Ala-Y3-TATE (18 ± 4.3, 15 and 9 ± 7.2, respectively). 68Ga-NOTA-PEG8-Y3-TATE showed the lowest tumor uptake of all compounds (SUV = 0.5, n = 1; see Figure 6); however, the 64Cu agent had higher uptake at 1 and 4 hours (2.6 ± 0.6 and 1.9 ± 0.4; see Figure 5).
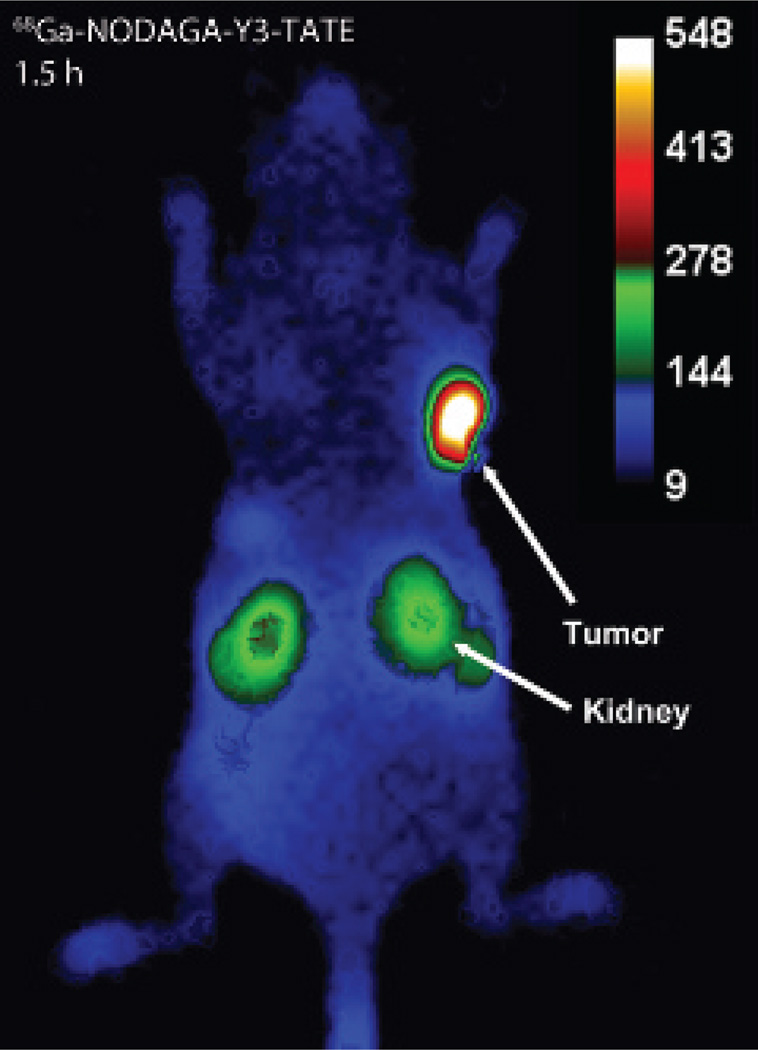
Cerenkov Imaging
To demonstrate the multimodal imaging utility of the three 68Ga-based probes, the HCT116-SSTR2-positive mice were also imaged by Cerenkov imaging in the bioluminescence imaging scanner from 1.5 to 2 hours following PET/CT. In mice imaged with 68Ga-NODAGA-Y3-TATE, the tumor images showed good contrast with minimal background other than the kidneys (Figure 7). Due to the semiquantitative nature of this 2D technique, exact values of uptake could not be calculated, but tumor to muscle ratios were measured through ROI analysis of the planar images generated by the Cerenkov emissions. Following the same trend as PET imaging at 1 hour, 68Ga-NODAGA-Y3-TATE showed a greater tumor to muscle ratio than 68Ga-NOTA-β-Ala-Y3-TATE and 68Ga-NOTA-PEG8-Y3-TATE (11 ± 4, 4.4 ± 7, and 4.4 [n = 1]). It should be noted that Cerenkov imaging was not attempted on mice injected with 64Cu-based probes, because the lower beta energy copper emissions produce significantly less Cerenkov luminescence, and is not optimal for this application.
Figure 7.
Cerenkov imaging 1.5 hours postinjection of 68Ga-NODAGA-Y3-TATE (3.9 MBq) in NCr nude mice bearing HCT116-SSTR2 tumors. Images were acquired for 300 seconds with binning: 8 × 8, F/stop: 1, field of view: 10 cm × 10 cm. The 68Ga-NODAGA-Y3-TATE Cerenkov image was selected due to its superior contrast between the tumor and background compared to the β-Ala and PEG8 analogues.
Discussion
The choice of bifunctional chelator, linker between the chelator and targeting molecule, and radiometal impacts the performance of SSTR-targeted imaging agents. This study focused on the incorporation of NOTA chelators to Y3-TATE, a well-characterized agonist of SSTR2, in comparison with CB-TE2A-Y3-TATE, which we previously showed to have high image contrast for imaging a SSTR2-positive tumor-bearing rat model.27,28 The NOTA chelator was selected due to its ability to stably incorporate both 64Cu and 68Ga. In addition, NODAGA and p-SCN-Bn-NOTA are commercially available analogues of NOTA that when conjugated provide three carboxylates for N3O3 coordination.17–19,35–37 The NODAGA chelator was conjugated directly to the N-terminus of Y3-TATE, resulting in an amide bond. NODAGA-Y3-TATE was previously synthesized, labeled with 68Ga in high specific activity, and evaluated in Rhesus monkey brain sections38,39; however, to our knowledge, 68Ga- or 64Cu-labeled NODAGA-Y3-TATE has not been evaluated in vivo, although NODAGA has been evaluated with the SSTR2 antagonist LM3.21 In a previously reported study, 64Cu-labeled NODAGA and CB-TE2A conjugates of the SSTR2 antagonist LM3 were compared, and the NODAGA analogue was deemed superior. One of the goals of this study was to determine if this trend was similar for the widely used SSTR2 agonist Y3-TATE. In addition, we compared these agents to other NOTA-Y3-TATE conjugates and evaluated them in an SSTR2-transfected human cell line, SSTR2-positive HCT116,29 which can be readily grown in nude mice and is a convenient model for evaluating new radiolabeled SSTR2-targeted agents.
The p-SCN-Bn-NOTA chelate was conjugated using two different linker groups; the linkers are necessary due to the instability of the thiourea bond at the α-amine. The placement of a thiourea directly on the α-amine of a peptide can cause an Edman’s degradation, resulting in removal of the terminal amino acid.40,41 The movement of the thiourea away from the α-amine can increase the stability of the thiourea when conjugated to a peptide. Banks and Paquette demonstrated that the conjugation through the ε-amine of a lysine versus the α-amine was more stable after 10 days at 37°C.42 Cooper and colleagues demonstrated that p-SCN-Bn-NOTA conjugated through the side chain on lysine forms a stable thiourea bond with no difference in the in vivo stability compared to an amide conjugated chelator.19 The β-Ala was selected to maintain a probe similar in size to the NODAGA and CB-TE2A analogues, whereas the PEG8 linker was selected to analyze the effects of linker length on the probe’s performance.
The 64Cu analogues were evaluated in vitro by saturation binding, competitive binding, and internalization assays using HCT116-SSTR2-positive cells. 64Cu/Cu-NODAGA-Y3-TATE (Kd = 0.5 ± 0.1 nM; Ki = 0.6 nM) demonstrated the highest binding affinity, similar to 64Cu-CB-TE2A-Y3-TATE (Kd = 0.5 ± 0.1 nM). The addition of p-Bn-SCN-NOTA through the β-Ala and PEG8 linkages decreased the affinity slightly (Kd = 1.8 ± 0.7 nM [Ki = 1.5 nM] and Kd = 2.3 ± 0.9 nM [Ki = 2.5 nM], respectively). The effect of the linker on affinity is mirrored in the cold gallium analogues as well: Ga-NODAGA-Y3-TATE (Ki = 0.6 nM), Ga-NOTA-β-Ala-Y3-TATE (Ki = 4.0 nM), and Ga-NOTA-PEG8-Y3-TATE (Ki = 16 nM). Similar to the results presented here, Rogers and colleagues noted a decrease in affinity with a PEGylated bombesin analogue compared to analogues with a smaller linker.43 Furthermore, the increase in linker length resulted in a reduced number of binding sites (Bmax) of the 64Cu-labeled analogues. We hypothesize that the larger PEG linkage created steric hindrance for binding of additional Y3-TATE-targeted agents by blocking potential binding pockets of SSTR2. 64Cu-NOTA-PEG8-Y3-TATE had the lowest Bmax, whereas the Bmax of 64Cu-NODAGA-Y3-TATE and 64Cu-NOTA-β-Ala-Y3-TATE were comparable. However, the Bmax of 64Cu-CB-TE2A-Y3-TATE was twofold higher than both analogues. 64Cu-NOTA-PEG8-Y3-TATE also demonstrated the lowest uptake and internalization in SSTR2-transfected HCT116 cells, with 64Cu-NODAGA-Y3-TATE and 64Cu-NOTA-β-Ala-Y3-TATE having similar, more superior internalization profiles. The in vitro results suggest that the linker length between the chelator and the peptide had an impact on their binding affinity and number of bound receptor sites. The use of the smaller β-Ala linker to maintain a similar linker length between the chelator and peptide when compared to direct conjugation appeared to have little to no effect on the in vitro performance of the radiotracers.
Based on the in vitro results we expected that the in vivo performance of NODAGA-Y3-TATE, NOTA-β-Ala-Y3-TATE, and CB-TE2A-Y3-TATE would be similar, whereas larger NOTA-PEG8-Y3-TATE would be less optimal. The tumor uptake and nontargeted organ clearance of 64Cu-NODAGA-Y3-TATE and 64Cu-CB-TE2A-Y3-TATE were comparable; however, 64Cu-CB-TE2A-Y3-TATE had a higher tumor to blood ratio at 4 hours. 64Cu-CB-TE2A-Y3-TATE demonstrated superior tumor to muscle/blood ratios over the NOTA analogues in the biodistribution studies at 1, 4, and 24 hours, except at 1 hour, where the tumor to blood/muscle ratio of 64Cu-NODAGA-Y3-TATE and all tumor to muscle ratios of 64Cu-labeled NOTA analogues at 4 hours were not statistically significant (p ≥ .07). When comparing 64Cu-NODAGA-Y3-TATE and 64Cu-CB-TE2A-Y3-TATE to the -β-Ala and -PEG8 p-SCN-NOTA analogues, it is clear that the length of the linker significantly affected the in vivo performance. The increased linker length of 64Cu-NOTA-PEG8-Y3-TATE likely resulted in significantly higher (p ≤ .05) uptake in the tumor at the 24-hour time point but with higher accumulation in the kidneys and liver at the 4- and 24-hour time points compared to the other analogues. The use of a small linker in 64Cu-NOTA-β-Ala-Y3-TATE demonstrated tumor uptake comparable to 64Cu-NODAGA-Y3-TATE and 64Cu-CB-TE2A-Y3-TATE at 1 hour; however, washout from the tumor occurred rapidly, with a significant reduction in tumor uptake at 4 and 24 hours. The superior in vivo performances of the similar-sized 64Cu-NODAGA-Y3-TATE and 64Cu-CB-TE2A-Y3-TATE suggest that direct conjugation of Y3-TATE to the α-amine provides a favorable interaction with SSTr2, resulting in increased uptake and retention in the SSTR2 tumor. This is further supported by the performance of the α-conjugated 68Ga-NODAGA-Y3-TATE, which had superior tumor to muscle/blood ratios in the biodistribution studies and tumor to muscle ratios in both PET/CT and Cerenkov imaging when compared to the -β-Ala and -PEG8 analogues. It should be noted that there were significant differences between the 64Cu- and 68Ga-labeled analogues with respect to the kidney uptake. We hypothesize that this is a result of differences in the overall charge of these chelated metals (64Cu: −1; 68Ga: neutral). The differences in the kidney uptake should be considered when selecting an imaging agent to minimize the radiation dose delivered to the kidneys. Finally, the choice of the radiometals (64Cu and 68Ga) with the exception of kidney uptake in the evaluation of the Y3-TATE analogues through biodistribution studies, PET/CT, and Cerenkov imaging did not demonstrate a significant impact on in vivo performance of the NOTA analogues.
Conclusion
Commercially available analogues of NOTA were investigated for radiolabeling with 64Cu and 68Ga for SSTR2-targeted PET/CT imaging. The size and method of conjugation had a greater impact on the performance of these SSTR2-targeted PET agents than changes in radiometal, which were determined insignificant. Direct conjugation of a NOTA chelator to the α-amine of Y3-TATE (NODAGA-Y3-TATE) demonstrated superior in vivo performance for 64Cu- and 68Ga-labeled analogues compared to the chelator conjugated through a linker. The in vivo performance of 64Cu-NODAGA-Y3-TATE was comparable to 64Cu-CB-TE2A-Y3-TATE, one of the gold standard agents that has been investigated in other SSTR2-positive tumor models. Although 64Cu-CB-TE2A-Y3-TATE was superior to 64Cu-NODAGA-Y3-TATE in tumor to blood ratios at 4 hours, an advantage of NODAGA-Y3-TATE is that this agent allows for incorporation of both 64Cu and 68Ga, incorporates 64Cu in high radiolabeling yields in a shorter period of time than 64Cu-CB-TE2A-Y3-TATE, and provides a versatile PET probe for imaging SSTR2-positive tumors.
Acknowledgments
We extend our gratitude to Kathryn E. Day for her assistance in the biodistribution and imaging experiments.
Financial disclosure of authors: This research was supported by NIH/National Cancer Institute (NCI) 5R01CA064475 and a joint grant from the Office of Science (Biological and Environmental Research), U.S. Department of Energy, and the NIH (National Institute of Biochemical Imaging and Bioengineering) (DE-SC0008833). Small-animal imaging performed at Washington University is supported in part by NCI Cancer Center Support Grant P30 CA91842. UPCI-shared resources (In Vivo Imaging Facility) were used in this research, and this facility is supported in part by award NCI P30CA047904.
Footnotes
Financial disclosure of reviewers: None reported.
References
- 1.Papotti M, Kumar U, Volante M, et al. Immunohistochemical detection of somatostatin receptor types 1–5 in medullary carcinoma of the thyroid. Clin Endocrinol (Oxf) 2001;54:641–649. doi: 10.1046/j.1365-2265.2001.01175.x. [DOI] [PubMed] [Google Scholar]
- 2.Weiner RE, Thakur ML. Radiolabeled peptides in oncology: role in diagnosis and treatment. BioDrugs. 2005;19:145–163. doi: 10.2165/00063030-200519030-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kulaksiz H, Eissele R, Rossler D, et al. Identification of somatostatin receptor subtypes 1, 2a, 3, and 5 in neuroendocrine tumours with subtype specific antibodies. Gut. 2002;50:52–60. doi: 10.1136/gut.50.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reubi JC, Waser B, Schaer JC, et al. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med. 2001;28:836–8/46. doi: 10.1007/s002590100541. [DOI] [PubMed] [Google Scholar]
- 5.Janson ET, Stridsberg M, Gobl A, et al. Determination of somatostatin receptor subtype 2 in carcinoid tumors by immunohistochemical investigation with somatostatin receptor subtype 2 antibodies. Cancer Res. 1998;58:2375–2378. [PubMed] [Google Scholar]
- 6.Hofland LJ, Liu Q, Van Koetsveld PM, et al. Immunohistochemical detection of somatostatin receptor subtypes sst1 and sst2a in human somatostatin receptor positive tumors. J Clin Endocrinol Metab. 1999;84:775–780. doi: 10.1210/jcem.84.2.5497. [DOI] [PubMed] [Google Scholar]
- 7.Schulz S, Schmitt J, Quednow C, et al. Immunohistochemical detection of somatostatin receptors in human ovarian tumors. Gynecol Oncol. 2002;84:235–240. doi: 10.1006/gyno.2001.6468. [DOI] [PubMed] [Google Scholar]
- 8.Schulz S, Pauli SU, Handel M, et al. Immunohistochemical determination of five somatostatin receptors in meningioma reveals frequent overexpression of somatostatin receptor subtype sst2a. Clin Cancer Res. 2000;6:1865–1874. [PubMed] [Google Scholar]
- 9.Kikutsuji T, Harada M, Tashiro S, et al. Expression of somatostatin receptor subtypes and growth inhibition in human exocrine pancreatic cancers. J Hepatobiliary Pancreat Surg. 2000;7:496–503. doi: 10.1007/s005340070021. [DOI] [PubMed] [Google Scholar]
- 10.Anderson CJ, Dehdashti F, Cutler PD, et al. 64Cu-TETA-octreotide as a PET imaging agent for patients with neuroendocrine tumors. J Nucl Med. 2001;42:213–221. [PubMed] [Google Scholar]
- 11.Rogers BE, McLean SF, Kirkman RL, et al. In vivo localization of [(111)In]-DTPA-D-phe1-octreotide to human ovarian tumor xenografts induced to express the somatostatin receptor subtype 2 using an adenoviral vector. Clin Cancer Res. 1999;5:383–393. [PubMed] [Google Scholar]
- 12.Wang ZZ, Qu W, Wang F, et al. Expression of somatostatin receptor reporter gene and its correlation with targeted imaging in vivo for detection of pancreas carcinoma. Zhonghua Zhong Liu Za Zhi. 2005;27:663–666. [PubMed] [Google Scholar]
- 13.Baum EM, Knox HD, Miller TR, Watson AM. Nuclides and isotopes chart of the nuclides. Schenectady (NY): Knolls Atomic Power Lab; 2009. [Google Scholar]
- 14.Fani M, Andre JP, Maecke HR. Ga-68-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol Imaging. 2008;3:53–63. doi: 10.1002/cmmi.232. [DOI] [PubMed] [Google Scholar]
- 15.Beattie BJ, Thorek DLJ, Schmidtlein CR, et al. Quantitative modeling of Cerenkov light production efficiency from medical radionuclides. PLoS One. 2012;7:e31402. doi: 10.1371/journal.pone.0031402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dothager RS, Goiffon RJ, Jackson E, et al. Cerenkov radiation energy transfer (CRET) imaging: a novel method for optical imaging of PET isotopes in biological systems. PLoS One. 2010;5:e13300. doi: 10.1371/journal.pone.0013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgado R, Sun YZ, Motekaitis RJ, et al. Stabilities of divalent and trivalent metal-ion complexes of macrocyclic triazatriacetic acids. Inorg Chem. 1993;32:3320–3326. [Google Scholar]
- 18.Eisenwiener KP, Prata MI, Buschmann I, et al. NODAGATOC, a new chelator-coupled somatostatin analogue labeled with [67/68Ga] and [111In] for SPECT, PET, and targeted therapeutic applications of somatostatin receptor (hsst2) expressing tumors. Bioconjug Chem. 2002;13:530–541. doi: 10.1021/bc010074f. [DOI] [PubMed] [Google Scholar]
- 19.Cooper MS, Ma MT, Sunassee K, et al. Comparison of (64)Cu-complexing bifunctional chelators for radioimmunoconjugation: labeling efficiency, specific activity, and in vitro/in vivo stability. Bioconjug Chem. 2012;23:1029–1039. doi: 10.1021/bc300037w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin M, Welch MJ, Lapi SE. Effects of chelator modifications on Ga-labeled [tyr ]octreotide conjugates. Mol Imaging Biol. 2013;15:606–613. doi: 10.1007/s11307-013-0627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fani M, Del Pozzo L, Abiraj K, et al. PET of somatostatin receptor-positive tumors using 64Cu- and 68Ga-somatostatin antagonists: the chelate makes the difference. J Nucl Med. 2011;52:1110–1118. doi: 10.2967/jnumed.111.087999. [DOI] [PubMed] [Google Scholar]
- 22.Alonso O, Gambini JP, Lago G, et al. In vivo visualization of somatostatin receptor expression with Ga-68-DOTA-TATE PET/CT in advanced metastatic prostate cancer. Clin Nucl Med. 2011;36:1063–1064. doi: 10.1097/RLU.0b013e31822920c9. [DOI] [PubMed] [Google Scholar]
- 23.Klinaki I, Al-Nahhas A, Soneji N, et al. 68Ga DOTATATE PET/CT uptake in spinal lesions and MRI correlation on a patient with neuroendocrine tumor: potential pitfalls. Clin Nucl Med. 2013;38:e449–e453. doi: 10.1097/RLU.0b013e31827a2325. [DOI] [PubMed] [Google Scholar]
- 24.Naji M, Zhao C, Welsh SJ, et al. 68Ga-DOTA-TATE PET vs. 123I-MIBG in identifying malignant neural crest tumours. Mol Imaging Biol. 2011;13:769–775. doi: 10.1007/s11307-010-0396-8. [DOI] [PubMed] [Google Scholar]
- 25.Thomas T, Zender S, Terkamp C, et al. Hypercortisolaemia due to ectopic adrenocorticotropic hormone secretion by a nasal para-ganglioma: a case report and review of the literature. BMC Res Notes. 2013;6:331. doi: 10.1186/1756-0500-6-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virgolini I, Ambrosini V, Bomanji JB, et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2010;37:2004–2010. doi: 10.1007/s00259-010-1512-3. [DOI] [PubMed] [Google Scholar]
- 27.Wadas TJ, Eiblmaier M, Zheleznyak A, et al. Preparation and biological evaluation of 64Cu-CB-TE2A-sst2-ANT, a somatostatin antagonist for PET imaging of somatostatin receptor-positive tumors. J Nucl Med. 2008;49:1819–1827. doi: 10.2967/jnumed.108.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprague JE, Peng Y, Sun X, et al. Preparation and biological evaluation of copper-64-labeled Tyr3-octreotate using a cross-bridged macrocyclic chelator. Clin Cancer Res. 2004;10:8674–8682. doi: 10.1158/1078-0432.CCR-04-1084. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen K, Parry JJ, Rogers BE, et al. Evaluation of copper-64-labeled somatostatin agonists and antagonist in sstr2-transfected cell lines that are positive and negative for p53: implications for cancer therapy. Nucl Med Biol. 2012;39:187–197. doi: 10.1016/j.nucmedbio.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achilefu S, Wilhelm RR, Jimenez HN, et al. A new method for the synthesis of tri-tert-butyl diethylenetriaminepentaacetic acid and its derivatives. J Org Chem. 2000;65:1562–1565. doi: 10.1021/jo991453t. [DOI] [PubMed] [Google Scholar]
- 31.Anderson CJ, Jones LA, Bass LA, et al. Radiotherapy, toxicity and dosimetry of copper-64-TETA-octreotide in tumor-bearing rats. J Nucl Med. 1998;39:1944–1951. [PubMed] [Google Scholar]
- 32.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 33.Shokeen M, Zheleznyak A, Wilson JM, et al. Molecular imaging of very late antigen-4 (alpha4beta1 integrin) in the premetastatic niche. J Nucl Med. 2012;53:779–786. doi: 10.2967/jnumed.111.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi J, Leahy RM. Resolution and noise properties of map reconstruction for fully 3-D PET. IEEE Trans Med Imaging. 2000;19:493–506. doi: 10.1109/42.870259. [DOI] [PubMed] [Google Scholar]
- 35.Eric T Clarke AEM. Stabilities of the Fe(III), Ga(III) and In(III) chelates of N,N,N′-triazacyclononanetriacetic acid. Inorg Chim Acta. 1991;181:273–280. [Google Scholar]
- 36.Martell AE, Motekaitis RJ, Clarke ET, et al. Stability constants of metal complexes of macrocyclic ligands with pendant donor groups. Supramol Chem. 1996;6:353–363. [Google Scholar]
- 37.Bevilacqua A, Gelb RI, Hebard WB, et al. Equilibrium and thermodynamic study of the aqueous complexation of 1,4,7-triazacyclononane-N,N′,N′-triacetic acid with protons, alkaline-earth-metal cations, and copper(II) Inorg Chem. 1987;26:2699–2706. [Google Scholar]
- 38.Velikyan I, Beyer GJ, Bergstrom-Pettermann E, et al. The importance of high specific radioactivity in the performance of 68Ga-labeled peptide. Nucl Med Biol. 2008;35:529–536. doi: 10.1016/j.nucmedbio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Velikyan I, Maecke H, Langstrom B. Convenient preparation of 68Ga-based PET-radiopharmaceuticals at room temperature. Bioconjug Chem. 2008;19:569–573. doi: 10.1021/bc700341x. [DOI] [PubMed] [Google Scholar]
- 40.Edman P. On the mechanism of the phenyl isothiocyanate degradation of peptides. Acta Chem Scand. 1956;10:761–768. [Google Scholar]
- 41.Edman P. Mechanism of the phenyl isothiocyanate degradation of peptides. Nature. 1956;177:667–668. [Google Scholar]
- 42.Banks PR, Paquette DM. Comparison of 3 common amine reactive fluorescent-probes used for conjugation to biomolecules by capillary zone electrophoresis. Bioconjug Chem. 1995;6:447–458. doi: 10.1021/bc00034a015. [DOI] [PubMed] [Google Scholar]
- 43.Rogers BE, Manna DD, Safavy A. In vitro and in vivo evaluation of a 64Cu-labeled polyethylene glycol-bombesin conjugate. Cancer Biother Radiopharm. 2004;19:25–34. doi: 10.1089/108497804773391649. [DOI] [PubMed] [Google Scholar]