Abstract
This study observes the therapeutic detoxification of quercetin, a well-known flavonoid, against carbon tetrachloride (CCl4) induced acute liver injury in vivo and explores its mechanism. Quercetin decreased CCl4-increased serum activities of alanine and aspartate aminotransferases (ALT/AST) when orally taken 30 min after CCl4 intoxication. The results of a histological evaluation further evidenced the ability of quercetin to protect against CCl4-induced liver injury. Quercetin decreased the CCl4-increased malondialdehyde (MDA) and reduced the glutathione (GSH) amounts in the liver. It also reduced the enhanced immunohistochemical staining of the 4-hydroxynonenal (4-HNE) in the liver induced by CCl4. Peroxiredoxin (Prx) 1, 2, 3, 5, 6, thioredoxin reductase 1 and 2 (TrxR1/2), thioredoxin 1 and 2 (Trx1/2), nuclear factor erythroid 2-related factor 2 (Nrf2), and heme oxygenase-1 (HO-1) all play critical roles in maintaining cellular redox homeostasis. Real-time polymerase chain reaction (PCR) results demonstrated that quercetin reversed the decreased mRNA expression of all those genes induced by CCl4. In conclusion, our results demonstrate that quercetin ameliorates CCl4-induced acute liver injury in vivo via alleviating oxidative stress injuries when orally taken after CCl4 intoxication. This protection may be caused by the elevation of the antioxidant capacity induced by quercetin.
Keywords: Hepatotoxicity, Oxidative stress, Peroxiredoxin (Prx), Nuclear factor erythroid 2-related factor 2 (Nrf2), TrxR, Trx, HO-1
1. Introduction
Flavonoids are widely distributed through red wine, fruits, vegetables, herbal tea and medicines. As flavonoids have various pharmacological activities, such as antioxidant, anti-inflammation, and anti-tumor, the nutritional value of flavonoids is well-known to people (Hollman and Katan, 1999; Park and Pezzuto, 2012). Some epidemiological surveys report that a negative correlation can be observed between dietary intake of flavonoids and cardiovascular disease or cancer (Muldoon and Kritchevsky, 1996; Yang et al., 2001). However, nutraceuticals, including flavonoids, may also have toxic effects depending on the dose and the redox status of the cell, which is also called as hormesis (Brambilla et al., 2008; Calabrese et al., 2010). Quercetin is a well-known flavonoid, and it is widely distributed in various Chinese medicinal herbs, such as Flos sophorae Immaturus (Kelly, 2011; Wu et al., 2012). Various reports show that quercetin has neuro-protection, anti-tumor, anti-inflammation, anti-oxidant, and anti-diabetic effects (Chirumbolo, 2010; Kelly, 2011; Dajas, 2012). Furthermore, some reports demonstrate that quercetin can ameliorate various toxin-induced liver injuries, such as ethanol and acetaminophen (Vicente-Sánchez et al., 2008; Liu et al., 2010; Yousef et al., 2010; de David et al., 2011; Domitrović et al., 2012). However, the concrete-engaged signals in regulating the protection of quercetin against liver injuries are not very clear.
Carbon tetrachloride (CCl4), a classic hepatotoxin, has been widely used in the industry. CCl4 can enter the body of humans and animals primarily through the respiratory and digestive tracts. Weber et al. (2003) reported that CCl4 could lead to a lipid peroxidation of unsaturated fatty membrane and organelle membrane after its metabolic activation by hepatic microsomal cytochrome P450, and thus lead to serious hepatocytes damage. CCl4-induced liver injury is one of the most widely used experimental models for exogenous toxins-induced hepatotoxicity (Ratziu and Zelber-Sagi, 2009; Chen et al., 2012).
It have already demonstrated that pretreatment of mice or rats with quercetin for five consecutive days can prevent CCl4-induced acute liver injury (Janbaz et al., 2004; Domitrović et al., 2012; Cui et al., 2014). However, whether quercetin can also alleviate CCl4-induced acute liver injury, when it is given after intraperitoneal (i.p.) injection of CCl4, is unknown.
Peroxiredoxins (Prxs) and thioredoxins (Trxs) are important liver antioxidant proteins and play important roles in maintaining liver redox homeostasis (Watson et al., 2004; Rhee et al., 2005). The present study is designed to observe the therapeutic detoxification of quercetin against CCl4-induced liver injury when it is orally given after CCl4 intoxication, and to further investigate the underlying mechanisms via focusing on Prxs, Trxs, and other antioxidant signals.
2. Materials and methods
2.1. Drugs and reagents
Quercetin was purchased from Sigma (USA). Kits for analysis of alanine and aspartate aminotransferases (ALT/AST) and malondialdehyde (MDA) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Anti-4-hydroxynonenal (4-HNE) antibody was purchased from Abcam (Cambridge, UK). The DAKO EnVision™ detection system was purchased from DAKO Corporation (USA). The Trizol reagent was purchased from Life Technology (USA). PrimeScript RT master mix and SYBR Premix Ex Taq™ were all purchased from TaKaRa (Japan). Other reagents, unless noted, were purchased from Sigma (USA).
2.2. Experimental animals
Specific pathogen free male ICR mice (18–22 g) were purchased from the Shanghai Laboratory Animal Center of Chinese Academy of Science (Shanghai, China). Animals were fed with a standard laboratory diet and given free access to tap water. The animal room was maintained at (22±1) °C and (65±5)% humidity, with a 12 h:12 h light-dark cycle. The animals received humane care in compliance with the institutional animal care guidelines approved by the Experimental Animal Ethical Committee of Shanghai University of Traditional Chinese Medicine, China.
2.3. Treatment of animals
Quercetin was dissolved in a sodium hydroxide solution and kept in a dark storage area. The solution was adjusted to pH 9.0. CCl4 was dissolved in olive oil to a 25% concentration.
Mice were randomly divided into four groups with eight mice each. The vehicle group (control) was first injected with olive oil i.p., and 30 min later was orally given a pH 9.0 vehicle solution. The CCl4-treated group was injected with CCl4 (15 ml/kg; i.p.), and 30 min later was orally given a pH 9.0 vehicle solution. The quercetin-treated groups were first injected with CCl4 solution i.p., and 30 min later were orally administered with quercetin 20 or 80 mg/kg, respectively. Mice were sacrificed 24 h after CCl4 injection, and plasma and livers were collected.
2.4. Analysis of ALT/AST activities
Fresh blood was obtained from mice in the four groups and put at room temperature for 60 min to clot. Serum was isolated following the centrifugation at 840g for 15 min. Serum ALT and AST activities were determined by kits according to the manufacturer’s instructions.
2.5. Histological examination
Live slices were fixed in 10% phosphate-buffered saline (PBS)-formalin and embedded in paraffin. Liver samples were subsequently sectioned (5 μm), stained with hematoxylin and eosin (H&E), and examined under a microscope (Olympus, Japan) to evaluate liver injuries.
2.6. Determination of tissue lipid peroxidation (LPO)
Liver tissues were homogenized in cold PBS. LPO was determined according to the method described by Högberg et al. (1974). MDA was formed as the product of LPO and served as an index for LPO. MDA reacts with 2-thiobarbituric acid to generate a pink-colored product, which has an absorbance at 532 nm. The MDA level was expressed as μmol/mg protein based on liver protein concentration.
2.7. Determination of liver glutathione (GSH)
The amounts of liver GSH and oxidative GSH (GSSG) were measured as described in our previous report (Liang et al., 2011).
2.8. Immunohistochemical staining of 4-HNE
Paraffin-embedded liver sections (5 μm) were first deparaffinized in xylene, and then rehydrated in a gradient of ethanol to distilled water. After quenching the endogenous peroxidase activity using 3% hydrogen peroxide, liver sections were further incubated with 5% bovine serum albumin to alleviate nonspecific binding, and then incubated with 4-HNE antibody at 4 °C overnight. The antigen-antibody reactions were detected using DAKO EnVision™ detection kits. All sections were counterstained with hematoxylin. Pictures were taken with a microscope (Nikon, Japan). Liver expression of 4-HNE was determined by image information object definition (IOD) values analyzed by Image-Pro Plus 6.0 (Media Cybernetics, USA).
2.9. Real-time polymerase chain reaction (PCR)
The total liver RNA was extracted by Trizol reagent as described by the manufacturer’s protocol. Complementary DNA (cDNA) was synthesized according to the instruction of the PrimeScript RT master mix kits. Real-time PCR was performed with STEPONE Plus (Carlsbad, CA) by a SYBR green premix according to the manufacturer’s guide. Relative expressions of target genes were standardized to β-actin, evaluated by the 2−ΔΔCT method and given as a ratio to control the experiments. The primer sequences used in this study are shown in Table 1.
Table 1.
List of primers for real-time PCR
| Target | Primer sequence (5'→3') |
| Prx1 | F: CACCCAAGAAACAAGGAGGA |
| R: AAAAAGGCCCCTGAAAGAGA | |
| Prx2 | F: TGATGAGGGCATTGCTTACAGG |
| R: GAGCGTCCCACAGGTAGGTCAT | |
| Prx3 | F: TTCCCACTTCAGTCATCTTGCC |
| R: ATGCCAGCACTTTCCAACAGC | |
| Prx4 | F: TTCCTGTTGCGGACCGAAT |
| R: TACACTTGTCCACCAGCGTAGAA | |
| Prx5 | F: GGAAGGCGACAGACTTATTATTGG |
| R: CCTTCACTATGCCGTTGTCTATCAC | |
| Prx6 | F: GCACCACAGAACTTGGCAGAG |
| R: CCAGGCAAGATGATCCTCAACA | |
| Trx1 | F: TCCCTCCCCGCAACAGCCAA |
| R: ACCGGAGAACTCCCCCACCTTT | |
| Trx2 | F: TCGCCAAGCAGCACGGGAAG |
| R: CGGTAGGCACAGCTGACACCTCA | |
| TrxR1 | F: TCGGCTCGCTGAACTGGGG |
| R: GCGATGA GGAACCGCTCTGCTG | |
| TrxR2 | F: GATCGGTGGGGGATCCGGTG |
| R: CCCACTTGGTGCCTCGGGGAG | |
| HO-1 | F: TCCCGAACATCGACAGCCCCA |
| R: AGGGGCAGTATCTTGCACCAGG | |
| Nrf2 | F: TCTCCTCGCTGGAAAAAGAA |
| R: AATGTGCTGGCTGTGCTTTA | |
| Actin | F: TTCGTTGCCGGTCCACACCC |
| R: GCTTTGCACATGCCGGAGCC |
F: forward primer; R: reverse primer
2.10. Statistical analysis
All experimental data were expressed as mean±standard error of the mean (SEM). Significant differences were determined by one-way analysis of variance (ANOVA). P<0.05 was considered as a statistically significant difference.
3. Results
3.1. Quercetin ameliorates CCl4-induced acute liver injury
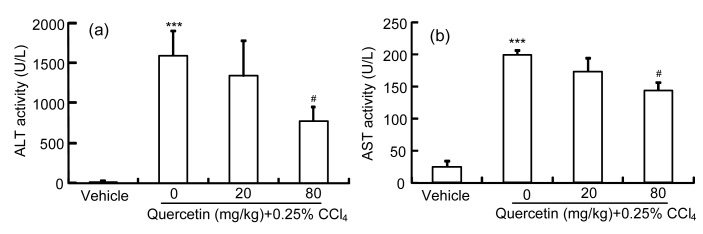
Serum activities of ALT and AST were increased in the CCl4 group compared with the control group (P<0.001; Fig. 1). After treatment with quercetin (80 mg/kg), CCl4-increased ALT and AST activities were decreased (P<0.05 and P<0.01, respectively). Quercetin (20 mg/kg) had no effect on CCl4-increased serum ALT or AST activity. In contrast to the control group, the mice in the CCl4 group showed serious liver damage, indicated by obvious hepatocyte necrosis and shrinkage nuclei (Fig. 2b). After treatment with quercetin (20 and 80 mg/kg), these abnormal changes were all alleviated (Figs. 2c and 2d).
Fig. 1.
Serum ALT (a) and AST (b) activities in mice
Data are shown as mean±SEM (n=8). *** P<0.001 vs. control; # P<0.05 vs. CCl4-treated group
Fig. 2.
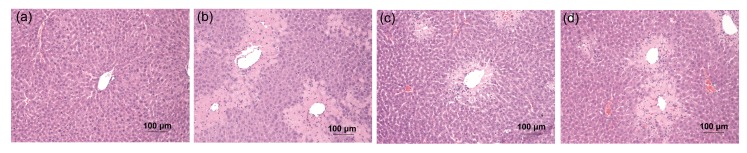
Histological evaluation of liver by H&E staining
(a) Vehicle group; (b) CCl4 group; (c) CCl4+20 mg/kg quercetin group; (d) CCl4+80 mg/kg quercetin group
3.2. Quercetin alleviates CCl4-induced liver oxidative injury
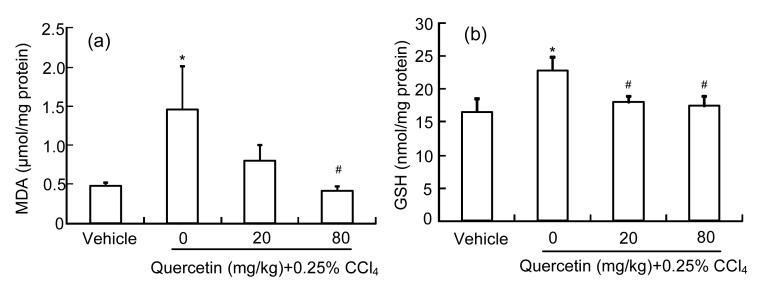
As the product of LPO, the MDA amount can reflect the liver LPO level (Högberg et al., 1974). Fig. 3a shows that the liver MDA amount increased significantly (P<0.05) in the CCl4-treated mice when compared with the control group. Quercetin (80 mg/kg) reduced the increased MDA amount induced by CCl4 (P<0.05). Quercetin (20 mg/kg) also reduced the increased MDA amount, but it showed no significant difference when compared with the CCl4 group (P>0.05).
Fig. 3.
Liver MDA (a) and GSH (b) amounts in mice
Data are shown as mean±SEM (n=8). * P<0.05 vs. control; # P<0.05 vs. CCl4-treated group
GSH is a cellular important antioxidant and it can protect cells against reactive oxygen species (ROS)-induced liver injury (Yuan and Kaplowitz, 2009). As shown in Fig. 3b, the liver GSH level in CCl4-treated mice was enhanced compared with the control group (P<0.05). Quercetin (20 or 80 mg/kg) decreased the CCl4-increased liver GSH level (P<0.05).
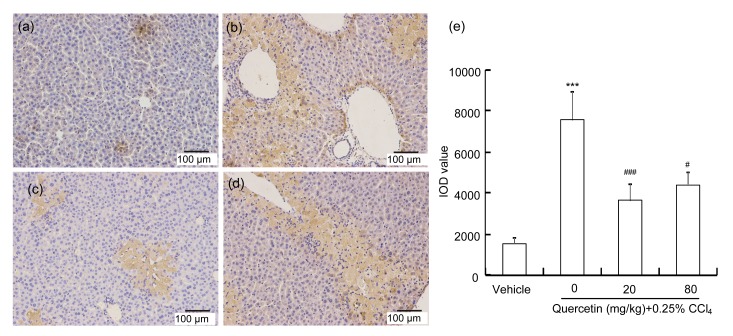
In addition, our results showed that the number of 4-HNE-stained hepatocytes increased in the CCl4-treated mice (Fig. 4b). Quercetin (20 or 80 mg/kg) reduced the CCl4-increased 4-HNE-stained cells (Figs. 4c and 4d). The statistical result in Fig. 4e showed that CCl4 enhanced the density of the immunohistochemical staining of 4-HNE (P<0.001), while quercetin (20 and 80 mg/kg) decreased this increase induced by CCl4 (P<0.001 and P<0.05, respectively).
Fig. 4.
Immunohistochemical staining of 4-HNE in mice livers and IOD values of the density of 4-HNE
Typical images selected from each group: (a) vehicle control; (b) CCl4; (c) CCl4+20 mg/kg quercetin; (d) CCl4+80 mg/kg quercetin. (e) IOD values of the density of 4-HNE staining analyzed in every section of at least three random fields. Data are expressed as mean(SEM (n=4). *** P<0.001 vs. control; # P<0.05, ### P<0.001 vs. CCl4-treated group
3.3. Effects of quercetin on Prx 1–6 mRNA expression
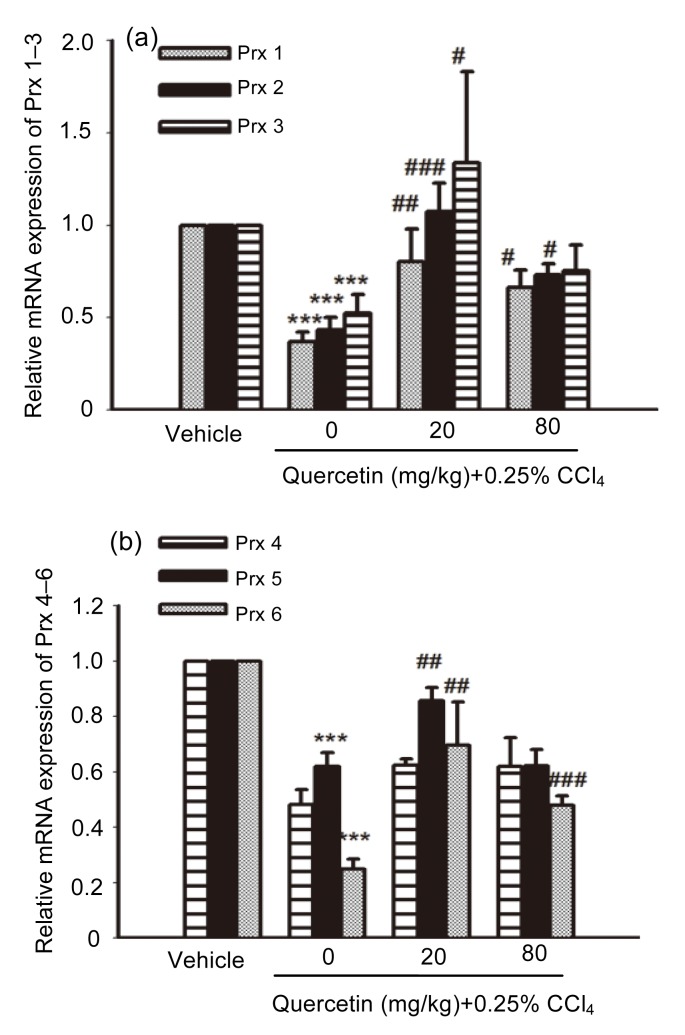
As shown in Fig. 5, the liver mRNA expressions of Prx 1–3, 5, 6 in CCl4-treated mice all decreased remarkably compared with the control group (P<0.001). After treatment with quercetin (20 mg/kg), the mRNA expressions of Prx 1, Prx 2, Prx 3, Prx 5, and Prx 6 all increased when compared with the CCl4-treated mice (P<0.01, P<0.001, P<0.05, P<0.01, and P<0.01, respectively). The mRNA expressions of Prx 1, Prx 2, and Prx 6 in 80 mg/kg quercetin-treated mice also increased when compared with the CCl4-treated mice (P<0.05, P<0.05, and P<0.001, respectively).
Fig. 5.
mRNA expressions of Prx 1–3 (a) and Prx 4–6 (b) in mice livers
Data are shown as mean±SEM (n=5–6). *** P<0.001 vs. control; # P<0.05, ## P<0.01, ### P<0.001 vs. CCl4-treated mice
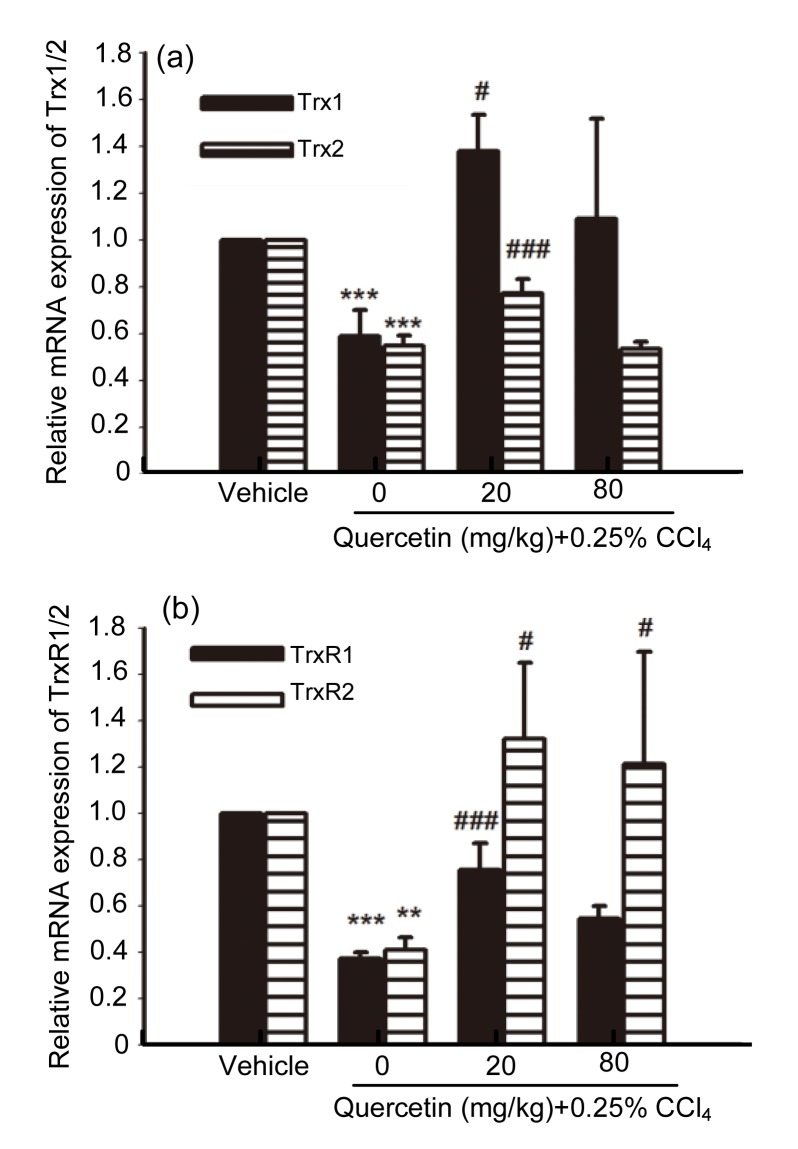
3.4. Effects of quercetin on Trx1/2 and TrxR1/2 mRNA expression
The liver mRNA expressions of other antioxidant enzymes, such as Trx1/2 and TrxR1/2, all decreased in the CCl4-treated mice when compared with the control group (P<0.01 and P<0.001, respectively) (Fig. 6). After treatment with 20 mg/kg quercetin, the mRNA expressions of Trx1/2 and TrxR1/2 were all elevated when compared with the CCl4-treated mice (P<0.05 and P<0.001, respectively). After treatment with 80 mg/kg quercetin, the mRNA expression of TrxR2 increased when compared with the CCl4-treated mice (P<0.05).
Fig. 6.
mRNA expressions of Trx1/2 (a) and TrxR1/2 (b) in mice livers
Data are shown as mean±SEM (n=5–6). ** P<0.01,*** P<0.001 vs. control; # P<0.05, ### P<0.001 vs. CCl4-treated mice
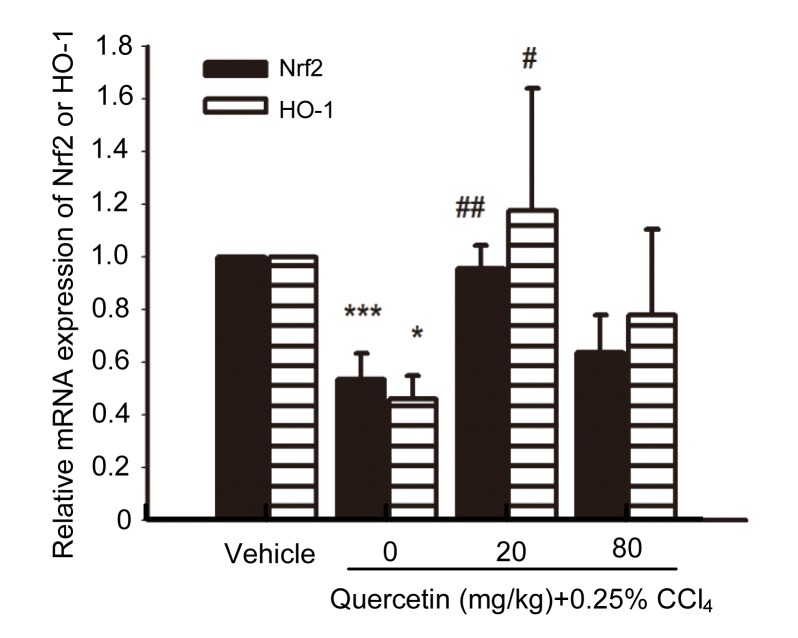
3.5. Effects of quercetin on the mRNA expression of HO-1 and nuclear factor erythroid 2-related factor 2 (Nrf2)
The liver mRNA expressions of HO-1 and Nrf2 in CCl4-treated mice decreased when compared with the control group (P<0.001 and P<0.05, respectively) (Fig. 7). After treatment with 20 mg/kg quercetin, the liver mRNA expressions of HO-1 and Nrf2 increased when compared with the CCl4-treated mice (P<0.01 and P<0.05, respectively).
Fig. 7.
Liver mRNA expressions of HO-1 and Nrf2
Data are shown as mean±SEM (n=5–6). * P<0.05, *** P<0.001 vs. control; # P<0.05, ## P<0.01 vs. CCl4-treated mice
4. Discussion
CCl4-induced acute liver injury in mice is widely used as an experimental animal model for screening hepato-protective drugs (Morio et al., 2001; Yuan et al., 2008). Some previous studies have already demonstrated that quercetin can prevent CCl4-induced acute liver injury when given several days before CCl4 intoxication (Janbaz et al., 2004; Domitrović et al., 2012; Cui et al., 2014). From the results of ALT/AST analysis and histological observations, our study demonstrates that quercetin can ameliorate CCl4-induced acute liver injury when it is given 30 min after CCl4 intoxication. Our study shows the potential therapeutic effects of quercetin against CCl4-induced acute liver injury, and its scientific significance is far more important than indicated in previous reports.
Generally, oxidative stress liver injuries are the main reason for CCl4-induced hepatotoxicity. 4-HNE and MDA are both the end-products of LPO (Högberg et al., 1974; Vatsyayan et al., 2011). Our results of liver MDA level and immunohistochemical staining of 4-HNE show that quercetin can decrease CCl4-induced LPO, which indicates that quercetin can ameliorate CCl4-induced liver oxidative injuries. GSH is the key antioxidant in liver, and its homeostasis is associated with various toxin-induced liver injuries (Kretzschmar and Klinger, 1990; Han et al., 2006). Further results show that CCl4 increased the cellular GSH level, which may contribute to the elevation of the body’s defensive capacity to protect CCl4-induced oxidative stress injuries. After treatment with quercetin, the increased liver GSH level was reduced, which suggests that oxidative stress injuries were ameliorated. There are already existing reports that state that quercetin has a free radical scavenging activity (Pascoal et al., 2011; di Meo et al., 2013), so we can speculate that quercetin itself may have the capacity to scavenge free radicals, and thus substitute for the function of GSH, which leads to a decrease of GSH levels in quercetin-treated mice.
The Prx family contains Prx 1–6, which are all antioxidant enzymes and have the capacity of scavenging free radicals in cells (Rhee et al., 2005). Previous reports have demonstrated the changed expression of the Prx family in exogenous toxin-induced liver injuries such as alcoholic liver injury and pyrazole-induced liver injury (Cohen et al., 2011; Bae et al., 2012). In addition, Hong et al. (2011) reported that an alkaline water named ENA actimineral resource A (ENA-A) could prevent CCl4-induced liver injury, and Prx1 is an increased component in the ENA-A group. Our results showed that the expressions of Prx 1–6 mRNAs in the CCl4-treated group all decreased when compared with the vehicle group. Except Prx 4, the mRNA expressions of all the other members of the Prx family increased in quercetin-treated mice. Our study suggests that quercetin can ameliorate liver oxidative injuries via elevating the expressions of Prx 1, 2, 3, 5, 6.
Trxs, including Trx1 and Trx2, are cellular important antioxidant proteins (Watson et al., 2004). Trx1/2 and Trx reductase 1/2 (TrxR1/2) constitute the Trx system, which plays a partially overlapping and highly complementary role to the GSH antioxidant system, and contributes to the maintenance of the cellular reducing environment (Williams et al., 2000). Our results show that the mRNA expressions of Trx1/2 and TrxR1/2 decreased in the CCl4-treated mice, but this decrease was reversed in the quercetin-treated mice. These results suggest that quercetin can ameliorate liver oxidative injuries via elevating the expression of the Trx system.
HO-1 is the inducible isoform of heme oxygenase, and it is reported to protect acute liver injuries (Sass et al., 2012). Our results showed that the mRNA expression of HO-1 decreased in the CCl4-treated mice, but this decrease was reversed in the quercetin-treated mice. This result suggests that the increased HO-1 expression may contribute to the alleviation of quercetion against CCl4-induced liver injury. It is well-known that HO-1/biliverdin reductase axis catalyzes the degradation of heme, and it can also reduce biliverdin to bilirubin, which is a free radical scavenger and exerts cytoprotective effects (Barone et al., 2009; Mancuso and Barone, 2009; Mancuso et al., 2012). Whether quercetin will affect the amounts of liver bilirubin needs further investigation.
Nrf2 is a critical transcription factor for regulating the expression of some antioxidant enzymes, such as HO-1, Prxs, and Trx1 (Young et al., 2010; Yang et al., 2011; Ke et al., 2013). Our results show that the mRNA expression of Nrf2 decreased in the CCl4-treated mice, but this decrease was reversed in the quercetin-treated mice. The above results suggest that CCl4 can regulate the expression of Nrf2 and quercetin can ameliorate the liver oxidative injuries via elevating the expression of Nrf2, which may contribute to the increased expression of its downstream antioxidant enzymes. Moreover, the increased expressions of these antioxidant enzymes by quercetin may not provide the appropriate regulation through Nrf2, which needs further investigation.
In conclusion, the present study demonstrates the therapeutic detoxification of quercetin against CCl4-induced acute liver injury in vivo. Our results show that quercetin can ameliorate CCl4-induced liver injury through inhibiting oxidative stress injury via elevating the expressions of the antioxidant transcription factor Nrf2 and some antioxidant enzymes, such as HO-1, Prx 1–3, Prx 5–6, Trx1/2, and TrxR1/2.
Footnotes
Project supported by the “Shu Guang” Project from Shanghai Municipal Education Commission and Shanghai Education Development Foundation (No. 13SG43), the National Natural Science Foundation of China (No. 81322053), and the Program for New Century Excellent Talents in University (No. NCET-11-1054), China
Compliance with ethics guidelines: Jia-qi ZHANG, Liang SHI, Xi-ning XU, Si-chong HUANG, Bin LU, Li-li JI, and Zheng-tao WANG declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Bae SH, Sung SH, Lee HE, et al. Peroxiredoxin III and sulfiredoxin together protect mice from pyrazole-induced oxidative liver injury. Antioxid Redox Signal. 2012;17(10):1351–1361. doi: 10.1089/ars.2011.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barone E, Trombino S, Cassano R, et al. Characterization of the S-denitrosylating activity of bilirubin. J Cell Mol Med. 2009;13(8b):2365–2375. doi: 10.1111/j.1582-4934.2008.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brambilla D, Mancuso C, Scuderi MR, et al. The role of antioxidant supplement in immune system, neoplastic, and neurodegenerative disorders: a point of view for an assessment of the risk/benefit profile. Nutr J. 2008;7(1):29. doi: 10.1186/1475-2891-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabrese V, Cornelius C, Trovato-Salinaro A, et al. The hormetic role of dietary antioxidants in free radical-related diseases. Curr Pharm Des. 2010;16(7):877–883. doi: 10.2174/138161210790883615. [DOI] [PubMed] [Google Scholar]
- 5.Chen P, Wang Z, Zeng L, et al. Protective effects of salecan against carbon tetrachloride-induced acute liver injury in mice. J Appl Toxicol. 2012;32(10):796–803. doi: 10.1002/jat.1694. [DOI] [PubMed] [Google Scholar]
- 6.Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm Allergy Drug Targets. 2010;9(4):263–285. doi: 10.2174/187152810793358741. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JI, Chen X, Nagy LE. Redox signaling and the innate immune system in alcoholic liver disease. Antioxid Redox Signal. 2011;15(2):523–534. doi: 10.1089/ars.2010.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui YM, Han Y, Yang XB, et al. Protective effects of quercetin and quercetin-5',8-disulfonate against carbon tetrachloride-caused oxidative liver injury in mice. Molecules. 2014;19(1):291–305. doi: 10.3390/molecules19010291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dajas F. Life or death: neuroprotective and anticancer effects of quercetin. J Ethnopharmacol. 2012;143(2):383–396. doi: 10.1016/j.jep.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 10.de David C, Rodrigues G, Bona S, et al. Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol Pathol. 2011;39(6):949–957. doi: 10.1177/0192623311418680. [DOI] [PubMed] [Google Scholar]
- 11.di Meo F, Lemaur V, Cornil J, et al. Free radical scavenging by natural polyphenols: atom versus electron transfer. J Phys Chem A. 2013;117(10):2082–2092. doi: 10.1021/jp3116319. [DOI] [PubMed] [Google Scholar]
- 12.Domitrović R, Jakovac H, Marchesi VV, et al. Differential hepatoprotective mechanisms of rutin and quercetin in CCl4-intoxicated BALB/cN mice. Acta Pharmacol Sin. 2012;33(10):1260–1270. doi: 10.1038/aps.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han D, Hanawa N, Saberi B, et al. Mechanisms of liver injury. III. Role of glutathione redox status in liver injury. Am J Physiol Gastrointest Liver Physiol. 2006;291(1):G1–G7. doi: 10.1152/ajpgi.00001.2006. [DOI] [PubMed] [Google Scholar]
- 14.Högberg J, Larson RE, Kristoferson A, et al. NADPH-dependent reductase solubilized from microsomes by peroxidation and its activity. Biochem Biophys Res Commun. 1974;56(3):836–842. doi: 10.1016/0006-291X(74)90681-0. [DOI] [PubMed] [Google Scholar]
- 15.Hollman PH, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37(9-10):937–942. doi: 10.1016/S0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 16.Hong IH, Ji H, Hwa SY, et al. The protective effect of ENA actimineral resource A on CCl4-induced liver injury in rats. Mar Biotechnol. 2011;13(3):462–473. doi: 10.1007/s10126-010-9317-8. [DOI] [PubMed] [Google Scholar]
- 17.Janbaz KH, Saeed SA, Gilani AH. Studies on the protective effects of caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomedicine. 2004;11(5):424–430. doi: 10.1016/j.phymed.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Ke B, Shen XD, Zhang Y, et al. KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of mouse liver transplants. J Hepatol. 2013;59(6):1200–1207. doi: 10.1016/j.jhep.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly GS. Quercetin. Altern Med Rev. 2011;16(2):172–194. [PubMed] [Google Scholar]
- 20.Kretzschmar M, Klinger W. The hepatic glutathione system-influences of xenobiotics. Exp Pathol. 1990;38(3):145–164. doi: 10.1016/S0232-1513(11)80201-X. [DOI] [PubMed] [Google Scholar]
- 21.Liang QN, Sheng YC, Jiang P, et al. The difference of glutathione antioxidant system in newly weaned and young mice liver and its involvement in isoline-induced hepatotoxicity. Arch Toxicol. 2011;85(10):1267–1279. doi: 10.1007/s00204-011-0664-7. [DOI] [PubMed] [Google Scholar]
- 22.Liu CM, Zheng YL, Lu J, et al. Quercetin protects rat liver against lead-induced oxidative stress and apoptosis. Environ Toxicol Pharmacol. 2010;29(2):158–166. doi: 10.1016/j.etap.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Mancuso C, Barone E. The heme oxygenase/biliverdin reductase pathway in drug research and development. Curr Drug Metab. 2009;10(6):579–594. doi: 10.2174/138920009789375405. [DOI] [PubMed] [Google Scholar]
- 24.Mancuso C, Barone E, Guido P, et al. Inhibition of lipid peroxidation and protein oxidation by endogenous and exogenous antioxidants in rat brain microsomes in vitro . Neurosci Lett. 2012;518(2):101–105. doi: 10.1016/j.neulet.2012.04.062. [DOI] [PubMed] [Google Scholar]
- 25.Morio LA, Chiu H, Sprowles KA, et al. Distinct roles of tumor necrosis factor-α and nitric oxide in acute liver injury induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2001;172(1):44–51. doi: 10.1006/taap.2000.9133. [DOI] [PubMed] [Google Scholar]
- 26.Muldoon MF, Kritchevsky SB. Flavonoids and heart disease. BMJ. 1996;312(7029):458–459. doi: 10.1136/bmj.312.7029.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park EJ, Pezzuto JM. Flavonoids in cancer prevention. Anti-Cancer Agents Med Chem. 2012;12(8):836–851. doi: 10.2174/187152012802650075. [DOI] [PubMed] [Google Scholar]
- 28.Pascoal AC, Ehrenfried CA, Eberline MN, et al. Free radical scavenging activity, determination of phenolic compounds and HPLC-DAD/ESI-MS profile of Campomanesia adamantium leaves. Nat Prod Commun. 2011;6(7):969–972. [PubMed] [Google Scholar]
- 29.Ratziu V, Zelber-Sagi S. Pharmacologic therapy of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13(4):667–688. doi: 10.1016/j.cld.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38(12):1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Sass G, Barikbin R, Tiegs G. The multiple functions of heme oxygenase-1 in the liver. Z Gastroenterol. 2012;50(1):34–40. doi: 10.1055/s-0031-1282046.. [DOI] [PubMed] [Google Scholar]
- 32.Vatsyayan R, Chaudhary P, Sharma A, et al. Role of 4-hydroxynonenal in epidermal growth factor receptor-mediated signaling in retinal pigment epithelial cells. Exp Eye Res. 2011;92(2):147–154. doi: 10.1016/j.exer.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vicente-Sánchez C, Egido J, Sánchez-González PD, et al. Effect of flavonoid quercetin on cadmium-induced hepatotoxicity. Food Chem Toxicol. 2008;46(6):2279–2287. doi: 10.1016/j.fct.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Watson WH, Yang X, Choi YE, et al. Thioredoxin and its role in toxicology. Toxicol Sci. 2004;78(1):3–14. doi: 10.1093/toxsci/kfh050. [DOI] [PubMed] [Google Scholar]
- 35.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33(2):105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 36.Williams CH, Jr, Arscott LD, Müller S, et al. Thioredoxin reductase: two modes of catalysis have evolved. Eur J Biochem. 2000;267(20):6110–6117. doi: 10.1046/j.1432-1327.2000.01702.x. [DOI] [PubMed] [Google Scholar]
- 37.Wu H, Chen M, Fan Y, et al. Determination of rutin and quercetin in Chinese herbal medicine by ionic liquid-based pressurized liquid extraction-liquid chromatography-chemiluminescence detection. Talanta. 2012;88:222–229. doi: 10.1016/j.talanta.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 38.Yang CS, Landau JM, Huang MT, et al. Inhibition of carcinogenesis by dietary polyphenolic compounds. Ann Rev Nutr. 2001;21(1):381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 39.Yang YC, Lii CK, Lin AH, et al. Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radic Biol Med. 2011;51(11):2073–2081. doi: 10.1016/j.freeradbiomed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Young R, Hayes JD, Brown K, et al. Peroxiredoxin gene expression signatures in liver reflect toxic insult. ASSAY Drug Dev Technol. 2010;8(4):512–517. doi: 10.1089/adt.2009.0246. [DOI] [PubMed] [Google Scholar]
- 41.Yousef MI, Omar SA, El-Guendi MI, et al. Potential protective effects of quercetin and curcumin on paracetamol-induced hitological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem Toxicol. 2010;48(11):3246–3261. doi: 10.1016/j.fct.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 42.Yuan L, Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med. 2009;30(1-2):29–41. doi: 10.1016/j.mam.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Yuan LP, Chen FH, Ling L, et al. Protective effects of total flavonoids of Bidens bipinnata L. against carbon tetrachloride-induced liver fibrosis in rats. J Pharm Pharmacol. 2008;60(10):1393–1402. doi: 10.1211/jpp/60.10.0016. [DOI] [PubMed] [Google Scholar]