All organisms capable of locomotion possess equilibrium receptor systems that use the gravitational field as a reference. In aquatic invertebrates, gravity receptors (statocysts) are considered to be the earliest known sense organs. Statocysts are composed of a dense mass (statolith or statoconia) that loads and mechanically stimulates sensory receptors, which are commonly cilia.
Ctenophores, or comb jellies, are gelatinous marine zooplankton with eight meridional rows of locomotory ciliary comb plates [1,2]. Their aboral statocyst contains a single massive statolith, which consists of numerous living cells (lithocytes), each with a nucleus and a large membrane-bound concretion [1,3].
The statolith is held upon the tips of four sickle-shaped groups of motile mechanoresponsive cilia (balancers), which act as pacemakers for the ciliary comb rows that cause swimming and geotaxis [1,2,4]. Each adult balancer consists of 150–200 cilia with individual ciliary membranes. Lithocytes are budded off from the epithelial floor close to the bases of the balancers [2,5], but how they come to be stuck together into a statolith at the tops of the balancers has remained a mystery. We report here that lithocytes are transported distally up the surface of the balancer cilia, independent of ciliary beating, to build the statolith.
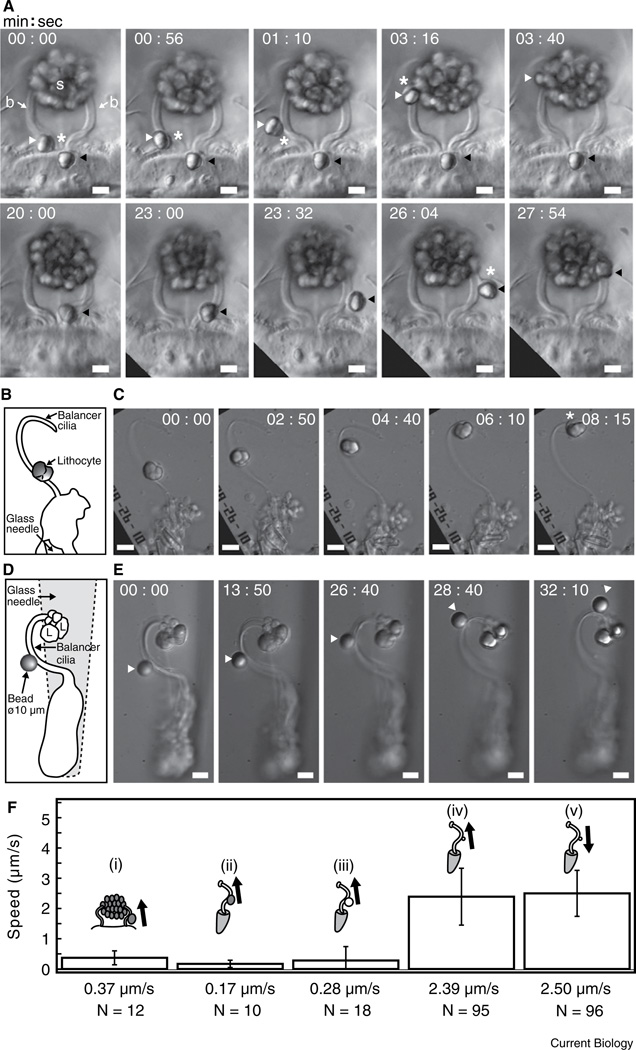
A freshly budded lithocyte in a larval Mnemiopsis leidyi first attaches to the base of a nearby balancer, as shown by its passive jiggling movements correlated with any beating of the balancer. The lithocyte then glides preferentially tipward at a speed of ~0.4 µm/s, regardless of whether or not the balancer beats, to join the other cells aggregated into the statolith (Figure 1A,F and Movie S1 in Supplemental Information, published with this article online). A brief pause may occur during transport of the lithocyte, often at a region of high curvature of the balancer.
Figure 1.
Lithocyte and microsphere movements on balancer cilia. (A) Lithocyte transport along a pair of adjacent balancers of a 3 mm larva of Mnemiopsis leidyi, viewed in the sagittal body plane. Two refractile lithocytes bud off sequentially from the epithelial floor and move up each balancer (b) into the statolith (s). Upper row: the left lithocyte (white arrowhead) is liberated first and moves up the left balancer. White asterisks indicate the peripheral cytoplasm surrounding the refractile concretion. Lower row: the right lithocyte (black arrowhead) buds off later and moves up the right balancer. The white asterisk shows a leading cytoplasmic rim. Scale bar, 10 µm. (B) Schematic drawing of an isolated adult balancer and an attached lithocyte. The cell bodies of the balancer are held by a glass needle. A dissociated adult lithocyte on the coverslip was translocated to the balancer by microscope stage controls and positioned above the curved base of the balancer. (C) The lithocyte moves up the balancer toward the tip at a speed of ~0.14 µm/s. The cytoplasmic rim of the lithocyte is visible in the last frame (white asterisk). Scale bar, 10 µm. (D,E) 10 µm microsphere transport along an isolated adult balancer. (D) Schematic drawing of the isolated balancer with a 10 µm microsphere attached about halfway up the balancer above its curved base. Several lithocytes (L) from the statolith remain at the balancer tip. The cell bodies of the balancer are held with a glass needle (out of focus). (E) The 10 µm microsphere moves up the balancer toward the tip. Scale bar, 10 µm. (F) Speed and direction histogram of lithocyte and microsphere movements on balancers from different preparations. Arrows show directions of tipward (anterograde) and baseward (retrograde) movements. The numbers of analyzed displacements are indicated by N. (i) Freshly budded lithocytes on intact larval balancers (12 displacements of 5 lithocyte movements on 5 balancers were analyzed); (ii) dissociated adult lithocytes on isolated adult balancers (10 displacements of 3 dissociated lithocyte movements on 3 isolated balancers were analyzed); (iii) 10 µm microspheres on isolated adult balancers (18 displacements of 7 microsphere movements on 7 isolated balancers were analyzed); (iv,v) 1 µm microspheres on isolated adult balancers (191 displacements of 11 microsphere movements on 10 isolated balancers were analyzed).
Lithocytes dissociated from statoliths of adult Mnemiopsis were positioned close to isolated adult balancers retaining their cell bodies, and were observed to move tipward at ~0.2 µm/s, slower than the speed of newly budded lithocytes on balancers of intact larvae (Figure 1B,C,F, and Movie S2). This finding is consistent with recent observations that lithocyte differentiation, budding and transport on balancers occur throughout the lifetime of Mnemiopsis, as well as in the cydippid ctenophore Pleurobrachia [5].
To distinguish between active or passive roles of lithocytes and balancers in generating the force for lithocyte movement, we found that 10 µm diameter inert polystyrene microspheres, similar in size to lithocytes, also moved preferentially tipward on isolated adult balancers of Mnemiopsis, at a speed similar to that of lithocytes on balancers of intact larvae (Figure 1D–F, and Movie S3).
In contrast, 1 µm diameter microspheres moved on isolated adult balancers bidirectionally in a saltatory fashion at a speed of ~2.5 µm/s in either direction (Figures 1F and S1, and Movie S4), similar to the speed of small bead movements on flagella of Chlamydomonas (see below).
These findings show that lithocytes are transported and added individually to the statolith by an active process of the balancer ciliary surface, in which the direction and speed of movement depend on the size of the cargo.
Various types of surface motility associated with the plasma membrane of microtubule-based cell extensions are well known, and include bidirectional movements of small beads on Chlamydomonas flagella, flagella-dependent whole cell gliding on a substrate, flagellamediated gamete interactions during mating in Chlamydomonas, and proximal transport of food organisms on non-axonemal singlet microtubule extensions of protists [6,7]. Chlamydomonas flagellar surface motilities are driven by intraflagellar transport (IFT), powered by kinesin and dynein motors in anterograde and retrograde directions, respectively [8]. We predict that a similar mechanism transports lithocytes on balancer cilia, and that this ctenophore system will prove useful for further understanding of IFT and surface motility in cilia and flagella.
To our knowledge this is the first report of eukaryotic cells serving as a cargo for transport by ciliary or flagellar surface motility, and the first example where ciliary surface motility plays a vital morphogenetic function in building the primary sensory/command organ of an animal. Since ctenophores are considered the sister lineage to all other animals [9,10], our observations are relevant to the origin and evolution of sensory and neural systems in Metazoa.
Supplementary Material
Acknowledgements
N.N. dedicates this work to Shinya Inoué. We are grateful to Rudolf Oldenbourg, Tomomi and Maki K. Tani, Ritsu Kamiya, Makoto Goda, Chris Inoué, Issei Mabuchi and Yuko K. Noda for their generous help and support. N.N. was supported by an NIH grant R01-EB002583 to Rudolf Oldenbourg.
Footnotes
Supplemental Information
Supplemental Information includes one figure and four movies and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2014.08.045.
References
- 1.Tamm SL. Ctenophora. In: Shelton GAB, editor. Electrical Conduction and Behavior in ‘Simple’ Invertebrates. Oxford: Clarendon Press; 1982. pp. 266–358. [Google Scholar]
- 2.Tamm SL. Cilia and the life of ctenophores. Invert. Biol. 2014;133:1–46. [Google Scholar]
- 3.Krisch B. Über das Apikalorgan (statocyste) der Ctenophore Pleurobrachia pileus. Z. Zellforsh. 1973;142:241–262. [PubMed] [Google Scholar]
- 4.Horridge GA. Relations between nerves and cilia in ctenophores. Am. Zool. 1965;5:357–375. doi: 10.1093/icb/5.3.357. [DOI] [PubMed] [Google Scholar]
- 5.Tamm SL. Formation of the statolith in the ctenophore Mnemiopsis leidyi. Biol. Bull. 2014;227 doi: 10.1086/BBLv227n1p7. in press. [DOI] [PubMed] [Google Scholar]
- 6.Bloodgood RA. Directed movements of ciliary and flagellar membrane components: a review. Biol. Cell. 1992;76:291–301. doi: 10.1016/0248-4900(92)90431-y. [DOI] [PubMed] [Google Scholar]
- 7.Bloodgood RA. The Chlamydomonas flagellar membrane and its dynamic properties. In: Witman GB, editor. The Chlamydomonas Sourcebook. 2nd Edition. Vol. 3. Academic Press; 2009. pp. 309–359. [Google Scholar]
- 8.Shih SM, Engel BD, Kocabas F, Bilyard T, Gennerich A, Marshall WF, Yildiz A. Intraflagellar transport drives flagellar surface motility. Elife. 2013;2:e00744. doi: 10.7554/eLife.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan JF, Pang K, Schnitzler CE, Nguyen AD, Moreland RT, Simmons DK, Koch BJ, Francis WR, Havlak P, et al. NISC Comparative Sequencing Program. The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution. Science. 2013;342:1242592. doi: 10.1126/science.1242592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moroz LL, Kocot KM, Citarella MR, Dosung S, Norekian TP, Povolotskaya IS, Grigorenko AP, Dailey C, Berezikov E, Buckley KM, et al. The ctenophore genome and the evolutionary origins of neural systems. Nature. 2014;510:109–114. doi: 10.1038/nature13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.