Abstract
Background
Brain metastases (BM) arising from Triple-negative breast cancer (TNBC) portend poor prognosis. TNBC is more common in premenopausal and African-American (AA) patients; both also confer poor prognosis. In a single institution cohort study, we sought to determine if inferior outcome of TN BCBM is more reflective of a higher-risk population or subtype itself.
Methods
The UNC Breast Cancer Database identified pts with BCBM diagnosed 1988 – 2008. BC subtype was assigned by IHC: HR+ (hormone receptor, ER+ and/or PR+)/HER2−, HR+/HER2+, HR−/HER2+ and TN (ER−/PR−/HER2−). Survival and recurrence patterns were evaluated by subtype, age (< vs ≥ 40 years) and race (AA vs non-AA) using the Kaplan-Meier method and Cox regression.
Results
Among 119 patients with BCBM, 33% were AA and 31% aged < 40 yrs. BC subtype was confirmed in 98 patients: 30% HR+/HER2−, 21% HR+/HER2+, 18% HR−/HER2+, 31% TN. Survival after BM was impacted by subtype (p=0.002), shortest for TNBC (0.24 yrs, CI 0.17 – 0.48). There were no age- (p=0.84) or race-specific (p=0.09) differences in survival after BM; stratification of subtypes by age and race revealed no difference (all, p > 0.1). Receipt of systemic therapy after BCBM was an important predictor of survival following BCBM (HR = 0.29, p=0.002) when adjusted for race, age, number of CNS lesions and BC subtype.
Conclusions
TNBC confers a high risk of death following BM regardless of race and age supporting the need for novel agents capable of controlling both intra- and extracranial TNBC across all races and ages.
INTRODUCTION
Brain metastases are a burgeoning clinical problem which negatively impact approximately 30% of patients diagnosed with advanced breast cancer1, 2. Despite local and systemic therapeutics, prognosis following a diagnosis of brain metastases arising from breast cancer is quite poor; historically, one year survival is approximately 20% across all breast cancer subtypes3. Breast cancer is no longer viewed as a homogenous disease process, but rather a compilation of reproducibly-identified intrinsic subtypes as defined by microarray -- Luminal A, Luminal B, Basal-like and HER2-enriched subtypes – each characterized by unique clinico-pathologic features and distinct prognosis4–6. Moreover, gene expression studies have identified subtype-specific predilection to distant metastatic sites7. Endocrine-sensitive tumors are more likely to recur in bone, while Basal-like and HER2-positive breast tumors in visceral sites, including the central nervous system (CNS) -- a site historically difficult to treat due to properties inherent to the blood brain barrier 8.
Building on these observations, several groups have sought to determine the contribution of intrinsic breast cancer subtype to the incidence, behavior, and prognosis of CNS metastases. Studies largely from eastern European and Asian patient populations suggest a high incidence and aggressive nature of brain metastases arising from Basal-like (usually identified as “Triple-negative” on clinical assays for HER2 and the estrogen [ER] and progesterone [PR] receptors) breast tumors9–12.
Basal-like/triple-negative breast cancer is a subtype of breast cancer commonly diagnosed in premenopausal and African American women – clinical factors which have both been correlated with poorer breast cancer outcome13–15. It is not clear whether or not the inferior outcome of Basal-like/triple-negative breast cancer brain metastases is a reflection of a higher-risk baseline population or of subtype itself. To our knowledge, the prognostic interplay of breast cancer subtype, race and age has yet to be explored in a cohort of racially-diverse patients diagnosed and treated for breast cancer and brain metastases.
In this single institution cohort study, we examined the clinico-pathologic characteristics and outcome of breast cancer patients diagnosed with brain metastases who received treatment at the University of North Carolina (UNC) at Chapel Hill between the years of 1988 – 2008. The goals of this study were: (1) to evaluate the clinical and pathologic characteristics of both primary breast and metastatic CNS tumors, (2) to report practice patterns (including both local and systemic therapies) among patients diagnosed with breast cancer-related brain metastases, and (3) to determine the contribution and interaction of breast cancer subtype, race and age on outcomes, particularly after CNS recurrence.
MATERIALS AND METHODS
Patient Population
This retrospective study was approved by the UNC Chapel Hill Office of Human Research Ethics Committee Institutional Review Board. Using an electronic clinical breast cancer database maintained at UNC Chapel Hill which includes >1,400 patients diagnosed with breast cancer between 1988 and 2008 who received a cancer-related treatment at UNC Hospitals, 119 patients (of which 25 patients were treated at UNC exclusively for brain metastases) were diagnosed with CNS metastases and were selected for study. A waiver of consent was granted by the UNC IRB to conduct this study given that all clinical data remained de-identified to study investigators.
The UNC clinical breast cancer database routinely includes clinical, treatment and outcome data from clinical and translational trial participants and patients treated with preoperative systemic therapy. Disease and vital status are updated yearly. Annual patient follow-up included a chart review of radiology reports, clinical notes, pathology reports and vital status (via the medical record, Social Security Death Index and obituaries).
Primary breast tumor characteristics
All 119 patients had a histologic confirmation of invasive breast cancer (with the exception of 2 initially diagnosed with ductal carcinoma in situ) and an eventual diagnosis of CNS metastases. The majority (77%, 92/119) of primary breast tumor pathologic specimens were reviewed by breast pathologists at UNC Chapel Hill. The remaining 23% (27/119) received their initial therapy at outside institutions and, therefore, did not have pathologic specimens reviewed by UNC breast pathologists. In these cases, pathology data was extracted from the medical record. Clinical variables included patient age, gender, race, stage of primary breast cancer at diagnosis, primary tumor size, lymph node stage at diagnosis, primary tumor histology including estrogen receptor (ER) status, progesterone receptor (PR) status, HER2 status, presence or absence of lymphovascular invasion (LVI) within the primary breast tumor, and treatment history (including local and systemic therapies).
Initial breast cancer staging was coded according to the 2003 Sixth Edition of the American Joint Committee on Cancer (AJCC). Primary tumor histological type (i.e. ductal or lobular) and grading of primary breast tumors were classified according to the Nottingham modification of the Scarff-Bloom-Richardson criteria. ER and PR status reported via the clinical database was performed by ligand binding assay (up to 1993) or immunohistochemistry (IHC, 1993-present). HER-2 status reported via the clinical database was determined either by IHC or fluorescence in situ hybridization (FISH). HER2 was reported as negative if either 0 or 1+ by IHC or FISH non-amplified. HER2 was reported as positive if 3+ by IHC or FISH amplified. In the rare case where HER2 was 2+ by IHC and FISH data was unavailable, samples were reported as HER2 negative (n = 7).
Central nervous system metastases-specific data
All patients included in the analysis were diagnosed with breast cancer metastatic to the CNS. CNS metastases were defined by the presence of metastatic disease to the brain parenchyma and/or the presence of leptomeningeal disease (LMD). CNS-specific data extracted from the database included number of CNS metastases, local CNS-specific therapies including neurosurgical resection, whole brain radiotherapy and focused brain radiotherapy. Presence or absence of extracranial disease was also recorded. Details on systemic therapies in the metastatic setting were extracted from the database.
Immunohistochemical Assays for ER, PR and HER2
Archival formalin-fixed, paraffin-embedded tumor tissues were available for 35 of the 119 patients and ER, PR and HER2 status were repeated via IHC for this subset of patients. Among these 35 patients, both primary breast tumor tissue and brain metastases tissue were available in the UNC Surgical Pathology Archives or Tissue Procurement Facility for 14 (matched pairs), brain metastases tissue only for 18, and primary breast tumor tissue only for 3. Approval to study clinically-annotated, de-identified tissues was obtained from the UNC Institutional Review Board.
IHC studies were performed on 5-micron formalin-fixed paraffin-embedded (FFPE) tissue sections on coated glass slides. A sequential immunohistochemical assay was performed on the Dako Autostainer platform (DakoCytomation). Respective monoclonal antibodies were applied for 30–60 minutes (with the exception of HER2 which was incubated overnight at 4C). Detection was completed by incubating with a biotinylated link. An avidin biotin complex (Vectastain Elite Kit 6102) was applied followed by diaminobenzidine (Innovex NB314SB) chromogen for nuclear localized antibodies (ER and PR); Vector SG Substrate SK-4700 for the membrane localized antibodies (HER2). Signal contrast was maximized by counterstaining in hematoxylin (DakoCytomation Mayer’s Hematoxylin S3309), rinsed in deionized water and placed in a bluing solution (Richard-Allan Scientific 7301) for the nuclear antibodies (ER and PR) and Vector Nuclear Fast Red H3403 for HER2. The following primary antibodies and dilutions were employed: Estrogen receptor (clone 1D5 1:50, Dako), Progesterone receptor (clone 16, 1:70, Vision BioSystems), and HER2 (clone CB11, 1:100, BioGenex). For each antibody, primary breast tumor tissue was used as a positive control.
Estrogen and progesterone receptor staining were scored using the Allred scoring system on a scale from 0–8 for nuclear staining only. The intensity score and percentage score are added to obtain the Allred score. Allred scores of 0–2 were classified as negative and Allred scores of 3–8 were classified as positive. HER2 was scored using the current ASCO/CAP guidelines16. Membranous immunoreactivity was scored (0 and 1+=negative, 2+=indeterminate, 3+=positive for overexpression) and percentage of tumor cells staining positive were given as raw score ranging from 0–100%. For the purposes of this analysis, 0 – 2+ was considered HER2 negative; 3+, HER2 positive.
Assignment of Breast Cancer Subtype
Clinical breast cancer subtype was assigned by IHC as previously described: HR+ (hormone receptor ER+ and/or PR+)/HER2−, HR+/HER2+, HR−/HER2+, and Triple-negative (ER−/PR−/HER2−)13. In the cases where primary breast ER, PR and HER2 status were known via both the clinical database and repeat IHC, repeat receptor status via IHC was prioritized over information from the database to assign subtype. If receptor status was unknown for primary breast specimens (via repeat IHC or from the clinical database), available ER, PR and HER2 from brain specimens was used to assign subtype, again prioritizing repeat IHC over information from the clinical database. Concordance between clinical database assignment of ER, PR and HER2 and that of repeat IHC among breast tumor samples where both were known was 91% (10/11), 73% (8/11), and 79% (11/14), respectively. Concordance between clinical database assignment of ER, PR and HER2 and repeat IHC among brain metastases samples where both were known was 88% (7/8), 44% (4/9), and 100% (12/12), respectively.
Statistical Analysis
The Kaplan-Meier method and Log-rank test were used to compare differences among survival curves, and Cox regression was used to evaluate possible predictors in the time-to-event outcomes. Overall survival (OS) was available for all patients included in this retrospective review, and was defined as the time from primary breast tumor diagnosis to death or last contact. Time to CNS recurrence (TTCNS) was defined as the time from primary breast tumor diagnosis to date of CNS metastasis; for those patients whose initial distant recurrence included CNS, this time is the same as time to distant recurrence. CNS-specific survival (CNS survival) was defined as the time from the date of CNS metastasis to the date of death or last contact. Due to missing data, the sample size available for multivariable modeling was limited, such that all variables (from univariable analysis) were not included in a single model. Thus, four multivariable models were constructed. The first included Triple-negative status with age, race, and > 3 brain metastases. The remaining three models included those four variables with the addition of one of the treatments after brain metastases: systemic therapy, neurosurgical resection, or radiation. Statistical analyses were performed with SAS 9.2 statistical software (SAS Institute Inc, Cary, NC).
RESULTS
Characteristics of Study Population
The clinical characteristics of the study population are presented in Table 1. The median age at breast cancer diagnosis was 44 years (range 23–83 years); 31% were aged less than 40 years. The majority of patients were female (118/119, 99%). Fifty-eight percent (62/106) were pre-menopausal at diagnosis, while 42% (44/106) were post-menopausal. Among the study population, 62% were Caucasian and 33% were African American.
Table 1.
Patient Demographics and Primary Breast Tumor Characteristics
| Clinico-pathologic Characteristics | All Patients | ER−/PR−/HER2− (n=30, 31%) | HR−/Her2+ (n=18, 18%) | HR +/HER2− (n=29, 30%) | HR +/HER2+ (n=21, 21%) | p-value |
|---|---|---|---|---|---|---|
| Age at Diagnosis | n = 119 | |||||
| Median (range) | 44 (23–83) | 48.5 (28–72) | 49 (29–63) | 41 (29–83) | 43 (32–63) | 0.2 |
| <40 yrs | 37 (31%) | 10 (33%) | 4 (22%) | 11 (38%) | 5 (24%) | 0.6 |
| ≥40 yrs | 82 (69%) | 20 (67%) | 14 (78%) | 18 (62%) | 16 (76%) | |
| Race | n = 119 | 0.16 | ||||
| Caucasian | 74 (62%) | 15 (50%) | 12 (67%) | 18 (62%) | 14 (67%) | |
| African American | 39 (33%) | 15 (50%) | 4 (22%) | 8 (28%) | 7 (33%) | |
| Hispanic | 3 (2%) | 2 (11%) | 1 (3%) | |||
| Other | 3 (2%) | 2 (7%) | ||||
| Stage at Diagnosis | n = 91 | 0.2 | ||||
| I | 3 (3%) | 1 (5%) | ||||
| II | 20 (22%) | 6 (25%) | 1 (7%) | 3 (14%) | 7 (44%) | |
| III | 43 (47%) | 12 (50%) | 7 (47%) | 11 (52%) | 4 (25%) | |
| IV | 25 (28%) | 6 (25%) | 7 (47%) | 6 (29%) | 5 (31%) | |
| Nodal Status at Diagnosis | n = 89 | 0.19 | ||||
| Negative | 24 (27%) | 8 (32%) | 2 (13%) | 4 (20%) | 7 (47%) | |
| Positive | 65 (73%) | 17 (68%) | 13 (87%) | 16 (80%) | 8 (53%) |
Clinical and pathological characteristics of primary breast carcinomas
The majority of patients (47%) presented with stage III disease; 28% with stage IV disease. Median tumor size was 3 cm (range 0 – 14 cm, 83 assessable patients) and 90% (78/87) of patients had primary breast tumors ≥ 2 cm. Seventy-three percent (65/89) of patients with known lymph node status had positive axillary lymph nodes at diagnosis. Thirty-five percent (30/85) of breast tumors exhibited lymphovascular invasion (LVI). There were no significant differences in primary breast cancer characteristics, including stage at diagnosis, tumor size, lymph node status, presence/absence of LVI, or grade by race (AA versus non-AA, all p > 0.05), age (< 40 versus ≥ 40 years, all p > 0.05) or subtype (all > 0.05).
Fifty-eight percent of (61/106) of primary breast tumors were ER negative and 65% (66/102) were PR negative, while 40% (38/94) were HER2 positive; no significant age- or race-specific differences in primary breast tumor ER, PR and HER2 status were seen. All clinical breast cancer subtypes were represented: 30% HR+/HER2−, 21% HR+/HER2+, 31% Triple-negative, and 18% HR−/HER2+, see Table 1. Consistent with prior reports13, a higher proportion of younger, AA patients were diagnosed with Triple-negative (versus non-Triple negative) breast cancer when compared to older, non-AA patients (64% [9/14] versus 29% [14/48], p = 0.03).
Local and systemic therapies for primary breast cancer diagnosis
As patients included in this analysis were treated either on a clinical trial or with preoperative systemic therapy, the large majority (89%, 104/117) received chemotherapy with curative intent. Half of patients treated with chemotherapy received both an anthracycline and taxane (50%, 50/101). Nineteen percent (22/115) received trastuzumab (Herceptin, Genentech/Roche) in the neoadjuvant or adjuvant setting, of which 45% had HR+/HER2+, 45% HR−/HER2+ breast tumors. One patient with HR+/HER2− and one with Triple-negative breast cancer also received trastuzumab. Forty-four percent (50/113) of patients received endocrine therapy. There were no significant race or age-specific differences in primary breast cancer systemic therapies with one exception -- a higher proportion of AA patients received chemotherapy in the neo-adjuvant setting (70% [21/30] versus 44% [25/57] non-AA, p = 0.04). As expected, a higher proportion of patients with HR+ (both HER2− and +) breast tumors received endocrine therapy (p < 0.001), while a higher proportion with HER2+ (both HR- and +) breast tumors received trastuzumab (p < 0.001).
Characteristics and treatment of CNS and non-CNS metastatic disease
Among the 119 breast cancer patients with CNS metastases, 88% (105/119) of CNS lesions were parenchymal, 9% leptomeningeal, and 3% both, see Table 2. At the time of metastatic presentation, 59% were diagnosed with extracranial disease only, 27% with intracranial disease only, and 14%, both. Forty-three percent of patients presented with a solitary brain metastasis while 38% presented with > 3 CNS metastatic lesions. There were no age-, race-, or subtype specific differences in number of CNS lesions or site of initial recurrence (all p > 0.05).
Table 2.
Central Nervous System (CNS)-specific Tumor Characteristics and Therapies
| Clinico-pathologic Characteritics | All Patients | ER−/PR−/HER2− (n=30, 31%) | HR−/Her2+ (n=18, 18%) | HR +/HER2− (n=29, 30%) | HR +/HER2+ (n=21, 21%) | p-value |
|---|---|---|---|---|---|---|
| Location of CNS metastases | n = 119 | 0.8 | ||||
| Brain Parenchyma | 105 (88%) | 26 (87%) | 16 (89%) | 24 (83%) | 19 (90%) | |
| Leptomeninges only | 10 (9%) | 4 (13%) | 1 (6%) | 3 (10%) | 1 (5%) | |
| Both | 4 (3%) | 1 (6%) | 2 (7%) | 1 (5%) | ||
| Sites of Disease at Initial Metastatic Presentation | n = 119 | 0.09 | ||||
| Extracranial Only | 70 (59%) | 13 (43%) | 13 (72%) | 19 (66%) | 13 (62%) | |
| Intracranial Only | 32 (27%) | 8 (27%) | 5 (28%) | 8 (28%) | 6 (29%) | |
| Intra & Extracranial | 17 (14%) | 9 (30%) | 2 (7%) | 2 (10%) | ||
| Number of Metastatic CNS Lesions | n = 113 | 0.3 | ||||
| 1 | 48 (43%) | 11 (41%) | 5 (28%) | 16 (57%) | 12 (60%) | |
| 2–3 | 22 (19%) | 6 (22%) | 6 (33%) | 3 (11%) | 3 (15%) | |
| 3+ | 43 (38%) | 10 (37%) | 7 (39%) | 9 (32%) | 5 (25%) | |
| Chemotherapy in the Metastatic Setting | n = 107 | 0.2 | ||||
| Yes | 80 (75%) | 16 (62%) | 15 (88%) | 22 (81%) | 15 (79%) | |
| No | 27 (25%) | 10 (38%) | 2 (12%) | 5 (19%) | 4 (21%) | |
| Lines of Chemotherapy in the Metastatic Setting | n = 80 | 0.15 | ||||
| 1 | 4 (5%) | 1 (7%) | 1 (4%) | |||
| 2–3 | 37 (46%) | 12 (75%) | 5 (33%) | 9 (41%) | 6 (40%) | |
| 3+ | 39 (49%) | 4 (25%) | 9 (60%) | 12 (55%) | 9 (60%) |
Among the 118 patients for which CNS local therapy information was available, 42% received whole brain radiation (WBRT), 10% surgical resection, 8% radiosurgery, 13% surgical resection and WBRT, 7% radiosurgery and WBRT, and 20% other. Numerically, a higher proportion of patients with HR+/HER2+ breast cancer underwent neurosurgical resection, see Table 3. There were no age, race or subtype-specific differences in receipt of intracranial radiation (all p > 0.05).
Table 3.
Systemic, Surgical and Radiation Treatments after CNS Recurrence by Subtype, Race and Age.
| Subtype | * Race | * Age | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Pts | ER−/PR−/HER2− (n=30, 31%) | HR−/Her2+ (n=18, 18%) | HR +/HER2− (n=29, 30%) | HR +/HER2+ (n=21, 21%) | Fisher’s p-value | Non-AA N=80 |
AA N=39 |
<40 N=37 |
>=40 N=82 |
|
| Any Systemic Therapy | 60/99 61% |
9/21 43% |
13/16 81% |
15/27 56% |
14/19 74% |
0.066 | 42/68 62% |
18/31 58% |
18/30 60% |
42/69 61% |
| Chemotherapy | 50/107 47% |
8/26 31% |
13/17 76% |
12/27 44% |
10/19 53% |
0.03 | 36/71 51% |
14/36 39% |
17/33 52% |
33/74 45% |
| Trastuzumab | 16/99 16% |
0/21 0% |
5/16 32% |
2/27 7% |
8/19 42% |
0.0005 | 13/68 19% |
3/31 10% |
6/30 20% |
10/69 14% |
| Endocrine Therapy | 15/99 15% |
1/21 5% |
0/16 0% |
7/27 26% |
4/19 21% |
0.04 | 12/68 18% |
3/31 10% |
2/30 7% |
13/69 19% |
| Neurosurgical Resection | 32/118 27% |
11/30 37% |
2/18 11% |
7/28 25% |
11/21 52% |
0.037 | 18/79 23% |
14/39 36% |
12/36 33% |
20/82 24% |
| Cranial Radiation | 89/119 75% |
18/30 60% |
15/18 83% |
20/29 69% |
15/21 71% |
0.4 | 63/80 79% |
26/39 67% |
29/37 78% |
60/82 73% |
All other p values > 0.14.
In the metastatic setting, 75% (80/107) of patients received chemotherapy, of which 95% (76/80) received more than one line of chemotherapy (median of 3 lines). Although no age-related differences were seen, a lower proportion of AA patients received > 3 lines of chemotherapy at any time during the metastatic disease course (19% [7/36] versus 47% [33/70] for non-AA patients, p = 0.02). Thirty-four percent (34/99) of patients received trastuzumab in the metastatic setting, while 38% (38/99) received endocrine therapy.
Focusing specifically on treatment following CNS recurrence, 61% (60/99) received systemic therapy after CNS recurrence among which subtype-specific differences were not present (p = 0.07, see Table 3). As expected, a higher proportion of patients with HER2+ (both HR− and +) received trastuzumab (p = 0.0005), while a higher proportion of patients with HR + (both HER2 − and +) received endocrine therapy (p = 0.04). Although a numerically higher proportion of patients with TNBC presented with both intra- and extracranial disease (see Table 2), a lower proportion of patients with TNBC received chemotherapy after brain metastases compared to other subtypes (p = 0.03). There were no race- or age-related differences in treatment after CNS recurrence (all p > 0.14).
Overall Survival and Natural History of CNS Recurrence
At a median follow-up of 6.2 years, 93 of 119 (78%) of patients had died. Across all ages, races and breast cancer subtypes, median overall survival (OS) was 4.03 years (95% CI, 3.09–5.05 years). Median OS differed significantly by subtype (p=0.017). Patients with Triple-negative breast cancer had shorter median OS, 2.12 years (CI, 1.14–3.45 years), when compared with other subtypes; HR−/HER2+ 3.52 years (CI, 1.61–7.5 years), HR+/HER2− 5.09 years (CI, 3.22–7.24 years), and HR+/HER2+ 5.19 years (CI, 2.1–10.05). Although there was no difference in OS by age (< or ≥ 40 years, p = 0.51), AA patients experienced shorter median OS when compared to non-AA patients (3.45 [CI, 1.92 – 4.98] versus 4.78 [CI, 3.52 – 6.04] years, respectively; p = 0.04).
The median time to the development of CNS metastases (TTCNS) across subtype, race and age was 2.62 years (CI, 1.85–3.23 years). TTCNS approached a significant association by breast cancer subtype (p = 0.08). TTCNS was shorter for patients with Triple-negative and HR−/HER2+ breast tumors (1.51 [CI, 1.08–2.31] and 1.65 [CI, 1.13 – 3.02] years, respectively). In contrast, TTCNS was 3.35 (CI, 1.75 – 4.98) years for HR+/HER2− and 4.15 (CI, 0.85 – 4.54) years for HR+/HER2+ subtypes. Although TTCNS recurrence did not differ by age (p = 0.43), TTCNS was shorter for AA patients when compared to non-AA patients (2.08 [CI, 1.30 – 3.21] versus 3.06 [CI, 1.85 – 3.82] years, respectively; p = 0.02).
CNS-specific Survival by Subtype, Race and Age
Across subtype, race and age, the median time to death following CNS metastases (CNS survival) was 0.65 years (0.48 – 0.98). CNS survival differed significantly by subtype (p=0.002, see Figure 1). CNS survival was 0.24 years (CI, 0.17 – 0.48), 1.19 years (CI, 0.27 – 3.02), 0.8 years (CI, 0.35 – 1.54), and 1.27 years (CI, 0.65 – 3.37) for Triple-negative, HR−/HER2+, HR+/HER2− and HR+/HER2+ subtypes, respectively. There was no difference in CNS survival by race (p = 0.09) or age (p = 0.84). Moreover, stratification by breast cancer subtype revealed no difference in CNS survival by race and age (all, p > 0.1).
Figure 1.
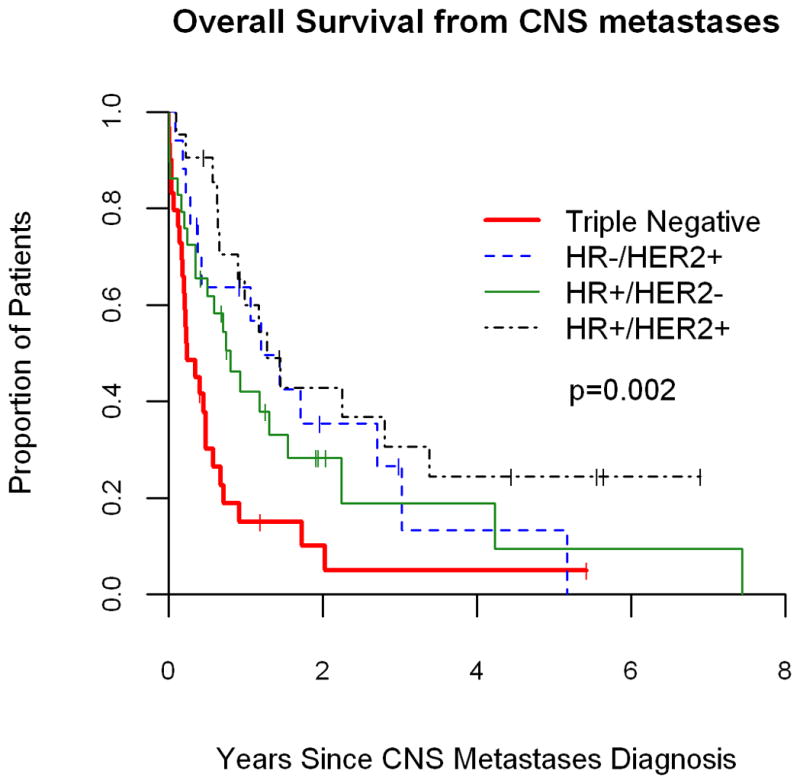
Survival from time of CNS metastases by clinical breast cancer subtype.
Impact of Subtype, Age, Race and Treatment on CNS-specific Survival
The triple-negative phenotype, presence of > 3 brain lesions, lack of neurosurgical resection, non-receipt of systemic or radiotherapy following CNS recurrence, and node positive status at primary breast cancer diagnosis predicted worse survival after brain metastases in univariable analysis, see Table 4. Triple-negative breast cancer (controlled for age, race, and > 3 brain metastases) was associated with worse survival following CNS recurrence (HR: 2.3 [95% CI: 1.33, 3.96], p=0.003) when compared to non-Triple-negative breast cancer in multivariable analysis.
Table 4.
Univariable and Multivariable Analysis of Risk Factors Predictive of Overall Survival Following Brain Metastases
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Clinicopathologic Variables | p | HR (95% CI) | p | HR (95% CI) |
| Triple Negative (vs. non–Triple Negative) | 0.0004 | 2.44 (1.50, 3.98) | 0.003 | 2.30 (1.33, 3.96) |
| Age ≥ 40 | 0.8 | 0.96 (0.61, 1.49) | 0.8 | 1.06 (0.63, 1.78) |
| Race (African-American) | 0.09 | 1.46 (0.94, 2.28) | 0.9 | 1.01 (0.59, 1.73) |
| >3 CNS Lesions | 0.001 | 2.01 (1.31, 3.09) | 0.0003 | 2.52 (1.54, 4.15) |
| Brain Resection (vs. none) | 0.001 | 0.42 (0.25, 0.71) | ||
| Brain XRT (vs none) | 0.04 | 0.60 (0.37, 0.97) | ||
| Systemic after CNS (vs none) | 0.0005 | 0.44 (0.27, 0.70) | ||
| Node Positive | 0.03 | 1.85 (1.08, 3.19) | ||
| Pre-Treatment Stage III–IV (vs. I–II) | 0.3 | 1.29 (0.76, 2.16) | ||
| T≥2cm | 0.2 | 1.79 (0.78, 4.15) | ||
| Brain as Initial Site of Recurrence | 0.16 | 0.74 (0.48, 1.12) | ||
| Leptomeningeal disease | 0.14 | 1.60 (0.85, 3.03) | ||
| Presence of Extracranial metastases | 0.5 | 1.2 (0.68, 2.13) | ||
Taking into account the significant univariable association between treatment for brain metastases and improved CNS-specific survival, multivariable modeling revealed the following. After controlling for age, race, and >3 brain metastases, receipt of any systemic treatment after brain metastases was the most important predictor of CNS-specific survival (HR = 0.4, p=0.002), above that of Triple-negative status (HR = 1.4, p = 0.3). However, Triple-negative status retained its significant association with CNS-specific survival after controlling for age, race, >3 brain lesions and resection (Triple-negative (HR = 2.8, p=0.0005), neurosurgical resection (HR = 0.4, p=0.005)) and radiation (Triple-negative (HR = 2.1, p=0.0009), radiation (HR = 0.5, p=0.028)).
DISCUSSION
Results from this single-institution, retrospective cohort study illustrate that Triple-negative breast cancer portends worse survival following a diagnosis of CNS metastases when compared to non-Triple-negative subtypes independently of AA race or young age. Moreover, within an inherently young, racially-diverse patient population treated in the United States, of which 33% were AA and 31% aged less than 40 years, the prognostic contribution of clinical breast cancer subtype persisted among patients diagnosed with breast cancer brain metastases. Interestingly, receipt of systemic therapy following CNS recurrence was an important predictor of survival following brain metastases. In contrast to that observed in non-Triple-negative subtypes, a recent series indicates the administration of chemotherapy following WBRT in the setting of Triple-negative breast cancer brain metastases does not improve survival10. Our data continues to highlight the dire need for novel systemic approaches capable of improving outcomes for patients diagnosed with Triple-negative breast cancer brain metastases across all racial and age groups.
In our series of patients treated at UNC Chapel Hill between 1988 – 2008, survival following brain metastases arising from Triple-negative breast cancer was 0.24 years compared to 1.19, 0.8 and 1.27 years for HR−/HER2+, HR+/HER2− and HR+/HER2+ subtypes, respectively. Our results are consistent with several, albeit few, reports evaluating the prognostic implication of subtype among patients with breast cancer brain metastases9, 10, 12. Among 222 patients treated at the Cancer Center, Warsaw, Poland from 2003 – 2006, Niwinski et al. reports median survival following brain metastases in Triple-negative, HER2-positive, and Luminal (endocrine sensitive) subtypes was 3.7, 9 and 15 months, respectively.11 A similar series by Nam et al. retrospectively evaluated 126 patients with breast cancer brain metastases treated at the National Cancer Center Hospital, Goyang, Korea from 2001 – 2006. Survival was shortest from brain metastases to death among patients with Triple-negative and Luminal A (HR+/HER2−) breast cancer (3.4 and 4.0 months) compared to HER2 (HR−/HER2+) and Luminal B (HR+/HER2+) counterparts (9.0 and 5.0 months; p = 0.0113)12. Our analysis also indicates poor prognosis following a diagnosis of brain metastases arising from HR+/HER2− breast tumors – an observation likely attributable to the fact that CNS recurrence is a late occurrence in the natural history of HR+/HER2− breast cancer.
Recognizing that younger women of AA descent are at higher risk for the development of Triple-negative breast cancer and both race and age differences are known to affect breast cancer survival,13–15, 17 one of the goals central to our analysis was to examine the prognostic influence of race and age following CNS recurrence. Examining race as a single prognostic factor, survival following CNS recurrence was not influenced by ethnicity across subtypes. However, AA patients experienced shorter OS and recurred within the CNS more rapidly than non-AA patients. Careful review of race-specific treatments indicates a lower proportion of AA women received > 3 lines of chemotherapy in the metastatic setting compared to non-AA patients. Although this study is not designed to identify causality for this observation, receipt of fewer lines of chemotherapy may partially explain both inferior OS and earlier recurrence in the CNS among AA patients. Similar to race, age as a single prognostic factor did not influence survival following CNS recurrence across subtypes. Interestingly, within this cohort of patients all of whom developed brain metastases from breast cancer, median age at breast cancer diagnoses was young, 44 years. Moreover, 31% of patients were aged < forty at initial diagnosis. Per the US Surveillance, Epidemiology and End Results (SEER) database, the median age at breast cancer diagnosis is 61 years (2002 – 2006) and 7% of women in the US diagnosed with breast cancer in are aged < 40 years17, 18. Although determining risk factors for the development of CNS recurrence was not the purpose of this study, prior reports indicate young age to be a risk factor for the development of breast cancer brain metastases and our data is supportive of this finding2.
Several limitations inherent to this study should be addressed. First, patients included in this analysis all developed CNS metastases from breast cancer and were treated at a single-center, academic institution largely on clinical protocols. As such, practice patterns (ie. routine use of neo-adjuvant chemotherapy, strong referral base for neurosurgical resection, etc.) may have introduced bias. Moreover, results from this self-contained dataset may not be generalizable across the entire breast cancer population, but add to the existing literature surrounding the high risk population who recur within the CNS. In addition, the database spans a long period (1998 – 2008) during which time newer therapies have been introduced into clinical practice. Thus, results may not be entirely applicable to the modern-day breast cancer patient population and should be interpreted within the confines of the study period. Secondly, an IHC surrogate was employed to assign breast cancer subtype from archival, paraffin-embedded tissues. As subtype assignment via gene expression is available only in research settings, this approach is generally accepted when fresh, frozen tissues samples are unavailable for analysis13. Breast cancer subtype assignment was based primarily on receptor status acquired from primary breast tumors. Although discordance between primary breast tumor and brain metastases ER, PR and HER2 status may occur19, routine biopsy of the CNS is not feasible (or necessary) in all cases. Moreover, receptor concordance between the clinical database and repeat IHC on available tissues was < 100%. As such, rare cases of subtype misclassification may have occurred. Finally, while the sample size of this retrospective analysis is comparable to works within the field, detection of small to moderate interactions between race, subtype, and survival may have been limited by the evaluation of subsets of the larger patient population.
In summary, this informative study is, to our knowledge, the first to examine the prognostic interplay of breast cancer subtype, race and age among patients with brain metastases in a racially-diverse US patient population. Results presented herein illustrate clinical breast cancer subtype maintains prognostic significance, independently of race and age, among patients with breast cancer brain metastases. Moreover, Triple-negative breast cancer, regardless of race or age confers the worst prognosis – a finding that is consistent with prior series and continues to highlight the need for the development of novel, targeted agents capable of controlling both intracranial and extracranial Triple-negative breast cancer.
Acknowledgments
Funding Sources: 5K12CA120780-03 (CA), NCI CA058223 (UNC SPORE) and Breast Cancer Research Foundation
Footnotes
Financial Disclosures: Stock ownership, Bioclassifier, LLC and Founder, University Genomics (CP), Advisory Board/Consultant, Sanofi-Aventis, BiPAR, Wyeth, Pfizer, Genentech, Bristol Myers Squibb, Novartis (LC, uncompensated)
References
- 1.Lee Y. Breast carcinoma: Pattern of metastasis at autopsy. J Surg Oncol. 1983;23:175–80. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 2.Tsukada Y, Fouad A, Pickren J, Lane W. Central nervous system metastasis from breast carcinoma. Autopsy study Cancer. 1983;52:2349–54. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Engel J, Eckel R, Aydemir U. Determinants and prognoses of locoregional and distant progression in breast cancer. Int J Radiat Oncol Biol Phy. 2003;55:1186–95. doi: 10.1016/s0360-3016(02)04476-0. [DOI] [PubMed] [Google Scholar]
- 4.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 5.Sorlie T, Perou C, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron J, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100(14):8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smid M, Wang Y, Zhang Y, Sieuwerts A, Yu J, Klijn J, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Research. 2008;68(9):3108–14. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 8.Lin N, Claus E, Sohl J, et al. Sites of Distant Recurrence and Clinical Outcomes in Patients With Metastatic Triple-negative Breast Cancer High Incidence of Central Nervous System Metastases. Cancer. 2008;113(10):2638–45. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heitz F, Harter P, Lueck H, Fissler-Eckhoff A, Lorenz-Salehi F, Scheil-Bertram S, et al. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. European Journal of Cancer. 2009;45(16):2792–98. doi: 10.1016/j.ejca.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Niwinska A, Murawska M, Lemanska I, Milewska J. The role of systemic treatment after whole brain radiotherapy (WBRT) in breast cancer patients with brain metastases: Differences depending on biological subtype. Journal of Clinical Oncology. 2009;27(15s):Abstract 1027. [Google Scholar]
- 11.Niwinska A, Murawska M, Pogoda K. Breast Cancer Brain Metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole brain radiotherapy (WBRT) Annals of Oncology. 2009 doi: 10.1093/annonc/mdp407.. [DOI] [PubMed] [Google Scholar]
- 12.Nam B-H, Kim SY, Han H-S, Kwon Y, Lee KS, Kim TH, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Research. 2008;10(R20) doi: 10.1186/bcr1870. http://breast-cancer-research.com/content/10/1/R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carey L, Perou C, Livasy C, Dressler L, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Malone K, Daling J. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Archives of Internal Medicine. 2003;163(1):49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg J, Chia Y, Plevritis S. The effect of age, race, tumor size, tumor grade, and disease stage on invasive ductal breast cancer survival in the U.S. SEER database. Breast Cancer Research and Treatment. 2005;89(1):47–54. doi: 10.1007/s10549-004-1470-1. [DOI] [PubMed] [Google Scholar]
- 16.Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Journal of Clinical Oncology. 2007;25(1):118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 17.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast Cancer Before Age 40 Years. Seminars in Oncology. 2009;36:237–49. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ries L, MD, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005. National Cancer Institute; Bethesda, MD: based on November 2007 SEER; http://seercancergov/csr/1975_2005/ [Google Scholar]
- 19.Simmons C, Miller N, Geddie W, Gianfelice D, Oldfield M, Dranitsaris G, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Annals of Oncology. 2009;20:1499–504. doi: 10.1093/annonc/mdp028. [DOI] [PMC free article] [PubMed] [Google Scholar]


