Abstract
Carbapenem-resistant Klebsiella pneumoniae (CRKP) is an increasing global threat. Here, we describe the prevalence and impact of tigecycline use in a cohort of patients with CRKP bacteriuria nested within a multicenter, prospective study. In the 21 month study period, 260 unique patients were included. Tigecycline was given to 80 (31%) patients. The use of tigecycline during the index hospitalization was significantly associated with the subsequent development of tigecycline resistance in the same patient (OR 6.13, 95%CI 1.15–48.65, p=0.03). In conclusion, the use of tigecycline with CRKP bacteriuria is common, and is associated with the subsequent development of tigecycline resistance.
Keywords: carbapenem resistant Enterobacteriaceae, multi-drug resistance, urinary tract infection
Resistance to carbapenems in Enterobacteriaceae (CRE) is increasing globally [1]. Among CRE, carbapenem-resistant Klebsiella pneumoniae (CRKP) is most prevalent. CRKP are generally resistant to multiple antibiotic classes. As a result, treatment options for CRKP include polymyxins, tigecycline, aminoglycosides, and fosfomycin, which are limited by concerns regarding efficacy and safety [2]. In addition, resistance to colistin and tigecycline is increasingly reported [3–5]. The molecular epidemiology of CRKP is currently being evaluated by the Carbapenem Resistance Consortium for Klebsiella pneumoniae (CRaCKle) [6]. This multicenter consortium is comprised of 5 health systems which include community-based hospitals and tertiary care referral centers ranging in size from 25 beds and over 700 annual admissions to over 1,400 beds and over 50,000 annual admissions. None of the hospitals performed screening for asymptomatic CRKP carriage during the study period. Here, we analyze tigecycline use in the cohort of patients with CRKP bacteriuria nested within CRaCKle.
CRKP are defined as K. pneumoniae isolates with decreased susceptibility to any of the carbapenems (MIC ≥ 2 mg/L). Bacterial identification and routine antimicrobial susceptibility testing was performed with MicroScan (Siemens Healthcare Diagnostics) or Vitek2 (BioMerieux), supplemented by GN4F Sensititre tray (Thermo Fisher) to confirm carbapenem results and to test tigecycline susceptibility. For tigecycline, European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints were used (susceptible MIC <2 mg/L, intermediate MIC=2 mg/L, and resistant MIC >2 mg/L). The Institutional Review Boards of all health systems involved approved the study.
Within the study period of 12/24/2011 until 10/1/2013, 260 unique patients with CRKP bacteriuria were included (Table); 73 patients met criteria for urinary tract infection (UTI) which was defined as outlined by Centers for Disease Control/National Healthcare Safety Network (CDC/NHSN) [7]. In addition, patients were deemed to have infection if they had a CRKP bloodstream infection within 48 hours of the positive urine culture even if CDC/NHSN criteria were not met.
Table.
All data are listed as n (%), unless otherwise indicated.
| All | Tigecycline | No Tigecycline | p | |
|---|---|---|---|---|
| n | 260 | 80 | 180 | |
| BASELINE CHARACTERISTICS | ||||
| Age, median (IQR) | 71 (61–81) | 72 (62–79) | 71 (60–83) | 0.80^^ |
| Gender, female | 162 (62) | 47 (58) | 115 (64) | 0.49 |
| Race | 0.54 | |||
| White, non Hispanic | 141 (54) | 42 (53) | 99 (55) | |
| Black, non Hispanic | 106 (41) | 34 (43) | 72 (40) | |
| Hispanic | 6 (2) | 3 (2) | 3 (2) | |
| Other | 7 (3) | 1 (1) | 6 (3) | |
| Diabetes mellitus | 141 (54) | 43 (54) | 98 (54) | 1.00 |
| Heart disease# | 139 (53) | 40 (50) | 99 (55) | 0.50 |
| Renal failure | 67 (26) | 16 (20) | 51 (28) | 0.17 |
| COPD | 72 (28) | 20 (25) | 52 (29) | 0.55 |
| Malignancy | 38 (15) | 11 (14) | 27 (15) | 0.85 |
| Dementia | 56 (22) | 17 (21) | 39 (22) | 1.00 |
| Immunocompromise† | 19 (7) | 5 (6) | 14 (8) | 0.80 |
| Charlson, median (IQR) | 3 (2–5) | 3 (2–6) | 3 (2–5) | 0.82 |
| Origin | 0.50^ | |||
| skilled nursing facility | 132 (51) | 46 (58) | 86 (48) | |
| home | 80 (31) | 20 (25) | 60 (33) | |
| hospital transfer | 32 (12) | 9 (11) | 23 (12) | |
| long term acute care | 16 (6) | 5 (6) | 11 (6) | |
| Infection | 73 (28) | 27 (34) | 46 (26) | 0.18 |
| Location at time of culture | 0.27 | |||
| emergency department | 90 (35) | 27 (34) | 63 (35) | |
| ward | 117 (45) | 32 (40) | 85 (47) | |
| intensive care unit | 53 (20) | 21 (26) | 32 (18) | |
| Pitt bacteremia score≥4 | 60 (23) | 26 (33) | 34 (19) | 0.025 |
| Days to first positive culture, median (IQR) | 0 (0–3) | 0 (0–2) | 0 (0–3) | 0.93 |
| Urinary drainage | 0.04^ | |||
| physiologic | 92 (35) | 19 (24) | 73 (41) | |
| Foley catheter | 129 (50) | 50 (63) | 79 (44) | |
| intermittent catheterization | 18 (7) | 5 (6) | 13 (7) | |
| permanent urinary diversion* | 21 (8) | 6 (8) | 15 (8) | |
| Urine wbc | 0.12^^^ | |||
| not performed | 71 (27) | 22 (28) | 49 (27) | |
| 0–5 cells/hpf | 13 (5) | 4 (5) | 9 (5) | |
| 5–10 cells/hpf | 13 (5) | 3 (4) | 10 (6) | |
| 10–25 cells/hpf | 33 (13) | 6 (8) | 27 (15) | |
| >25 cells/hpf | 130 (50) | 45 (56) | 85 (47) | |
| OUTCOMES | ||||
| CRKP in other sites*** | ||||
| none | 224 (86) | 60 (75) | 164 (91) | 0.0009 |
| blood | 14 (5) | 7 (9) | 7 (4) | 0.14 |
| respiratory | 9 (3) | 4 (5) | 5 (3) | 0.46 |
| wound | 12 (5) | 9 (11) | 3 (2) | 0.002 |
| other | 2 (1) | 0 (0) | 2 (1) | 1.00 |
| Length of stay, days, median (IQR) | 9 (5–15) | 12 (7–18) | 8 (5–14) | 0.001^^ |
| ICU admission | 119 (46) | 46 (57) | 73 (41) | 0.015 |
| days in ICU, median (IQR)** | 6 (3–13) | 10 (5–16) | 3 (4–9) | |
| Disposition | 0.23^^^^ | |||
| death/hospice | 29 (11) | 13 (16) | 16 (9) | |
| home | 59 (23) | 11 (14) | 48 (27) | |
| skilled nursing facility | 127 (49) | 34 (43) | 93 (52) | |
| long term acute care | 41 (16) | 21 (26) | 20 (11) | |
| transfer other hospital | 4 (2) | 1 (1) | 3 (2) | |
coronary artery disease and/or heart failure,
10mg/day prednisone or equivalent corticosteroid dosing, solid organ or stem cell transplant recipient,
includes suprapubic catheter and ileal conduit.
in those patients with an ICU admission.
during index admission (totals do not add to 100%, as some patients had CRKP isolated from more than one additional anatomical site). CRKP: carbapenem-resistant K. pneumoniae.
pearson.
median test,
ordinal logistic for those with urinalysis performed,
multivariate nominal logistic model adjusted for infection, Pitt score, drainage, and additional CRKP source.
Eighty (31%) patients received tigecycline during their index hospitalization. Each patient was only included once at the time of their first urine culture from which CRKP was isolated. Severe acute illness (defined as a Pitt bacteremia score greater or equal to 4 points, on the day of the index urine culture [8]) was more common in patients treated with tigecycline. At the time of culture, most patients had non-physiologic urinary drainage, defined as indwelling urinary catheter, intermittent catheterization or permanent urinary diversion (65%). A difference in distribution of method of urinary drainage was observed between patients treated with tigecycline; in the tigecycline group 24% had physiologic drainage, as compared to 41% of others (p=0.04). During the index hospitalization, 36 (14%) patients had CRKP isolated from other anatomical sites in addition to the urine. Tigecycline treatment was associated with CRKP isolated from other sites; 25% of patients treated with tigecycline had CRKP in other sites as compared to 9% those who did not receive tigecycline (p=0.0009).
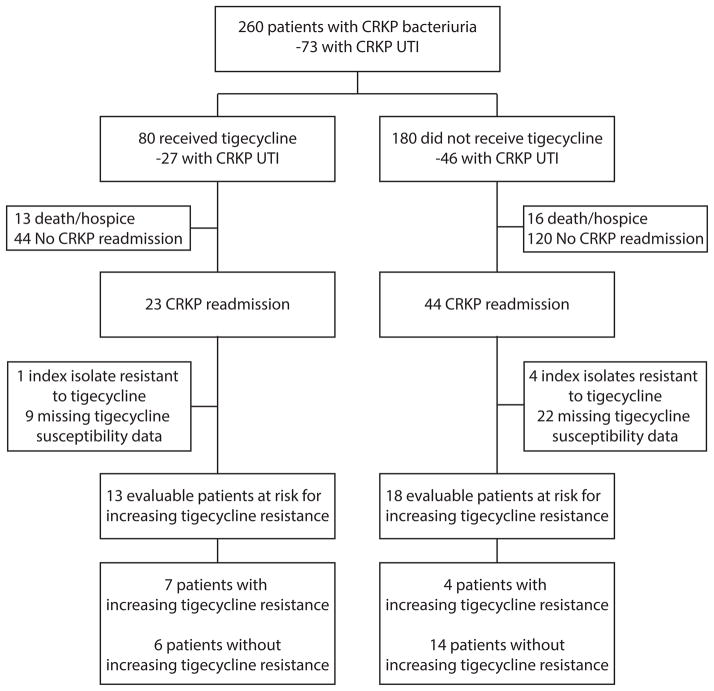
Tigecycline treatment was not associated with a change in the rate of readmissions during which CRKP was again isolated in culture (Figure). Tigecycline susceptibility data were available for the index culture and subsequent CRKP isolates in 36 patients with readmissions (Figure). Five of the index isolates were resistant to tigecycline. In the remaining 31 patients, increasing resistance was noted in 11/31 (35%) of patients; 6 patients had a susceptible index isolate and a resistant isolate upon readmission, 1 isolate went from intermediate to resistant, and 4 isolates went from susceptible to intermediate. The use of tigecycline was independently associated with the development of subsequent tigecycline resistance (OR 6.13, 95%CI 1.15–48.65, p=0.03). Tigecycline resistance developed in 4/18 (22%) who did not receive tigecycline, as compared to 7/13 (54%) of patients who were treated with tigecycline. In these 7 patients, the median time from index culture to first isolation of a more resistant isolate was 65 days (IQR 31–77 days).
Figure.
Diagram outlining patient flow throughout the study. CRKP carbapenem resistant Klebsiella pneumoniae. UTI urinary tract infection. CRKP readmission is defined as any readmission during which CRKP was again isolated from a clinical culture from any anatomical site.
A non-significant trend was seen towards more development of tigecycline resistance in patients with spontaneous physiologic urinary drainage (OR 4.56, 95% CI 0.83–36.24, p=0.08) as compared to patients with indwelling bladder catheters and other methods of non-physiologic urinary drainage such as intermittent straight catheterization. Both the susceptible and the non-susceptible strains were available for strain typing in 3 of 11 patients in whom tigecycline resistance developed. Repetitive extragenic palindromic (rep)-PCR was used (DiversiLab Strain typing system, Bacterial BarCodes, bioMerieux, Athens, GA) with ≥ 95% similarity considered as the same rep-PCR type [9]. In all 3 patients the strains were identical.
In this cohort, we noted frequent use of tigecycline, as well as a concerning baseline rate of tigecycline resistance. The association between tigecycline use and subsequent tigecycline resistance was previously evaluated in a case control study in K. pneumoniae isolates that produced either extended spectrum beta lactamases (ESBL) and/or K. pneumoniae carbapenemases (KPC); a similar magnitude of effect between tigecycline use and tigecycline resistance (OR 6.00) was reported [10]. The current study provides concordant evidence; important strengths of the current study are that we show the longitudinal and directional development of tigecycline resistance with prospectively collected data, and the molecular analysis confirming that initial susceptible strains and subsequent resistant strains in a given patient were identical.
Limitations include the potential of confounding by indication, and the lack of an objective method to distinguish asymptomatic bacteriuria from UTI. We chose to include all patients with CRKP bacteriuria, as treatment is common in those patients who do not meet the CDC/NHSN criteria for UTI which were primarily designed for surveillance purposes. Lastly, of the total 260 patients included in this study, only 31 could be included in the analysis of the influence of tigecycline use on subsequent tigecycline resistance. Independent confirmation from other cohorts will be required for ultimate validation of our findings.
In summary, the use of tigecycline in patients with CRKP bacteriuria – while infrequently indicated – was relatively common in this population. We have shown that the use of tigecycline in patients with CRKP bacteruria is associated with rapid development of in vitro resistance.
Acknowledgments
The authors wish to thank all the patients who were described in this paper and their families.
FUNDING:
This work was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number UM1AI104681, and by funding to DVD and FP from the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. In addition, this work was supported in part by the Veterans Affairs Merit Review Program (RAB), the National Institutes of Health (Grant AI072219-05 and AI063517-07 to RAB), and the Geriatric Research Education and Clinical Center VISN 10 (RAB), the Research Program Committees of the Cleveland Clinic (DVD), an unrestricted research grant from the STERIS Corporation (DVD)
TRANSPARENCY:
DvD has received research funding from Steris Inc, and has served on the Speaker’s Bureau for Astellas.
RAB has received research funding from Astra Zeneca, Merck and Checkpoints and has served on a Tetraphase drug safety monitoring board.
References
- 1.Braykov NP, Eber MR, Klein EY, Morgan DJ, Laxminarayan R. Trends in resistance to carbapenems and third-generation cephalosporins among clinical isolates of klebsiella pneumoniae in the united states, 1999–2010. Infect Control Hosp Epidemiol. 2013;34:259–268. doi: 10.1086/669523. [DOI] [PubMed] [Google Scholar]
- 2.van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant enterobacteriaceae: A review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013;75:115–120. doi: 10.1016/j.diagmicrobio.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sader HS, Farrell DJ, Flamm RK, Jones RN. Tigecycline activity when tested against bacterial strains from united states medical centers: Variation in potency and spectrum since approval for clinical use (2006–2012) Antimicrob Agents Chemother. 2014 doi: 10.1128/AAC.02684-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang TD, Berhin C, Bogaerts P, Glupczynski Y. In vitro susceptibility of multidrug-resistant enterobacteriaceae clinical isolates to tigecycline. J Antimicrob Chemother. 2012;67:2696–2699. doi: 10.1093/jac/dks288. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Camacho E, Gomez-Gil R, Tobes R, et al. Genomic analysis of the emergence and evolution of multidrug resistance during a klebsiella pneumoniae outbreak including carbapenem and colistin resistance. J Antimicrob Chemother. 2014;69:632–636. doi: 10.1093/jac/dkt419. [DOI] [PubMed] [Google Scholar]
- 6.van Duin D, Perez F, Rudin SD, et al. Surveillance of carbapenem-resistant klebsiella pneumoniae: Tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother. 2014 doi: 10.1128/AAC.02636-14. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horan TC, Andrus M, Dudeck MA. Cdc/nhsn surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: A commentary. Int J Antimicrob Agents. 1999;11:7–12. doi: 10.1016/s0924-8579(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 9.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. Multilocus sequence typing of klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43:4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nigo M, Cevallos CS, Woods K, et al. Nested case-control study of the emergence of tigecycline resistance in multidrug-resistant klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:5743–5746. doi: 10.1128/AAC.00827-13. [DOI] [PMC free article] [PubMed] [Google Scholar]