Abstract
AIM: To analyze the cytokine production by peripheral blood cells from cirrhotic patients with and without TLR4 D299G and/or T399I polymorphisms.
METHODS: The study included nine patients with cirrhosis and TLR4 D299G and/or T399I polymorphisms, and 10 wild-type patients matched for age, sex and degree of liver failure. TLR4 polymorphisms were determined by sequence-based genotyping. Cytokine production by peripheral blood cells was assessed spontaneously and also after lipopolysaccharide (LPS) and lipoteichoic acid (LTA) stimulation.
RESULTS: Patients with TLR4 polymorphisms had a higher incidence of previous hepatic encephalopathy than wild-type patients (78% vs 20%, P = 0.02). Spontaneous production of interleukin (IL)-6 and IL-10 was lower in patients with TLR4 polymorphisms than in wild-type patients [IL-6: 888.7 (172.0-2119.3) pg/mL vs 5540.4 (1159.2-26053.9) pg/mL, P < 0.001; IL-10: 28.7 (6.5-177.1) pg/mL vs 117.8 (6.5-318.1) pg/mL, P = 0.02]. However, the production of tumor necrosis factor-α, IL-6 and IL-10 after LPS and LTA stimulation was similar in the two groups.
CONCLUSION: TLR4 polymorphisms were associated with a distinctive pattern of cytokine production in cirrhotic patients, suggesting that they play a role in the development of cirrhosis complications.
Keywords: Inflammatory response, Hepatic encephalopathy, Genetic factors, Infections
Core tip: The relationship between toll-like receptors (TLR) polymorphisms and the immune response is the focus of intensive research in chronic inflammatory conditions. This is the first study demonstrating that the presence of certain TLR4 polymorphisms is associated with a characteristic pattern of cytokine production. These polymorphisms could have a relevant role in the development of complications in patients with cirrhosis.
INTRODUCTION
A pro-inflammatory state is a frequent phenomenon in patients with cirrhosis and it has been related to several factors. One of these factors is the exposure to pathogen-associated molecular patterns (PAMPs) [i.e., endotoxin or lipopolysaccharide (LPS) from gram-negative bacteria] due to intestinal bacterial translocation[1-6]. There is growing evidence that the inflammatory response in these patients is implicated in the prognosis and development of complications[1,2,7,8], especially hepatic encephalopathy[9-11]. Moreover, due to bacterial translocation and alterations in immune response, patients with cirrhosis are predisposed to develop bacterial infections, mainly caused by enteric pathogens[1,2].
Toll-like receptors (TLR) recognize molecules broadly shared by pathogens but distinguishable from host molecules, collectively referred to as PAMPs. TLR2 recognizes multiple bacterial cell wall components (peptidoglycan, lipoteichoic acid and lipoproteins) by forming heterodimers with TLR1 and TLR6 and cooperating with CD14, CD36 and Dectin-1. In association with CD14 and its co-receptor MD-2, TLR4 recognizes LPS, a major component of gram-negative bacteria. It was reported that CD14 is also involved in TLR2-mediated lipoteichoic acid (LTA) stimulation[12]. On the other hand, TLR2 seems to be required for LPS-induced TLR4 signaling[13]. After the binding of TLR ligands, pro-inflammatory cytokines are released by the immune cell and systemic inflammatory response occurs[3,14,15].
Two main non-synonymous TLR4 genetic polymorphisms can usually be found in co-segregation in Caucasians[14-16]. The first of these is D299G, an A/G polymorphism that causes the amino-acid change from Asp to Gly at position 299, and the other is T399I, a C/T polymorphism that causes the change from Thr to Ile at position 399[14-16]. These polymorphisms have been shown to change the ligand-binding site of the receptor[17]. They can modify the inflammatory response[16,17] and predispose to bacterial infections[14,15,18]. The presence of TLR4 polymorphisms can therefore influence disease progression and development of complications in patients with cirrhosis[19,20].
We observed that cirrhotic patients with the TLR4 D299G polymorphism were more predisposed to bacterial infections and hepatic encephalopathy than wild-type patients[19]. One possible explanation for these findings is that the inflammatory response in patients with the TLR4 D299G polymorphism differs from that in wild-type patients.
This study was designed to compare the cytokine production by peripheral blood cells from cirrhotic patients with TLR4 D299G and/or T399I polymorphisms to the cytokine production in patients without these polymorphisms.
MATERIALS AND METHODS
Patients
From April 2006 to May 2011 we screened 258 consecutive hospitalized patients with cirrhosis and ascites and found TLR4 D299G and/or T399I polymorphisms in 28 patients (11%). The present study was performed between February 2009 and July 2011 and included those patients with TLR4 polymorphisms who survived free of liver transplant and fulfilled the inclusion and exclusion criteria. Patients with TLR4 polymorphisms (polymorphism group) were matched for age, sex and degree of liver failure with cirrhotic patients without these polymorphisms (wild-type group). All the patients were Caucasian and cirrhosis was diagnosed by biopsy or from clinical, analytical and ultrasonographic findings. They all had decompensated cirrhosis as they had previously presented at least one decompensation.
All were outpatients coming to the hospital for follow-up visits or to the day hospital for therapeutic paracentesis. The exclusion criteria were human immunodeficiency virus infection or any other immunodeficiency, treatment with immunosuppressive or immunomodulatory drugs, hepatocellular carcinoma or other neoplasia, active alcohol intake in the previous year, liver transplantation, and active infection or any other decompensation of cirrhosis requiring hospitalization in the previous month.
At inclusion in the study, we recorded clinical and analytical data as well as previous decompensations of cirrhosis, with special emphasis on hepatic encephalopathy episodes, and we collected blood samples for laboratory analysis/assays.
A group of healthy Caucasian donors was also included to compare their cytokine production with that of patients with cirrhosis.
The study was a priori approved by the Clinical Research Ethics Committee at Hospital de la Santa Creu i Sant Pau and conforms to the ethical guidelines of the Helsinki Declaration of 1975 (6th revision, 2008). All patients gave written consent to be included after receiving appropriate verbal and written information.
Samples
Whole blood samples were collected from healthy donors, patients from the polymorphism group (TLR4 D299G and/or T399I) and wild-type patients. Samples were processed within one hour after collection and maintained at room temperature. For cytokine production analysis, blood was collected into BD Vacutainer® tubes (BDbiosciences, San Jose, CA, United States) containing sodium-heparin. For the genomic DNA extraction, blood was collected into separate BD Vacutainer® tubes containing ethylene diamine tetra-acetic acid.
Genomic DNA extraction and TLR4 and TLR2 polymorphisms genotyping
Genomic DNA was extracted from buffy-coat fraction using QIAmp DNA blood minikit (Qiagen Inc., Valencia, CA, United States). The sequencing primer used for TLR4 Asp299Gly (D299G, rs4986790) was 5’-TGGAATGCTGGAAATCCAGA-3’, and for Thr399Ile (T399I, rs4986791) was 5’-CTCTAGAGGGCCTGTGCA-3’.
As TLR2 polymorphisms can influence the cytokine production[15,16], we assessed the presence of Arg753Gln (R753Q, rs5743708) and Arg677Trp (R677W) TLR2 polymorphisms in our study population. The sequencing primer used for TLR2 R753Q was 5’-GCCTACTGGGTGGAGAACCT-3’ and for R677W was 5’-GGCCACTCCAGGTAGGTCTT-3’.
The polymerase chain reaction consisted of an initial denaturation at 95 °C for 3 min, followed by 34 cycles of 45 s at 95 °C, 30 s at 60 °C for TLR4 and 61.2 °C for TLR2, 1 min at 72 °C, and a final extension of 5 min at 72 °C. Once the amplification was confirmed, sequencing was performed by Macrogen Inc, Korea using BigDye (Applied Biosystem) chemistry.
Lipopolysaccharide binding protein levels
Serum was tested for lipopolysaccharide binding protein (LBP) concentration to assess exposure to bacteria and their endotoxins as an index of bacterial translocation[5], using specific ELISA (Biometec GmbH, Greifswald, Germany) according to the manufacturer’s instructions. LBP was quantified with standard curves provided by the corresponding ELISA kit. The detection limit was 5 ng/mL.
Whole blood cell culture
Heparinized whole blood (2.5 mL) was cultured in 5 ml polypropylene tubes (BDbiosciences, San Jose, CA, United States) with: (1) 1 mL of RPMI 1640 medium (Biowhittaker, Cologne, Germany); (2) 650 μL of RPMI 1640 medium with 350 μL of ultrapure LPS (0.01 μg/mL) (Ultra pure lipopolysaccharide from Escherichia coli 0111:B4 strain- TLR4 ligand cat# tlrl-pelps InvivoGen, San Diego, CA, United States); and (3) 350 μL of LTA (1 μg/mL) (Lipoteichoic acid from Staphylococcus aureus- TLR2 ligand cat #tlrl-psltal InvivoGen). The cultures were maintained at 37 °C with 5% CO2 for 20 h.
Cytokine levels
Culture supernatants were tested for TNF-α, IL-10 (BD Pharmingen, Frankin Lakes, NJ, United States) and IL-6 (ImmunoTools, Friesoythe, Germany) concentrations using specific ELISAs, according to the manufacturer’s instructions. All cytokines were quantified with standard curves provided by the corresponding ELISA kit. The detection limits were: 30 pg/mL for TNF-α and IL-10, and 10 pg/mL for IL-6.
Statistical analysis
Comparisons between patients with cirrhosis and healthy donors and between patients with TLR4 polymorphisms and wild-type patients were performed using Fisher’s exact test for categorical variables and Mann-Whitney test for quantitative data. A two-sided P value < 0.05 was considered statistically significant. Data are expressed as frequencies or median (range).
RESULTS
Patient characteristics
From the 28 patients with TLR4 D299G and/or T399I polymorphisms, 19 were not included for the following causes: 8 died before the present study was performed, 2 underwent liver transplantation, 6 were lost to follow-up, 2 developed hepatocellular carcinoma, and 1 had macroglobulinemia. Therefore, 9 patients with cirrhosis and TLR4 D299G and/or T399I polymorphisms (polymorphism group) were included in the study and compared to 10 matched cirrhotic patients without these polymorphisms (wild-type group). Five healthy donors were also included.
In the polymorphism group, eight of the nine patients showed both TLR4 D299G and T399I polymorphisms in heterozygosis, while the remaining patient showed only the T399I polymorphism in heterozygosis. Neither of the TLR2 polymorphisms was detected in the polymorphism group nor in the wild-type group. No TLR4 or TLR2 polymorphisms were detected in any of the healthy donors.
Table 1 shows clinical and analytical characteristics of the two groups of patients. We did not find statistical differences in demographics, etiology of cirrhosis, degree of liver insufficiency, blood total leukocyte, monocyte and lymphocyte count, pharmacological treatment, or presence of ascites. There was a trend towards more patients on prophylaxis with norfloxacin in the polymorphism group.
Table 1.
Clinical and analytical characteristics of patients with TLR4 polymorphisms and wild-type patients n (%)
| Polymorphisms (n = 9) | Wild-type (n = 10) | P value | |
| Age (yr) | 63.2 (40-78) | 61.0 (42-79) | 0.93 |
| Sex (male/female) | 6 (67)/3 (33) | 6 (60)/4 (40) | 1.00 |
| Etiology (alcohol/HCV/alcohol + HCV/other) | 3 (33)/3 (33)/2 (22)/1 (11) | 5 (50)/2 (20)/3 (30)/0 (0) | 0.5 |
| Child-Pugh score | 7 (5-9) | 6 (5-8) | 0.49 |
| MELD score | 11 (6-16) | 12 (6-18) | 0.62 |
| Serum bilirubin (μmol/L) | 17.0 (7-41) | 27.5 (9-60) | 0.56 |
| Serum albumin (g/L) | 32.8 (22.7-49.4) | 35.9 (30.0-43.9) | 0.34 |
| INR | 1.10 (1.01-1.48) | 1.22 (0.96-1.39) | 0.68 |
| Blood total leukocyte count (× 109/L) | 5.89 (2.75-11.48) | 5.67 (3.84-8.57) | 0.93 |
| Blood neutrophil count (× 109/L) | 3.30 (2.00-7.78) | 3.21 (1.61-5.63) | 0.74 |
| Blood monocyte count (× 109/L) | 0.59 (0.20-0.98) | 0.66 (0.38-1.00) | 0.46 |
| Blood lymphocyte count (× 109/L) | 1.00 (0.38-2.15) | 1.41 (0.35-2.22) | 0.39 |
| Present ascites | 3 (33) | 3 (30) | 1.00 |
| Diuretics | 6 (67) | 9 (90) | 0.30 |
| Beta-blockers | 4 (44) | 5 (50) | 1.00 |
| Norfloxacin prophylaxis | 5 (55) | 4 (40) | 0.65 |
HCV: Hepatitis C virus; MELD: Model for end-stage liver disease; INR: International normalized ratio.
The follow up from the time of the first decompensation of cirrhosis until inclusion in the study was 18.0 (4.5-97.0) months in the polymorphism group and 30.7 (2.5-59.5) in the wild-type group (P = 0.36). Table 2 shows the previous complications of cirrhosis during this period. There was a non-significant trend to a higher incidence of variceal bleeding and bacterial infections in the polymorphism group than in the wild-type group. The incidence of hepatic encephalopathy was higher in the polymorphism group than in the wild-type group (78% vs 20%, P = 0.02). Patients from the polymorphism group presented a higher number of hepatic encephalopathy episodes per patient than the wild-type group. Infection was the most frequent precipitating factor in the two groups: 8 episodes in the polymorphism group and 5 in the wild-type group. Other precipitating events were: diuretics (4 and 0 episodes), electrolyte disturbances (1 and 2), gastrointestinal bleeding (1 and 0), constipation (2 and 0), and benzodiazepine treatment (2 and 0). No precipitating events could be identified in 2 episodes from the polymorphism group and in 1 episode from the wild-type group.
Table 2.
Data of previous complications of cirrhosis in patients with TLR4 polymorphisms and wild-type patients n (%)
| Polymorphisms (n = 9) | Wild-type (n = 10) | P value | |
| Previous ascites | 9 (100) | 10 (100) | 1.00 |
| Previous variceal bleeding | 4 (44) | 2 (20) | 0.35 |
| Previous bacterial infections | 8 (89) | 5 (50) | 0.14 |
| Number of infections/patient | 2.0 (0-4) | 0.5 (0-5) | 0.14 |
| Previous SBP | 5 (55) | 3 (30) | 0.37 |
| Previous hepatic encephalopathy | 7 (78) | 2 (20) | 0.02 |
| Number of episodes of encephalopathy | 17 | 8 | |
| Number of episodes of encephalopathy/patient | 1 (0-5) | 0 (0-7) | 0.03 |
| Degree of encephalopathy (episodes) 1/2/3/4 | 5/9/1/2 | 3/4/0/1 | |
| Degree of encephalopathy1 | 2.0 (1-4) | 1.9 (1-4) | 0.74 |
Median (range) of the degree of hepatic encephalopathy in patients with this complication. SBP: Spontaneous bacterial peritonitis.
LBP plasma levels
LBP plasma levels showed a trend to be higher in all patients with cirrhosis than in healthy donors [21.7 (2.2-49.7) μg/mL vs 9.3 (6.0-16.2) μg/mL, P = 0.28]. Patients with TLR4 polymorphisms showed a trend to have higher LBP plasma levels than wild-type patients [29.4 (14.7-49.7) μg/mL vs 7.4 (2.2-32.9) μg/mL, P = 0.07].
Cytokine production
Table 3 shows cytokine production by peripheral blood cells from healthy donors and all patients with cirrhosis. Spontaneous TNF-α and IL-6 production was significantly higher in patients with cirrhosis than in healthy donors. The cytokine production of TNF-α after LTA stimulation and IL-6 after LPS and LTA stimulation was significantly higher in patients with cirrhosis than in healthy donors.
Table 3.
Spontaneous and stimulated cytokine production by peripheral blood cells in healthy donors and all patients with cirrhosis
| Healthy donors (n = 5) | All patients with cirrhosis (n = 19) | P value | |
| Spontaneous TNF-α (pg/mL) | < 30 | 602.4 (96.1-3920.1) | 0.001 |
| TNF-α after LPS (pg/mL) | 1693 (1185-3709) | 2904 (931-5078) | 0.150 |
| TNF-α after LTA (pg/mL) | 638.3 (49.7-1958) | 2440 (241-4798) | 0.006 |
| Spontaneous IL-6 (pg/mL) | < 10 | 1537.8 (172.0-26053.9) | 0.001 |
| IL-6 after LPS (pg/mL) | 20493 (19499-23042) | 30219 (1362-32680) | 0.006 |
| IL-6 after LTA (pg/mL) | 22157 (17390-27010) | 30165 (3269-32707) | 0.005 |
| Spontaneous IL-10 (pg/mL) | < 30 | 43.6 (6.5-318.1) | 0.210 |
| IL-10 after LPS (pg/mL) | 1309 (590.2-1578) | 939.9 (120.8-2100) | 0.890 |
| IL-10 after LTA (pg/mL) | 499.3 (268.7-816.9) | 800.4 (80.72-2077) | 0.150 |
TNF-α: Tumour necrosis factor-alpha; LPS: Lipopolysaccharide; LTA: Lipoteichoic acid; IL: Interleukin.
Figure 1 shows spontaneous and stimulated cytokine production in the two groups of patients with cirrhosis. There was a non-significant trend for a lower TNF-α spontaneous production in the polymorphism group than in the wild-type group. Spontaneous production of IL-6 [888.7 (172.0-2119.3) pg/mL vs 5540.4 (1159.2-26053.9) pg/mL, P < 0.001] and IL-10 [28.7 (6.5-177.1) pg/mL vs 117.8 (6.5-318.1) pg/mL, P = 0.02] was lower in the polymorphism group than in the wild-type group. The cytokine production after stimulation with LPS and LTA was similar in the two groups. Therefore, the increase in stimulated cytokine production with respect to spontaneous cytokine production, expressed as fold change, was higher in the polymorphism group than in the wild-type group (Table 4).
Figure 1.
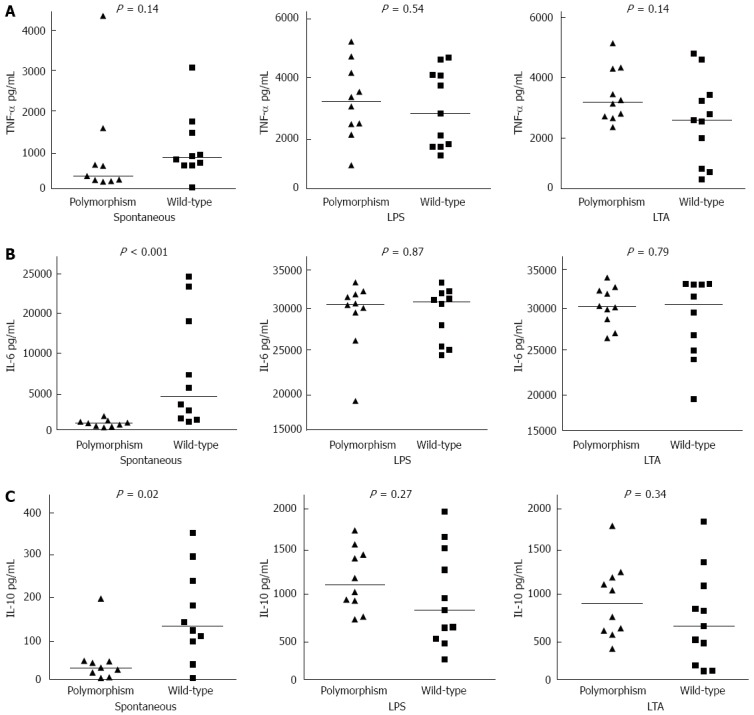
Cytokine production in patients with cirrhosis and TLR4 polymorphisms and wild-type patients. A: TNF-α concentration in supernatants of unstimulated and LPS and LTA stimulated peripheral blood cells; B: IL-6 concentration in supernatants of unstimulated and LPS and LTA stimulated peripheral blood cells; and C: IL-10 concentration in supernatants of unstimulated and LPS and LTA stimulated peripheral blood cells. TNF-α: Tumour necrosis factor-alpha; LPS: Lipopolysaccharide; LTA: Lipoteichoic acid; IL: Interleukin.
Table 4.
Increment in cytokine production expressed as stimulated/spontaneous production fold change in patients with cirrhosis and TLR4 polymorphisms and wild-type patients
| Polymorphisms (n = 9) | Wild-type (n = 10) | P value | |
| Increment TNF-α after LPS | 5.3 (1.0-17.4) | 3.8 (1.1-17.0) | 0.280 |
| Increment TNF-α after LTA | 6.5 (1.2-14.4) | 2.7 (0.2-5.1) | 0.040 |
| Increment IL-6 after LPS | 26.0 (14.7-189.9) | 3.3 (1.1-17.0) | < 0.001 |
| Increment IL-6 after LTA | 35.2 (15.4-183.6) | 3.0 (1.2-25.6) | < 0.001 |
| Increment IL-10 after LPS | 37.8 (9.3-185.1) | 6.3 (0.5-143.9) | 0.006 |
| Increment IL-10 after LTA | 32.0 (11.3-70.4) | 3.7 (1.0-23.7) | 0.002 |
TNF-α: Tumour necrosis factor-alpha; LPS: Lipopolysaccharide; LTA: Lipoteichoic acid; IL: Interleukin.
Table 5 shows cytokine production by peripheral blood cells from patients without prophylactic norfloxacin. The differences in the cytokine production between patients with TLR4 polymorphisms and wild-type patients in this subgroup were similar to those observed in the whole series.
Table 5.
Spontaneous and stimulated cytokine production by peripheral blood cells in patients with cirrhosis not treated with norfloxacin
| Polymorphisms (n = 4) | Wild-type (n = 6) | P value | |
| Spontaneous TNF-α (pg/mL) | 464.3 (223.6-3920.1) | 813.1 (591-1573) | 0.25 |
| TNF-α after LPS (pg/mL) | 3166 (931.8-5078) | 2936 (1272-4547) | 0.76 |
| TNF-α after LTA (pg/mL) | 2766 (2291-4798) | 1927 (241.3-4455) | 0.17 |
| Spontaneous IL-6 (pg/mL) | 699.4 (320.8-1145.6) | 5540 (1538-26053.9) | < 0.001 |
| IL-6 after LPS (pg/mL) | 27880 (19100-30070) | 29000 (24960-32670) | 0.47 |
| IL-6 after LTA (pg/mL) | 29660 (27970-30290) | 29860 (3269-32170) | 1.00 |
| Spontaneous IL-10 (pg/mL) | 13.2 (6.5-39.1) | 135.6 (36.2-318.1) | 0.02 |
| IL-10 after LPS (pg/mL) | 949.5 (655-1474) | 1467 (334.5-2100) | 0.35 |
| IL-10 after LTA (pg/mL) | 606.8 (376-1234) | 897.6 (86.66-1530) | 0.91 |
TNF-α: Tumour necrosis factor-alpha; LPS: Lipopolysaccharide; LTA: Lipoteichoic acid; IL: Interleukin.
DISCUSSION
The main finding in the present study is that peripheral blood cells from patients with cirrhosis and TLR4 D299G and/or T399I polymorphisms spontaneously produced lower levels of IL-6 and IL-10 than wild type patients but showed a similar IL-6, TNF-α and IL-10 production after stimulation.
Patients with cirrhosis presented a higher spontaneous production of pro-inflammatory cytokines than healthy donors. This finding agrees with the pro-inflammatory state described in cirrhosis[5,6], thought to be mainly related to bacterial translocation and increased plasma endotoxin[1,2,4-6]. When continuously exposed to endotoxin, the immune system can develop tolerance, leading to blunted cytokine production after stimulation[21-23]. However, our results do not support this hypothesis because we observed a higher pro-inflammatory cytokine production after LPS and LTA stimulation in patients with cirrhosis than in controls. A higher pro-inflammatory cytokine production after LPS stimulation has also been observed previously in patients with cirrhosis[24] and in rats with carbon tetrachloride-induced cirrhosis[25].
Despite similar clinical and analytical characteristics, patients with cirrhosis and TLR4 D299G and/or T399I polymorphisms showed lower IL-6 spontaneous production than wild-type patients. In the setting of cirrhosis and increased endotoxemia, this finding could be the consequence of a decrease in the cellular responsiveness to endotoxin associated with these TLR4 polymorphisms, as observed in other populations[16,26]. Such impaired responsiveness could lead to a defective clearance of the increased plasma endotoxin, which in turn would enhance synthesis of LBP, an index of long-term exposure to endotoxin[5]. Moreover, increased LBP could further decrease the synthesis of IL-6[27]. In support of this hypothesis, we observed a trend for higher LBP plasma levels in patients with TLR4 polymorphisms than in wild-type patients. However, when peripheral blood cells were stimulated with high concentrations of LPS or LTA, patients with cirrhosis and TLR4 polymorphisms and wild type-patients showed a similar cytokine production. Our findings are in keeping with previous data showing that the functional consequences of TLR4 polymorphisms are more evident at low agonist concentrations than at high agonist concentrations[28]. Changes in the anti-inflammatory cytokine IL-10 paralleled those observed in IL-6 and can be explained as a compensatory mechanism to attenuate the effects of this pro-inflammatory cytokine[23].
Previous studies in non-cirrhotic populations have reported contradictory results about the relationship between TLR4 D299G and T399I polymorphisms and immune response[15,16,18,26,29-32], as hypo-[16,26], normo-[29-32] and also hyper-responsiveness[29] have all been associated with these polymorphisms. These contradictory findings can be attributed to differences between the studies, such as the characteristics and status of patients or cells evaluated, stimulation protocols and the doses of ligands used, and interactions between several coincident polymorphisms[15,18,28,29]. For example, in the Dogon population, the TLR4 D299G polymorphism is usually observed without the T399I polymorphism, and it has been associated with a higher TNF-α production after LPS stimulation than in wild-type individuals[29]. In Caucasians, however, the D299G polymorphism is almost always associated with the T399I polymorphism, and these two polymorphisms are associated with a TNF-α production after LPS stimulation that is similar to that in wild-type individuals[29]. To our knowledge, no studies have previously evaluated the influence of TLR4 D299G or T399I polymorphisms on peripheral blood cell function in the complex immunological setting of patients with cirrhosis.
Antibiotic treatment can influence the function of immune cells by decreasing bacterial translocation[5,22,33]. To avoid this confounding factor, we further analyzed patients without prophylactic norfloxacin separately. We found that patients with TLR4 polymorphisms presented lower spontaneous IL-6 production than wild-type patients, as observed in the whole series.
The influence of genetic factors in the evolution of patients with liver diseases is currently gaining interest[19-20,34-36]. The different cytokine production profile observed in our patients could help to explain some associations between TLR4 polymorphisms and the development of complications in cirrhosis. The lower spontaneous pro-inflammatory cytokine production in our study reflects a less marked basal pro-inflammatory state in vivo in patients with TLR4 D299G and/or T399I polymorphisms than in wild-type patients. This finding could be related to the predisposition of patients with these TLR4 polymorphisms to develop bacterial infections[14,15,19], but it could also explain the protective effect of TLR4 polymorphisms against progression of liver disease[19,20]. The inflammatory response has been increasingly implicated in the development of hepatic encephalopathy, and it is now considered that inflammation acts synergistically with ammonia toxicity in the pathogenesis of this complication[9-11]. Moreover, as we also observed in the present study, infection is a main triggering factor of hepatic encephalopathy[9-11]. The different cytokine production profile observed in our study suggests that cirrhotic patients with TLR4 polymorphisms may show in vivo a greater difference between basal and stimulated (i.e., during bacterial infection[1-5,37]) cytokine production than wild-type patients. This higher cytokine production gradient in TLR4 polymorphisms patients could be related to their predisposition to develop hepatic encephalopathy, as has been observed here and in a previous study with a higher number of patients[19].
Our study has certain limitations. The first is the small number of patients studied and their heterogeneity, as etiologies of cirrhosis were diverse and some patients were on norfloxacin treatment. It was difficult to recruit a larger, more homogeneous series of patients because the prevalence of TLR4 D299G and T399I polymorphisms is only about 10% of the population[14,15,19]. Besides, we found that our results were similar when we analyzed only patients without antibiotic treatment. Furthermore, the etiology was related to alcohol and/or hepatitis C virus in most patients in both groups. Previous studies have shown that the presence of hepatitis C virus appears to have no relevant effects on the cytokine production evaluated in the present study[38,39]. A second limitation is that we determined cytokine production in whole blood cell cultures. This is a physiological model to study the overall peripheral immune response ex vivo[29,31,40], but it is unable to identify the subsets of cells responsible for the different cytokine production profile. And third, the relationship between a higher cytokine production gradient and the development of hepatic encephalopathy in patients with TLR4 polymorphisms is only speculative because no data were available on cytokine production during hepatic encephalopathy episodes.
In conclusion, spontaneous IL-6 and IL-10 production was lower in patients with cirrhosis and TLR4 D299G and/or T399I polymorphisms than in wild-type patients. Furthermore, the production of IL-6, TNF-α and IL-10 after TLR stimulation was similar in patients with TLR4 polymorphisms and in wild-type patients. This different pattern of cytokine production could play a role in the development of complications, such as hepatic encephalopathy, in patients with these polymorphisms.
ACKNOWLEDGMENTS
The authors thank Carolyn Newey for English language assistance. This study was performed within the PhD immunology program at Universitat Autònoma de Barcelona.
COMMENTS
Background
Due to bacterial translocation and several alterations in the immune response, patients with cirrhosis are predisposed to develop infections caused by enteric bacteria. Pathogen-associated molecular patterns in these bacteria are usually recognized by transmembrane receptors (TLRs) found mainly on the surface of leukocytes. When this occurs, TLRs trigger the innate inflammatory response. The authors previously observed that cirrhotic patients with the TLR4 D299G polymorphism were more predisposed to bacterial infections and hepatic encephalopathy than wild-type patients. One possible explanation for these findings is that the inflammatory response in patients with the TLR4 D299G polymorphism differs from that in wild-type patients.
Research frontiers
The influence of genetic factors in the evolution of patients with liver diseases is currently gaining interest. The profile of the innate immune response in patients with TLR polymorphisms could help to explain the development of complications in cirrhosis.
Related publications
Guarner-Argente C, Sanchez E, Vidal S, Roman E, Concepcion M, Poca M, Sanchez D, Juarez C, Soriano G, Guarner C. Toll-like receptor 4 D299G polymorphism and the incidence of infections in cirrhotic patients. Aliment Pharmacol Ther 2010; 31(11): 1192-1199
Innovations and breakthroughs
No studies have previously evaluated the influence of TLR4 polymorphisms on peripheral blood cell function in the complex immunological setting of patients with cirrhosis. Cirrhotic patients with TLR4 D299G and/or T399I polymorphisms showed a less marked basal pro-inflammatory state than wild-type patients, predisposing them to develop bacterial infections. Cirrhotic patients with these TLR4 polymorphisms showed a higher difference between basal and bacteria stimulated cytokine production than wild-type patients. Since infection is a main triggering factor of hepatic encephalopathy, this higher cytokine production gradient in patients with these polymorphisms could be related to their predisposition to develop this cirrhotic complication.
Applications
The main application is the identification of predisposing biomarkers for cirrhosis complications: bacterial infections and hepatic encephalopathy.
Terminology
Bacterial translocation is defined as the passage of bacteria or their products from the intestinal lumen to extraintestinal sites, such as mesenteric lymph nodes. Hepatic encephalopathy is the occurrence of confusion, altered level of consciousness and coma as a result of liver failure.
Peer review
The studies presented the data on the cytokine [interleukin (IL)-6 and IL-10] and tumor necrosis factor production by peripheral blood cells from cirrhotic patients with and without TLR4/D299G and /or T399I polymorphism. The cytokine production was assessed before and after lipopolysaccharide and lipoteichoic acid stimulation. Based on the presented data the authors suggest that the presence of TLR4/D299G and/or T399I polymorphism may be associated with a distinct pattern of cytokine production in patients with cirrhosis and, hence could be responsible for the development of complications. This is quite informative, well written, and relatively well presented study. However, there are several omissions that detract the reader.
Footnotes
Supported by Health research fund “Fondo de Investigaciones Sanitarias” (to SV); Catalan Ministry of Health Stabilization Program; and Institute of Health Carlos III, Ministry of Science and Innovation, Grant No. PI09/00357, Madrid, Spain
P- Reviewer: Slomiany BL S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Bellot P, Francés R, Such J. Pathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implications. Liver Int. 2013;33:31–39. doi: 10.1111/liv.12021. [DOI] [PubMed] [Google Scholar]
- 2.Guarner C, Soriano G. Bacterial translocation and its consequences in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:27–31. doi: 10.1097/00042737-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704–720. doi: 10.1136/gut.2008.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francés R, Zapater P, González-Navajas JM, Muñoz C, Caño R, Moreu R, Pascual S, Bellot P, Pérez-Mateo M, Such J. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology. 2008;47:978–985. doi: 10.1002/hep.22083. [DOI] [PubMed] [Google Scholar]
- 5.Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208–217. doi: 10.1053/jhep.2003.50038. [DOI] [PubMed] [Google Scholar]
- 6.Tazi KA, Quioc JJ, Abdel-Razek W, Tellier Z, Guichard C, Ogier-Denis E, Lebrec D, Moreau R. Protein array technology to investigate cytokine production by monocytes from patients with advanced alcoholic cirrhosis: An ex vivo pilot study. Hepatol Res. 2009;39:706–715. doi: 10.1111/j.1872-034X.2009.00498.x. [DOI] [PubMed] [Google Scholar]
- 7.Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, Monnet E, Di Martino V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol. 2012;56:1299–1304. doi: 10.1016/j.jhep.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Zapater P, Francés R, González-Navajas JM, de la Hoz MA, Moreu R, Pascual S, Monfort D, Montoliu S, Vila C, Escudero A, et al. Serum and ascitic fluid bacterial DNA: a new independent prognostic factor in noninfected patients with cirrhosis. Hepatology. 2008;48:1924–1931. doi: 10.1002/hep.22564. [DOI] [PubMed] [Google Scholar]
- 9.Córdoba J, Mínguez B. Hepatic encephalopathy. Semin Liver Dis. 2008;28:70–80. doi: 10.1055/s-2008-1040322. [DOI] [PubMed] [Google Scholar]
- 10.Butterworth RF. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology. 2011;53:1372–1376. doi: 10.1002/hep.24228. [DOI] [PubMed] [Google Scholar]
- 11.Shawcross DL, Shabbir SS, Taylor NJ, Hughes RD. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2010;51:1062–1069. doi: 10.1002/hep.23367. [DOI] [PubMed] [Google Scholar]
- 12.Knapp S, von Aulock S, Leendertse M, Haslinger I, Draing C, Golenbock DT, van der Poll T. Lipoteichoic acid-induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. J Immunol. 2008;180:3478–3484. doi: 10.4049/jimmunol.180.5.3478. [DOI] [PubMed] [Google Scholar]
- 13.Good DW, George T, Watts BA. Toll-like receptor 2 is required for LPS-induced Toll-like receptor 4 signaling and inhibition of ion transport in renal thick ascending limb. J Biol Chem. 2012;287:20208–20220. doi: 10.1074/jbc.M111.336255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schröder NW, Schumann RR. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious disease. Lancet Infect Dis. 2005;5:156–164. doi: 10.1016/S1473-3099(05)01308-3. [DOI] [PubMed] [Google Scholar]
- 15.Misch EA, Hawn TR. Toll-like receptor polymorphisms and susceptibility to human disease. Clin Sci (Lond) 2008;114:347–360. doi: 10.1042/CS20070214. [DOI] [PubMed] [Google Scholar]
- 16.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 17.Rallabhandi P, Bell J, Boukhvalova MS, Medvedev A, Lorenz E, Arditi M, Hemming VG, Blanco JC, Segal DM, Vogel SN. Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J Immunol. 2006;177:322–332. doi: 10.4049/jimmunol.177.1.322. [DOI] [PubMed] [Google Scholar]
- 18.Ferwerda B, McCall MB, Verheijen K, Kullberg BJ, van der Ven AJ, Van der Meer JW, Netea MG. Functional consequences of toll-like receptor 4 polymorphisms. Mol Med. 2008;14:346–352. doi: 10.2119/2007-00135.Ferwerda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guarner-Argente C, Sánchez E, Vidal S, Román E, Concepción M, Poca M, Sánchez D, Juárez C, Soriano G, Guarner C. Toll-like receptor 4 D299G polymorphism and the incidence of infections in cirrhotic patients. Aliment Pharmacol Ther. 2010;31:1192–1199. doi: 10.1111/j.1365-2036.2010.04291.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Shiffman ML, Friedman S, Venkatesh R, Bzowej N, Abar OT, Rowland CM, Catanese JJ, Leong DU, Sninsky JJ, et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007;46:297–306. doi: 10.1002/hep.21695. [DOI] [PubMed] [Google Scholar]
- 21.Francés R, Rodríguez E, Muñoz C, Zapater P, De la ML, Ndongo M, Pérez-Mateo M, Such J. Intracellular cytokine expression in peritoneal monocyte/macrophages obtained from patients with cirrhosis and presence of bacterial DNA. Eur J Gastroenterol Hepatol. 2005;17:45–51. doi: 10.1097/00042737-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Testro AG, Gow PJ, Angus PW, Wongseelashote S, Skinner N, Markovska V, Visvanathan K. Effects of antibiotics on expression and function of Toll-like receptors 2 and 4 on mononuclear cells in patients with advanced cirrhosis. J Hepatol. 2010;52:199–205. doi: 10.1016/j.jhep.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Lin CY, Tsai IF, Ho YP, Huang CT, Lin YC, Lin CJ, Tseng SC, Lin WP, Chen WT, Sheen IS. Endotoxemia contributes to the immune paralysis in patients with cirrhosis. J Hepatol. 2007;46:816–826. doi: 10.1016/j.jhep.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Devière J, Content J, Denys C, Vandenbussche P, Schandene L, Wybran J, Dupont E. Excessive in vitro bacterial lipopolysaccharide-induced production of monokines in cirrhosis. Hepatology. 1990;11:628–634. doi: 10.1002/hep.1840110416. [DOI] [PubMed] [Google Scholar]
- 25.Ramírez MJ, Ibáñez A, Navasa M, Casals E, Morales-Ruiz M, Jiménez W, Arroyo V, Rodés J. High-density lipoproteins reduce the effect of endotoxin on cytokine production and systemic hemodynamics in cirrhotic rats with ascites. J Hepatol. 2004;40:424–430. doi: 10.1016/j.jhep.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Montes AH, Asensi V, Alvarez V, Valle E, Ocaña MG, Meana A, Carton JA, Paz J, Fierer J, Celada A. The Toll-like receptor 4 (Asp299Gly) polymorphism is a risk factor for Gram-negative and haematogenous osteomyelitis. Clin Exp Immunol. 2006;143:404–413. doi: 10.1111/j.1365-2249.2005.03002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller M, Stamme C, Draing C, Hartung T, Seydel U, Schromm AB. Cell activation of human macrophages by lipoteichoic acid is strongly attenuated by lipopolysaccharide-binding protein. J Biol Chem. 2006;281:31448–31456. doi: 10.1074/jbc.M605966200. [DOI] [PubMed] [Google Scholar]
- 28.Prohinar P, Rallabhandi P, Weiss JP, Gioannini TL. Expression of functional D299G.T399I polymorphic variant of TLR4 depends more on coexpression of MD-2 than does wild-type TLR4. J Immunol. 2010;184:4362–4367. doi: 10.4049/jimmunol.0903142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferwerda B, McCall MB, Alonso S, Giamarellos-Bourboulis EJ, Mouktaroudi M, Izagirre N, Syafruddin D, Kibiki G, Cristea T, Hijmans A, et al. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc Natl Acad Sci USA. 2007;104:16645–16650. doi: 10.1073/pnas.0704828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erridge C, Stewart J, Poxton IR. Monocytes heterozygous for the Asp299Gly and Thr399Ile mutations in the Toll-like receptor 4 gene show no deficit in lipopolysaccharide signalling. J Exp Med. 2003;197:1787–1791. doi: 10.1084/jem.20022078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Aulock S, Schröder NW, Gueinzius K, Traub S, Hoffmann S, Graf K, Dimmeler S, Hartung T, Schumann RR, Hermann C. Heterozygous toll-like receptor 4 polymorphism does not influence lipopolysaccharide-induced cytokine release in human whole blood. J Infect Dis. 2003;188:938–943. doi: 10.1086/378095. [DOI] [PubMed] [Google Scholar]
- 32.van der Graaf C, Kullberg BJ, Joosten L, Verver-Jansen T, Jacobs L, Van der Meer JW, Netea MG. Functional consequences of the Asp299Gly Toll-like receptor-4 polymorphism. Cytokine. 2005;30:264–268. doi: 10.1016/j.cyto.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Zapater P, Caño R, Llanos L, Ruiz-Alcaraz AJ, Pascual S, Barquero C, Moreu R, Bellot P, Horga JF, Muñoz C, et al. Norfloxacin modulates the inflammatory response and directly affects neutrophils in patients with decompensated cirrhosis. Gastroenterology. 2009;137:1669–79.e1. doi: 10.1053/j.gastro.2009.07.058. [DOI] [PubMed] [Google Scholar]
- 34.Appenrodt B, Grünhage F, Gentemann MG, Thyssen L, Sauerbruch T, Lammert F. Nucleotide-binding oligomerization domain containing 2 (NOD2) variants are genetic risk factors for death and spontaneous bacterial peritonitis in liver cirrhosis. Hepatology. 2010;51:1327–1333. doi: 10.1002/hep.23440. [DOI] [PubMed] [Google Scholar]
- 35.Romero-Gómez M, Jover M, Del Campo JA, Royo JL, Hoyas E, Galán JJ, Montoliu C, Baccaro E, Guevara M, Córdoba J, et al. Variations in the promoter region of the glutaminase gene and the development of hepatic encephalopathy in patients with cirrhosis: a cohort study. Ann Intern Med. 2010;153:281–288. doi: 10.7326/0003-4819-153-5-201009070-00002. [DOI] [PubMed] [Google Scholar]
- 36.Minmin S, Xiaoqian X, Hao C, Baiyong S, Xiaxing D, Junjie X, Xi Z, Jianquan Z, Songyao J. Single nucleotide polymorphisms of Toll-like receptor 4 decrease the risk of development of hepatocellular carcinoma. PLoS One. 2011;6:e19466. doi: 10.1371/journal.pone.0019466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navasa M, Follo A, Filella X, Jiménez W, Francitorra A, Planas R, Rimola A, Arroyo V, Rodés J. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: relationship with the development of renal impairment and mortality. Hepatology. 1998;27:1227–1232. doi: 10.1002/hep.510270507. [DOI] [PubMed] [Google Scholar]
- 38.Riordan SM, Skinner N, Nagree A, McCallum H, McIver CJ, Kurtovic J, Hamilton JA, Bengmark S, Williams R, Visvanathan K. Peripheral blood mononuclear cell expression of toll-like receptors and relation to cytokine levels in cirrhosis. Hepatology. 2003;37:1154–1164. doi: 10.1053/jhep.2003.50180. [DOI] [PubMed] [Google Scholar]
- 39.Peng C, Liu BS, de Knegt RJ, Janssen HL, Boonstra A. The response to TLR ligation of human CD16+CD14- monocytes is weakly modulated as a consequence of persistent infection with the hepatitis C virus. Mol Immunol. 2011;48:1505–1511. doi: 10.1016/j.molimm.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 40.von Baehr V, Döcke WD, Plauth M, Liebenthal C, Küpferling S, Lochs H, Baumgarten R, Volk HD. Mechanisms of endotoxin tolerance in patients with alcoholic liver cirrhosis: role of interleukin 10, interleukin 1 receptor antagonist, and soluble tumour necrosis factor receptors as well as effector cell desensitisation. Gut. 2000;47:281–287. doi: 10.1136/gut.47.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]


