Abstract
Selective IgM deficiency (sIGMD) is very rare; it may be associated with celiac disease (CD). We present the case of an 18-year-old man with sIGMD masking seronegative CD. Symptoms included abdominal pain, diarrhea and weight loss. Laboratory tests showed reduced IgM, DQ2-HLA and negative anti-transglutaminase. Villous atrophy and diffuse immature lymphocytes were observed at histology. Tissue transglutaminase mRNA mucosal levels showed a 6-fold increase. The patient was treated with a gluten-free diet (GFD) and six months later the symptoms had disappeared, the villous architecture was restored and mucosal tissue transglutaminase mRNA was comparable to that of healthy subjects. After 1 year of GFD, a complete restoration of normal IgM values was observed and duodenal biopsy showed a reduction of immature lymphocytes and normal appearance of mature immune cells.
Keywords: Selective IgM deficiency, Seronegative celiac disease, Tissue transglutaminase, Gluten-free diet
Core tip: Selective IgM deficiency is a rare condition, associated in 5% of cases to Celiac Disease (CD). The CD diagnosis in patients with immunoglobulin deficiencies is based only on response to a Gluten-Free Diet (GFD). Deposits of tissue transglutaminase are found in duodenal samples of seronegative CD. Our case (IgM deficiency/seronegative CD) disclosed novel insights: (1) it is possible to observe a selective association of IgM deficiency/seronegative CD; (2) the histological diagnosis of CD may be complex due to poor lymphocyte maturation; (3) mRNA coding for tissue transglutaminase in duodenal mucosa could be a useful diagnostic tool; and (4) GFD may reverse IgM deficiency by promoting lymphocyte maturation.
TO THE EDITOR
Usually, immunoglobulin deficiencies underlie an immune deregulation, with a severe lack of B-cell differentiation[1]. Selective IgM deficiency (sIGMD) is a very rare disease (less than 300 cases described in literature) and is defined by low levels of IgM (< 0.40 g/L according to current guidelines)[2]. It is characterized by recurrent infections and treatment consists of immunoglobulin administration. The prevalence of this condition is estimated to be about 0.26% in the general population, while in association with celiac disease (CD) it is reported to be about 5%. However, a sIGMD-seronegative CD association has never been described, to the best of our knowledge, and so there may be an underestimation of the prevalence. Indeed, seronegative celiac patients with immunoglobulin deficiency are a controversial diagnostic problem, since histological aspects of these two disorders may overlap. In this case, the only convincing diagnostic tool is the response to a gluten-free diet (GFD)[1]. However, some reports suggest the possibility of supporting the diagnosis of seronegative CD by laboratory techniques, such as mucosal tissue transglutaminase mRNA assay[3] or immunoglobulin An anti-tissue transglutaminase deposits detected by immunofluorescence in frozen biopsy samples[4]. In a recent work we describe a similar increase of mucosal mRNA coding for tissue transglutaminase in both seropositive and seronegative CD[5].
On these bases, we believe it is of interest to report the case of a patient with sIGMD and seronegative CD, who showed a marked clinical and histological improvement after GFD and ultimately, restored IgM levels. In this patient, we used tissue transglutaminase mucosal mRNA to unmask CD, and mucosal lymphocytes characterization during follow-up for 1 year.
An 18-year male patient was admitted to our Unit for abdominal pain and diarrhea; these symptoms had been present for one year. In the last year he had suffered 13 kg weight loss, although his body mass index was 21.1 at the time of admission. Family history was notable for celiac disease in a sister. Among laboratory results we found a reduction of IgM (0.30 g/L) with normal values of IgA-IgG (total and subclasses). Anti transglutaminase IgA and IgG and anti-endomysium were negative despite the presence of DQ2 HLA[6]. No positive autoantibody was found. We ruled out Crohn’s disease, ulcerative colitis, Helicobacter pylori infection, intestinal bacterial overgrowth, allergy to food proteins other from gluten, connective tissue diseases, chronic use of non-steroidal anti-inflammatory drugs or Olmesartan. Moreover, in accordance with American Gastroenterological Association Guidelines, infective causes of small bowel inflammation were excluded by specific investigations for bacteria (Salmonellae species, Shigella, Campylobacter jejuni, enterotoxic E. coli, Yersinia enterocolitica), viruses (Rotavirus, Norovirus, Adenovirus, Astrovirus, Calicivirus and Coronavirus) and intestinal parasites (Giardia Lamblia). Colonoscopy with histological examination did not reveal any specific picture. No alterations were found at Magnetic Resonance study of the small bowel, nor 99Tc Scintigraphy (to exclude the presence of Meckel’s diverticulum).
Esophagogastroduodenoscopy showed mild duodenal hyperaemia, while histology revealed a lymphocyte infiltration of the distal duodenal mucosa with nodular lymphoid aggregates. A marked villous atrophy was also evident (Figure 1A). Immunohistochemical lymphocyte characterization showed a predominance of immature B forms (CD 79a) (Figure 2A) while there was a reduction of MUM1 (marker of B-cell differentiation and T-cell activation) positive cells (Figure 3A) and CD3 and CD8. This doubtful picture (i.e., villous atrophy in the absence of a number of intraepithelial CD3 lymphocytes diagnostic for CD) and the possible sIGMD/CD association as well as the family history, led us to perform mucosal tissue transglutaminase mRNA detection by a widely validated method based on reverse transcriptase polymerase chain reaction[7]. A duodenal sample from a healthy subject and one from a patient with seropositive CD were used as negative and positive control, respectively. A marked mucosal increase of tissue transglutaminase expression (6.2 fold compared to negative control) and of CD (7.1 fold) was observed in our patient. On these bases the patient was discharged with instructions to follow a GFD for 6 mo.
Figure 1.
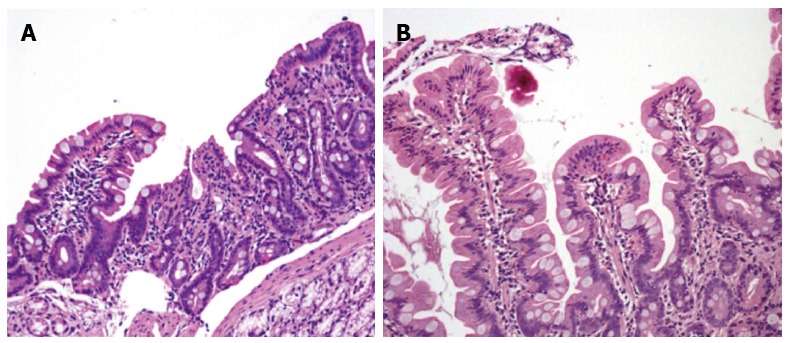
Duodenal biopsy specimens before (A) and 6 mo after (B) gluten-free diet in our patient with Selective IgM deficiency and seronegative celiac disease. A subtotal villous atrophy with an almost flat mucosa is evident before, and complete villous restoration after the dietary therapy (HE: × 200).
Figure 2.
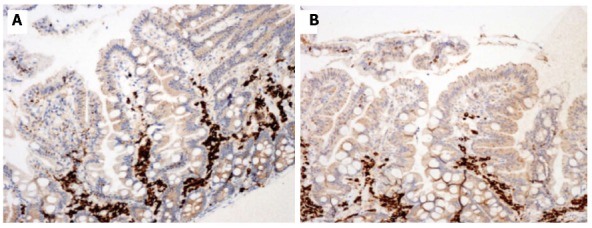
Duodenal biopsy specimens before (A) and 12 mo after (B) gluten-free diet in our patient with Selective IgM deficiency and seronegative celiac disease. Immunohistochemistry showed a mild reduction of CD 79a immature lymphocytes. Positive cells are stained brown and counterstained blue (× 200).
Figure 3.
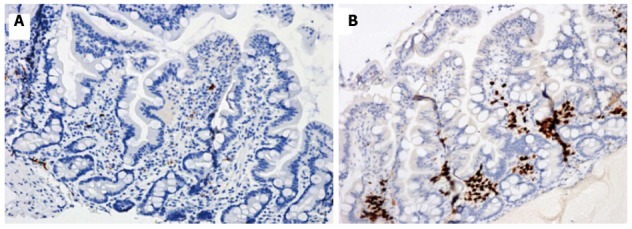
Duodenal biopsy specimens before (A) and 12 mo after (B) gluten-free diet in our patient with Selective IgM deficiency and seronegative celiac disease. Immunohistochemistry showed scarse MUM1 plasma cells before the diet and a marked positivity 12 mo later. Positive cells are stained brown and counterstained blue (× 200).
In the 6-mo follow-up, the patient gained 8 kg weight and had a total remission of the gastrointestinal symptoms. A second esophagogastroduodenoscopy showed, at histological examination of duodenal biopsy, normalization of the villous architecture (Figure 1B) although immunohistochemical lymphocyte (CD3, CD8, CD 79a and MUM1) characterization was unchanged. Laboratory investigations confirmed sIGMD without further alterations. Finally, tissue transglutaminase mRNA assay showed a marked decrease (1.3 fold compared to negative control). After one year of GFD, the patient showed normal physical examination and laboratory test results, including a surprising normalization of IgM values (1.10 g/L). Duodenal biopsy was performed and showed the persistence of a normal villous architecture, a mild reduction of mucosal CD 79a (Figure 2B) and a marked increase of MUM1 positive cells (Figure 3B). Transglutaminase mRNA confirmed normal values.
Our case may be considered a very rare observation in the complex scenario of CD, immunodeficiency and autoimmunity[8]. There is debate in literature about the association of CD and primary immunoglobulin deficiencies, such as common variable immunodeficiency (CVID)[1]. The conclusion of a recent study was that only the response to a GFD offers a reliable diagnostic tool for defining the presence of CD in a patient with CVID[1]. Moreover, an association of CD has been described in 20% of IgA deficiency cases. The main novelty in our case is the sIGMD-seronegative CD association. In accordance with current guidelines, clinical and histological response to GFD was the main diagnostic tool of CD in our patient. Additionally, IgM levels were restored after one year of GFD and this unexpected finding suggests that the immunoglobulin selective deficiency might not have been only a primary condition, as suggested by mucosal immature lymphocytes. It is possible that CD and consequent alterations of mucosal permeability could have induced enteric protein loss, lowering serum IgM that were then restored after healing of the duodenal mucosa. Supporting this view, immature lymphocytes were reduced after 12 mo GFD and MUM1 cells strongly increased, suggesting a further reason for the IgM increase. In a future perspective, finally, it is possible that the detection of mRNA coding for tissue transglutaminase in duodenal mucosa may be, despite the known specificity limitations, a useful diagnostic tool both for identifying seronegative forms of CD when this condition is suggested by histological criteria and HLA class assessment, and for evaluating mucosal restoration after a GFD.
Footnotes
P- Reviewer: Ianiro G S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Biagi F, Bianchi PI, Zilli A, Marchese A, Luinetti O, Lougaris V, Plebani A, Villanacci V, Corazza GR. The significance of duodenal mucosal atrophy in patients with common variable immunodeficiency: a clinical and histopathologic study. Am J Clin Pathol. 2012;138:185–189. doi: 10.1309/AJCPEIILH2C0WFYE. [DOI] [PubMed] [Google Scholar]
- 2.Dati F, Schumann G, Thomas L, Aguzzi F, Baudner S, Bienvenu J, Blaabjerg O, Blirup-Jensen S, Carlström A, Petersen PH, et al. Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP Reference Material (CRM 470). International Federation of Clinical Chemistry. Community Bureau of Reference of the Commission of the European Communities. College of American Pathologists. Eur J Clin Chem Clin Biochem. 1996;34:517–520. [PubMed] [Google Scholar]
- 3.Esposito C, Paparo F, Caputo I, Porta R, Salvati VM, Mazzarella G, Auricchio S, Troncone R. Expression and enzymatic activity of small intestinal tissue transglutaminase in celiac disease. Am J Gastroenterol. 2003;98:1813–1820. doi: 10.1111/j.1572-0241.2003.07582.x. [DOI] [PubMed] [Google Scholar]
- 4.Tosco A, Maglio M, Paparo F, Rapacciuolo L, Sannino A, Miele E, Barone MV, Auricchio R, Troncone R. Immunoglobulin A anti-tissue transglutaminase antibody deposits in the small intestinal mucosa of children with no villous atrophy. J Pediatr Gastroenterol Nutr. 2008;47:293–298. doi: 10.1097/MPG.0b013e3181677067. [DOI] [PubMed] [Google Scholar]
- 5.Buffelli F, Ierardi E, Losurdo G, Di Leo A, Giorgio F, Amoruso A, Montenegro L, Piscitelli D, Fiore MG, Resta L. Tissue transglutaminase (ttg) levels paralleles intraepithelial lymphocytes (IELS) in patients suitable with gluten related seronegative problems: preliminary report. Pathologica. 2013;105:296. [Google Scholar]
- 6.Rostami-Nejad M, Romanos J, Rostami K, Ganji A, Ehsani-Ardakani MJ, Bakhshipour AR, Zojaji H, Mohebbi SR, Zali MR, Wijmenga C. Allele and haplotype frequencies for HLA-DQ in Iranian celiac disease patients. World J Gastroenterol. 2014;20:6302–6308. doi: 10.3748/wjg.v20.i20.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ierardi E, Giorgio F, Rosania R, Zotti M, Prencipe S, Della Valle N, De Francesco V, Panella C. Mucosal assessment of tumor necrosis factor alpha levels on paraffined samples: a comparison between immunohistochemistry and real time polymerase chain reaction. Scand J Gastroenterol. 2010;45:1007–1008. doi: 10.3109/00365521.2010.483739. [DOI] [PubMed] [Google Scholar]
- 8.La Villa G, Pantaleo P, Tarquini R, Cirami L, Perfetto F, Mancuso F, Laffi G. Multiple immune disorders in unrecognized celiac disease: a case report. World J Gastroenterol. 2003;9:1377–1380. doi: 10.3748/wjg.v9.i6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]


