Introduction
Infections have long been known to complicate care in patients following traumatic injury frequently leading to excess morbidity and mortality.1, 2 In no setting is this more well-recognized than the challenging environment of combat casualty care. During the current military conflicts in Iraq and Afghanistan, Operations Iraqi and Enduring Freedom (OIF/OEF), major advances resulting in increased survival among wounded personnel have been observed. These include enhanced training of medics, forward deployment of surgical assets, rapid medical evacuation, and improvements in body armor.3–5 The significant advances leading to survival are coupled with major challenges in care due to the extensive nature of the injuries, profound bone and soft tissue disruption, and extensive wound contamination.6, 7 In addition, the rapid transit of these patients through multiple echelons of medical care places significant obstacles on infection control in an era of increasing risk due to hospital-associated multidrug resistant (MDRO) organisms.8, 9
The U.S. Department of Defense (DoD) has implemented a range of measures to improve combat casualty care and mitigate risk of infectious complications. A Joint Theater Trauma System and Joint Theater Trauma Registry (JTTR) have been developed to benchmark metrics and to provide a timely assessment of performance improvement interventions.5, 10, 11 Efforts to prevent infection include the development of guidelines for the prevention of infection related to combat injuries through comprehensive review of current evidence and consensus review by military and civilian experts in trauma, infectious disease, infection control, preventive medicine, and surgical specialties.12 In addition, standardized infection control measures across echelons of care accompanied by enhanced MDRO surveillance and serial evaluation have also been implemented.13, 14
Despite the growing literature describing infectious complications of combat-related trauma, there is still a lack of prospectively collected standardized infection data that includes specific therapy, microbiological findings and clinical outcomes across treatment facilities. This report describes the initial data and current status of an ongoing 5-year prospective observational cohort study of infectious complications associated with traumatic injury sustained during deployment, the DoD-Department of Veterans Affairs (VA) Trauma Infectious Disease Outcomes Study (TIDOS).
Material and Methods
TIDOS Project Design
The TIDOS project is an observational cohort study of short and long term infectious disease outcomes following a deployment-related traumatic injury. TIDOS cohort eligibility criteria include the following: active duty personnel or DoD beneficiary, ≥ 18 years of age, wounded or injured during deployment requiring return via Level IV at Landstuhl Regional Medical Center (LRMC), Landstuhl, Germany to a participating DoD hospital in the U.S., and providing informed consent or surrogate consent through a legally authorized representative. Participating Level V clinical sites include the National Naval Medical Center (NNMC), Bethesda, MD, Brooke Army Medical Center (BAMC), San Antonio, TX, and Walter Reed Army Medical Center (WRAMC), Washington DC. Consenting patients are enrolled in the study prior to discharge from one of the participating Level V hospitals. Participants are contacted periodically at 1, 3, 6, 12, 18, 24 months and then yearly for 5 years after discharge. Follow-up methods include subject interviews, web-based questionnaires, and query of DoD and Department of Veterans Affairs electronic healthcare databases.
Patient trauma history, injury severity scoring, and surgical management is obtained through selected data elements retrieved from the DoD Joint Theater Trauma Registry (JTTR).5 The JTTR was established by the Assistant Secretary of Defense for Health Affairs as a means to provide an effective performance improvement tool to assess combat casualty care epidemiology, treatment, and outcome. An infectious diseases (ID) module to augment the JTTR was developed to capture infection-specific data throughout levels of care at participating hospitals. This ID-specific information includes diagnoses, treatments, and outcomes of bloodstream infections (BSI), clinical sepsis, bone and joint infections, skin and soft tissue infections (SSTI), central nervous system infections (CNS), intra-thoracic/pulmonary infections, and intra-abdominal infection. The JTTR/ID module captures data on all active duty military trauma patients admitted to Level IV and selected Level V facilities. A comprehensive assessment of the overall population and the representativeness of the TIDOS cohort were performed by analyzing de-identified JTTR/ID module data from non-enrolled trauma patients admitted through Level IV during the period. The study is approved by the institutional review boards of the Uniformed Services University of the Health Sciences and the St. Louis Veterans Administration Medical Center (VAMC).
This report includes subjects admitted to Level IV during the first three months of the study (June 1 – August 31, 2009) along with subject experience through Level V hospitalization (for those admitted to participating Level V sites) and up to 6-months of follow-up post Level V discharge (for those consenting to follow-up).
Study definitions/endpoints
Trauma care within the military is typically described based on levels defined as follows: Level I, point of injury/first responder care within the combat zone; Level II, resuscitation and surgical stabilization at medical units (not hospitals) within the combat zone (which may be augmented with surgical assets); Level III, medical/surgical care at combat support or other theater hospitals (highest available care in the combat zone); Level IV, regional medical center care located in communication zone (e.g., LRMC), and Level V, definitive treatment/rehabilitation at major tertiary care medical centers in United States.
Injury parameters include type of injury (blunt or penetrating), mechanism of injury, anatomic site, early interventions, and delayed care management. Severity scoring systems are used to provide internal and external comparability of this traumatized patient population both for initial assessment of the traumatic injury and subsequent interval health assessments. The Injury Severity Score (ISS) uses anatomic classification for injury classification and severity scoring.15 Interval assessments of general health are undertaken using the Sequential Organ Failure Assessment (SOFA).16 The SOFA score is composed of scores from six organ systems, graded from 0 to 4 according to the degree of dysfunction/failure.
Infectious disease events are classified using a combination of clinical findings, laboratory and other test results, as shown in Table 1, available through medical record review, applying standardized definitions for nosocomial infections used by the National Healthcare Safety Network (NHSN).17 In addition, a physician’s clinical diagnosis in the absence of meeting a priori defined criteria was also counted as an ID event provided there was initiation of directed antimicrobial therapy with continuation of this therapy for ≥ 5 days. An ID event is excluded if the medical record states an alternative diagnosis is determined accompanied by discontinuation of directed antimicrobial therapy.
Table 1.
TIDOS Criteria for Infectious Disease Syndromes1
| ID Classification | Diagnostic Criteria |
|---|---|
| Colonization | The presence of microorganisms on skin, mucous membranes, open wounds, or in excretions or secretions but without clinical signs or symptoms compatible with an infectious disease diagnosis. |
| Systemic inflammatory response syndrome (SIRS)35 | Patient has ≥ 2 of the following [Temp > 38°C or Temp < 36°C, HR > 90 beats/min, RR > 20 breaths/min, PaCO2 < 32 mmHg, WBC > 12000 cells/mm3, WBC < 4000 cells/mm3, WBC < 10% immature (band forms)] |
| Clinical sepsis | Sepsis35 - SIRS plus ≥ 1 of the following: positive culture, Gram stain of sterile body fluid, or visual evidence of infection Septic Shock36 - sepsis criteria plus ≥ 1 of the following: systemic mean BP <60 mmHg (<80 if previous hypertension) after 20–30 mL/kg starch or 40–60 mL/kg serum saline solution, PCWP 12–20 mmHg, need for dopamine at >5 mcg/kg/min to maintain BP >60 mmHg, or need for norepinephrine or epinephrine at <0.25 mcg/kg/min to maintain BP >60 mmHg |
| Bloodstream infection (BSI) | Recognized pathogen cultured from ≥ 1 blood cultures or patient has ≥ 2 of the SIRS criteria and ≥ 1 of the following: 1) common skin contaminant cultured from ≥ 2 blood cultures drawn on separate occasions or 2) common skin contaminant cultured from ≥ 1 blood culture from a patient with an intravascular line and physician instituted antimicrobial therapy |
| Osteomyelitis | Definite: Positive bone culture or evidence on direct examination of bone during surgical procedure or histopathology exam Probable: Patient has ≥ 2 signs/symptoms from group A [temp > 38°C, localized swelling, localized tenderness, localized heat, drainage] and at least one from group B [organisms cultured from blood or radiographic evidence of infection] Possible: Patient meets all criteria in Group A [open fracture or exposed bone with environmental contamination at time of injury, deep wound tissue growing any organism, evidence of local inflammation (purulence or necrotic tissue) or systemic inflammation (fever, leukocytes ≥ 12000/µL, elevated C-reactive protein or elevated erythrocyte sedimentation rate) or all criteria in Group B [non-union fracture on follow-up, Evidence of systemic inflammation (fever, leukocytes ≥ 12000/µL, elevated C-reactive protein or elevated erythrocyte sedimentation rate)] |
| Joint/bursa infection | Definite: organisms cultured from joint fluid/synovial biopsy or evidence of joint/bursa infection seen during a surgical operation or histopathology Possible: Patient has ≥ 2 signs/symptoms from group A [joint pain, swelling, tenderness, erythema, heat, evidence of effusion or limitation of motion] and at least one from group B [organisms and WBC seen on Gram stain of joint fluid, WBC ≥ 12000/µL in joint fluid with no other underlying rheumatologic disorder, or radiographic evidence of infection] |
| Disc space infection | Definite: positive culture from vertebral disc space tissue obtained during surgery or needle aspiration or evidence seen during surgical procedure or histopathology Possible: radiographic evidence and ≥ 1 of the following: fever without other recognized cause or pain at involved vertebral disc space |
| Wound/surgical site infections/skin & soft tissue infections (SSTI) | Superficial: Involves only skin/subcutaneous tissue and ≥ 1 of the following from each area: 1) Pain/tenderness, localized swelling, redness, or heat AND 2) purulent site drainage, organisms isolated from aspirate/aseptically obtained culture of fluid/tissue, organisms isolated from purulent drainage, or visual evidence of superficial incisional SSI or SSTI Deep: ≥ 1 of the following: purulent site drainage, organisms isolated from aspirate/aseptically obtained culture of fluid/tissue, organisms isolated from purulent drainage, deep wound/incision spontaneously dehisces or is deliberately opened by a surgeon in the presence of fever or localized tenderness, unless the wound culture is negative, abscess or other evidence of infection |
| Central nervous system infection | Intracranial infection: [brain abscess, subdural or epidural infection, encephalitis] or [positive culture from brain tissue or dura] or [abscess/evidence of intracranial infection seen during surgical procedure or histopathology] or [patient has at least one of the following from each group: (headache, dizziness, fever, localizing neurological signs, or change in level of consciousness or confusion) or (organisms seen on microscopy of brain/abscess tissue obtained by needle aspiration/biopsy or radiographic evidence of infection)] Meningitis or ventriculitis: Positive CSF culture or ≥ 1 of the following from each area: (fever, headache, stiff neck, meningeal signs, cranial nerve signs, or irritability) and (increased white cells, elevated protein, and/or decreased glucose in CSF, positive CSF Gram stain, positive blood culture, positive CSF, blood, or urine antigen test) Spinal abscess without meningitis: positive culture from abscess in the spinal epidural/subdural space or abscess in the spinal epidural/subdural space seen during surgery or ≥ 1 of the following from each area: (fever, back pain, focal tenderness, radiculitis, paraparesis, or paraplegia) and (positive blood culture or radiographic evidence) |
| Intrathoracic/pulmonary infection | Pneumonia: both must be present – (New or progressive infiltrate seen on radiology film and evidence of infection (temp >38°C or <36°C or WBC ≥12000 or <4000)) and (new onset purulent sputum, change in character of sputum, increase respiratory secretions, new onset worsening cough, dyspnea, or tachypnea, rales/bronchial breath sounds, worsening gas exchange, same organism growing from respiratory secretions as found in blood cultures, positive culture from minimally contaminated lower respiratory tract specimen, ≥5% BAL-obtained cells contain intracellular bacteria on direct microscopy, or laboratory evidence of uncommon pathogen) Empyema: ≥ 1 of the following: positive culture from pleural fluid biochemical analysis consistent with empyema, empyema seen during surgery or histopathology, or radiographic evidence consistent with empyema Lung abscess: ≥ 1 of the following: organism seen on smear or cultured from lung tissue or fluid, including pleural fluid, lung abscess seen during a surgical operation or histopathology, or abscess cavity seen on radiographic examination of lung |
| Intraabdominal infection | Includes gall bladder, bile ducts, liver, spleen, pancreas, peritoneum, subphrenic or subdiaphragmatic space, or other intra-abdominal tissue or area not otherwise specified; positive culture of purulent material from intra-abdominal space obtained during surgery/needle aspiration or abscess or other evidence of intra-abdominal infection seen during surgery/histopathology exam or ≥ 2 of the following: temp >38°C, nausea, vomiting, abdominal pain, jaundice, or paraparesis plus or ≥ 1 of the following: organisms cultured from drainage from surgically placed drain, organisms seen on Gram stain of drainage/tissue obtained during surgery or needle aspiration, or radiographic evidence of infection |
Diagnostic criteria derived from standardized definitions for nosocomial infections used by the National Healthcare Safety Network (NHSN).17
Clinical microbiology
Microbiological evaluations to diagnose infections are performed at the discretion of the clinical providers. Infection control policy dictates the timing and methods for active surveillance for colonization. Targeted surveillance includes admission swabs of the groin and axilla (axillary swabs discontinued during the period reported) as well as external nares culture and/or PCR to detect methicillin resistant S. aureus (MRSA). Antibiotic susceptibility results, performed by each hospital’s clinical microbiology laboratory, are interpreted in accordance with Clinical and Laboratory Standards Institute (CLSI) if available. MDRO classification is defined as any bacteria resistant to ≥ 3 classes of antibiotics (aminoglycosides, β-lactams, carbapenems, and fluoroquinolones), presence of extended spectrum β-lactamase (ESBL) or Klebsiella pneumoniae carbapenemase (KPC) production, MRSA, or vancomycin-resistant Enterococcus (VRE).18 Following completion of identification and susceptibility testing at the clinical microbiology laboratory (per standard procedures at each site), research staff collect bacterial and fungal isolates for archival for future phenotypic and genotypic analysis. All participating sites use either BD PhoenixTM (BD Biosciences, Sparks, MD, USA) or Vitek® 2 (bioMérieux Inc., Hazelwood, MO) automated systems for speciation and antibiotic susceptibility testing along with standardized resistance testing through disc diffusion and E-tests. Bacterial and fungal isolates to archive all multidrug resistant organisms (MDRO), Acinetobacter isolates, sterile site and operative procedure isolates, and other clinically relevant organisms (nonsterile site isolates are collected with restriction to no duplicates accepted within a 72-h interval).
Statistical analysis
Statistical analyses include descriptive statistics shown as counts (percentages) for categorical variables, and medians (interquartile ranges; IQR) for continuous variables. Group comparisons were conducted using Fisher’s Exact test and the Wilcoxon-Mann-Whitney test of no difference between groups for the categorical and continuous variables, respectively. Odds ratios and confidence intervals were computed using a method recently proposed by Fay.19 Continuous variables with skewed distribution were compared using the exact Wilcoxon test.20
ID events are classified as described above with the following additional steps to assure each event is unique and to prevent overestimation: 1) patients with ID events of the same syndromic type were verified to have occurred ≥ 1 month apart and not represent a continuation of the initial event; 2) patients with multiple ID events classified as either SSTI or osteomyelitis were verified to be from different anatomic regions; and 3) SSTI and osteomyelitis ID events occurring at the same anatomic location with date of diagnosis ± 3 days were coded as a single ID event for rate calculation (however both syndrome types are recorded). ID events occurring within the same level of care were individually reviewed by the principal investigator for confirmation. Incidence density rates were calculated by number of ID events from time of trauma through discharge at last hospital facility during initial inpatient period (Level IV or V) with exact Poisson confidence intervals. All analyses were conducted using R version 2.11 (R foundation; www.r-project.org) and SAS version 9.2 (SAS Corporation; Cary, NC).
Results
TIDOS Enrollment
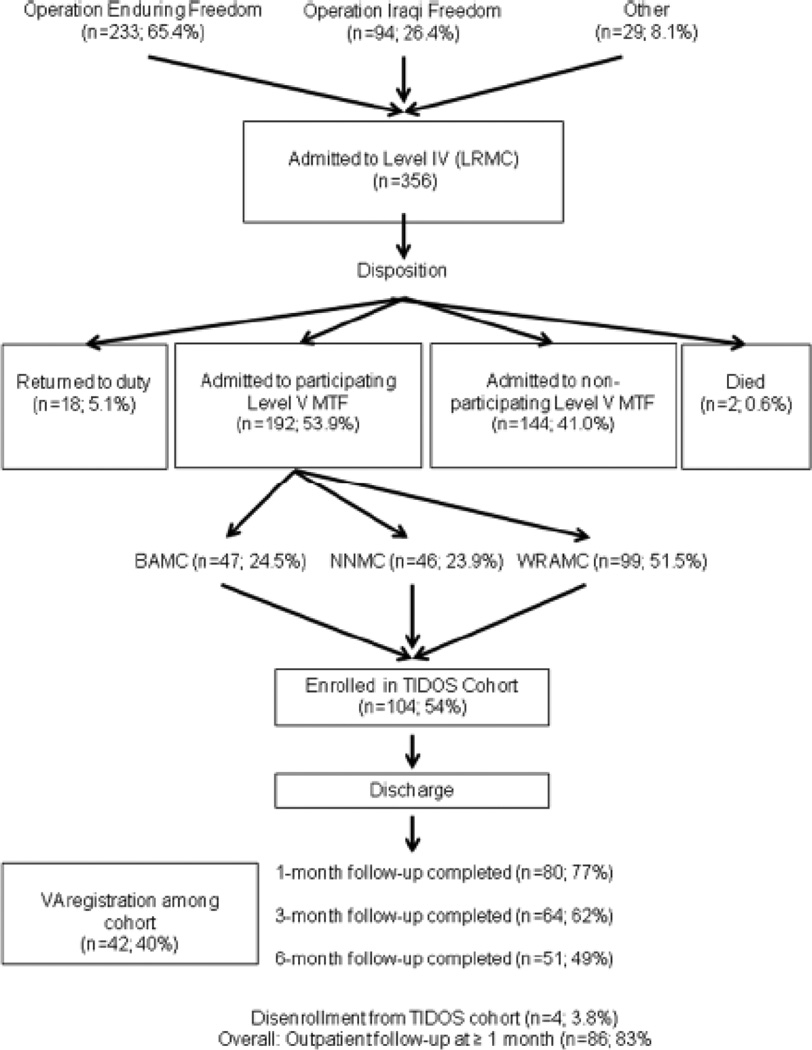
Cohort enrollment and data collection began June 2009. The patient flow is outlined in Figure 1 from the operational theater, admission at Level IV care, Level IV discharge disposition, distribution across participating U.S. military hospitals, and subsequent enrollment, follow-up, and VA registration for the TIDOS cohort. During this period, there were 356 (approx. 65% in support of OEF) Level IV trauma admissions with 192 (53.9%) of these patients transferred to participating U.S. military hospitals, most commonly to the WRAMC with 99 patients (51.5%). TIDOS cohort enrollment of potentially eligible patients is at 54% with approximately 30–40% of the patients not contacted to discuss enrollment frequently due to short inpatient stays and/or weekend discharges. A relatively high proportion of the TIDOS cohort (40%) have left active duty service and registered for care in the Veterans Administration Medical Center (VAMC) system in less than one year since date of injury.
Figure 1.
Flow diagram of military personnel movement through the healthcare system following traumatic injury during deployment and subsequent enrollment and follow-up in the TIDOS project
Note: LRMC (Landstuhl Regional Medical Center), CONUS (Continental United States), MTF (Medical Treatment Facility), BAMC (Brooke Army Medical Center), NNMC (National Naval Medical Center). WRAMC (Walter Reed Army Medical Center), VA (Veterans Administration)
Overall population comparison to TIDOS cohort
As detailed on Table 2, there were no significant differences between operational theater, gender, or age when comparing patient characteristics either by participating versus non-participating U.S. hospitals or by TIDOS enrollment status. The distribution by branch of service among enrolled vs. non-enrolled patients showed a higher proportion of Army (OR 2.2, 95% C.I. 1.1, 4.4) and lower Marine Corps (OR 0.4, 95% C.I. 0.2, 0.8) service among the cohort. The mechanism of trauma was less likely to be caused by an explosive device in the patients transferred to the nonparticipating hospitals. This group of patients also had a higher proportion of injuries caused by gunshot wounds (OR 0.4, 95% C.I. 0.2, 0.8). The group with the highest injury severity scores was the TIDOS enrolled cohort (P < 0.0001).
Table 2.
Demographic and injury characteristics of patients admitted through Level IV (Landstuhl Regional Medical Center) (June–August 2009) following traumatic Injury during deployment
| Trauma patients admitted at Level IV | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Level IV → RTD (N=18) |
Level IV → Level V (other sites) (N=144) |
Level IV → Level V (TIDOS participating sites) |
Total (%) | ||
| TIDOS enrolled (N=104) |
Not enrolled (N=88) |
|||||
| JTTR Registrants | No. with available data | 17 | 140 | 102 | 87 | 346 (97.2) |
| Operational theater (%) | Operation Iraqi Freedom | 4 (23.5) | 41 (29.3) | 26 (25.5) | 19 (21.8) | 90 (26.0) |
| Operation Enduring Freedom | 0 (0) | 90 (64.3) | 75 (73.5) | 63 (72.4) | 228 (65.9) | |
| Non-OIF/OEF deployment | 13 (76.5) | 9 (6.4) | 1 (1.0) | 5 (5.8) | 28 (8.1) | |
| Gender, % male | 82.4 | 97.9 | 99.0 | 96.6 | 97.1 | |
| Age, median (IQR) | n=346 | 25 (23–29) | 24 (21–30.5) | 24 (21–29) | 24 (21–30) | 24 (21–30) |
| Branch of service (%) | Army | 11 (64.7) | 108 (77.1) | 82 (80.4) | 57 (65.5) | 258 (74.6) |
| Marine | 0 (0) | 23 (16.4) | 12 (11.8) | 24 (27.6) | 59 (17.1) | |
| Air Force | 4 (23.5) | 5 (3.6) | 4 (3.9) | 1 (1.1) | 14 (4.1) | |
| Navy | 1 (5.9) | 3 (2.1) | 3 (2.9) | 5 (5.8) | 12 (3.5) | |
| Other | 1 (5.9) | 1 (0.7) | 1 (1.0) | 0 (0) | 3 (0.9) | |
| Military grade/rank (%) | Enlisted (E1–E5) | 14 (82.4) | 105 (75.0) | 77 (75.5) | 65 (74.7) | 261 (75.4) |
| Enlisted (E6–E9) | 1 (5.9) | 20 (14.3) | 16 (15.7) | 16 (18.4) | 53 (15.3) | |
| Warrant/Officer | 1 (5.9) | 14 (10.0) | 8 (7.8) | 6 (7.0) | 29 (8.4) | |
| Civilian | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) | 1 (0.3) | |
| Mechanism of Injury | Gunshot wound | 0 (0) | 32 (22.9) | 11 (10.8) | 9 (10.3) | 52 (15.0) |
| Motor vehicle crash | 4 (23.5) | 8 (5.7) | 5 (4.9) | 7 (8.1) | 24 (6.9) | |
| Blast | 0 (0) | 61 (43.6) | 70 (68.6) | 49 (56.3) | 180 (52.0) | |
| % explosive device | 0 | 76.9 | 84.9 | 84.3 | 81.7 | |
| Other | 12 (70.6) | 31 (22.1) | 11 (10.8) | 12 (13.8) | 66 (19.1) | |
| Injury Severity Score15 | ISS2005, median(IQR) | 4.5 (4–12) | 5 (3.4–9) | 12.3 (5.67–21.5) | 10 (6.5–10) | 9 (4.25–9) |
Note: Numbers represent patient numbers with percentages in parentheses unless otherwise indicated.
Joint Theater Trauma Registry (JTTR); Injury Severity Score (ISS); interquartile range (IQR)
The median (IQR) time (in days) from trauma to Level IV admission was similar irrespective of eventual Level IV disposition: return to duty (RTD) 3.0 (1–6), non-participating Level V 3.0 (2–4), and participating Level V 3.0 (2–4). As expected the median hospitalization at Level IV was significantly less than at the U.S. hospitals, 2 compared to 15 days (Table 3). The TIDOS enrolled group had longer hospitalizations than non-enrolled patients, 16 vs. 10 days. The patients transferred to participating U.S. hospitals had significantly higher rates of ICU admissions, mechanical ventilation, proportion with massive blood transfusion requirement, admission SOFA scores, and use of other invasive interventions while at Level IV compared to patients transferred to nonparticipating hospitals. There was however no significant differences in these rates between TIDOS enrolled versus non-enrolled patients. Of the 94 Level IV ICU admits, 82 (87.2%) were transferred to a participating U.S. hospital. At discharge from participating U.S. hospitals, there was no statistical difference, based on TIDOS enrollment, for the numbers of patients with indwelling orthopedic hardware, intravascular lines, or ongoing antimicrobial therapy.
Table 3.
Hospitalization characteristics of patients admitted through Level IV (Landstuhl Regional Medical Center) (June–August 2009) following traumatic injury
| Trauma patients admitted at Level IV | ||||||
|---|---|---|---|---|---|---|
| Level IV → Level V (TIDOS participating sites) |
||||||
| Characteristic | Level IV → RTD (N=18) |
Level IV →Level V (other sites) (N=144) |
TIDOS enrolled (N=104) |
Not enrolled (N=88) |
||
| Level IV Admission |
Level V Admission |
Level IV Admission |
Level V Admission |
|||
| Days Hospitalized, median (IQR) | 3 (1–10) | 4 (3–6) | 2 (1–3) | 16 (6–45) | 2 (1–4) | 10 (3–21) |
| ICU admission (%)1 | 4 (22.2) | 25 (16.8) | 46 (44.2) | 37 (41.6) | 48 (57.1) | 20 (37.0) |
| Days in ICU, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 3 (4–6) | 2 (1–4) | 3 (2–11) |
| Mechanical ventilation (%)1 | 0 (0) | 10 (6.7) | 26 (25.0) | 9 (10.1) | 30 (35.7) | 7 (13.0) |
| Days on Ventilator, median (IQR) | - | 1 (1–4) | 1 (1–2) | 2 (2–3) | 2 (1–3) | 7 (5–13) |
| Median number operating room visits/week (IQR) | 1(1–2) | 1(1-1) | 1 (1-1) | 3(2–7) | 1(1-1) | 2.5(1–4) |
| Level III/IV - 24 h blood transfusion ≥ 10 units (%)1 | 0 (0.0) | 1 (0.7) | 7 (6.9) | - | 9 (10.3) | - |
| Admission SOFA2 – Mean (std dev) | 0.56(1.10) | 0.76(2.0) | 2.45 (3.18) | 0.98(2.04) | 2.67(3.19) | 1.46(2.75) |
| Abnormal SOFA score component (%) | ||||||
| Respiration (PaO2/FiO2 < 400) | 1 (5.6) | 4 (2.7) | 14 (13.5) | 6 (5.8) | 10 (11.4) | 13 (14.8) |
| Coagulation (platelets < 150 103/mm3) | 2 (11.1) | 15 (10.3) | 50 (48.1) | 20 (19.2) | 41 (46.6) | 15 (17.0) |
| Hepatic Function (bilirubin > 1.2mg/dl) | 1 (5.6) | 21 (14.4) | 27 (26.0) | 11 (10.6) | 25 (28.4) | 11 (12.5) |
| Cardiovascular (mean arterial pressure < 70 mmHg) | 2 (11.1) | 23 (15.8) | 17 (16.3) | 10 (9.6) | 8 (9.1) | 4 (4.5) |
| Neurological Function (Glascow coma scale < 15) | 1 (5.6) | 7 (4.8) | 30 (28.8) | 11 (10.6) | 31 (35.2) | 17 (19.3) |
| Renal Function (creatinine > 1.2 mg/dl) | 1 (5.6) | 11 (7.5) | 8 (7.7) | 2 (1.9) | 12 (13.6) | 3 (3.4) |
| Clinical course/intervention (%) | ||||||
| Central line | 3 (16.7) | 8 (5.6) | 34 (32.7) | 33 (31.7) | 33 (37.5) | 30 (34.1) |
| Enteric feeding tube | 0 | 0 | 13 (12.5) | 11 (10.6) | 17 (19.3) | 17 (19.3) |
| Chest tube | 0 | 3 (2.1) | 6 (5.8) | 6 (5.8) | 13 (14.8) | 13 (14.8) |
| Ventriculostomy | 0 | 0 | 0 | 0 | 5 (5.7) | 6 (6.8) |
| Total parenteral nutrition | 0 | 0 | 1 (1.0) | 5 (4.8) | 0 | 3 (3.4) |
| Vasopressor therapy | 0 | 2 (1.4) | 4 (3.8) | 1 (1.0) | 5 (5.7) | 5 (5.7) |
| Discharge Status (%) | ||||||
| Indwelling orthopedic hardware | 6 (33.3) | 19 (13.2) | 23 (22.1) | 50 (48.1) | 16 (18.2) | 30(34.1) |
| Indwelling intravascular line | 2 (11.1) | 12 (8.3) | 22 (21.2) | 13(12.5) | 17 (19.3) | 7 (8.0) |
| Antimicrobial therapy3 | 6 (33.3) | 86 (59.7) | 94 (90.4) | 43 (41.3) | 74 (84.1) | 37(42.0) |
Note: Numbers represent patient numbers with percentages in parentheses unless otherwise indicated.
Denominator based on JTTR registrant available data.
Sequential Organ Failure Assessment (SOFA)16
Antimicrobial therapy does not include anti-malarial prophylaxis post-deployment (i.e. doxycycline, mefloquine, atovaquone/proguanil)
Infectious disease events during initial hospitalization (Level IV and V)
The proportion of patients experiencing ≥ 1 ID event increased from 5.1% at Level IV to 26.6% at Level V (Table 4). The number of patients experiencing infection at Level IV by discharge disposition is as follows: patients returning to duty – 3 (16.7%), transferring to non-participating level V facility – 0 (0.0%), participating level V without TIDOS enrollment – 8 (9.1%), and participating level V with enrollment – 7 (6.7%). A total of 117 unique ID events were documented at Level IV and V (Table 5); 95 (81.2%) by meeting a priori criteria and 22 (18.8%) by infection suspicion (not meeting criteria) plus treated with ≥ 5 days directed antimicrobial therapy. There was no statistical difference between the proportions classified as ID events meeting criteria at Level IV vs. Level V, 71.4 vs. 83.3%, respectively. The specific ID event type along with the relative percentage (in descending order) and the number of patients affected for all of the 117 unique ID events was as follows: wound/SSTI - 51 (43.6%; 37 patients), BSI - 22 (18.8%; 18 patients), osteomyelitis - 23 (19.7%; 18 patients), intrathoracic/pneumonia - 17 (14.5%; 15 patients), sepsis (without focal infection) - 2 (1.7%; 2 patients), intraabdominal - 6 (5.1%; 5 patients), CNS - 2 (1.7%; 2 patients), and miscellaneous infections - 11 (9.4%; 10 patients).
Table 4.
Incident infectious complications during hospitalization among patients admitted through Level IV (Landstuhl Regional Medical Center) (June–August 2009) following traumatic injury
| Level IV | Level V | |||||
|---|---|---|---|---|---|---|
| Characteristic | ICU admission |
Ward admission |
All | ICU admission |
Ward admission |
All |
| Number of patients | 94 | 262 | 356 | 64 | 128 | 192 |
| No. of patients with infections | 15 (16.0) | 3 (1.1) | 18 (5.1)1 | 32 (50.0) | 19 (14.8) | 51 (26.6)1 |
| No. of patients with selected infectious syndromes (%) | ||||||
| Bloodstream infection | 2 (2.1) | 0 | 2 (0.6) | 13 (20.3) | 4 (3.1) | 17 (8.9) |
| Skin and soft tissue infections/wounds | 2 (2.1) | 2 (0.7) | 4 (1.1) | 22 (34.4) | 17 (13.3) | 39 (20.3) |
| Pneumonia | 10 (10.6) | 0 | 10 (2.8) | 7 (10.9) | 0 (0) | 7 (3.7) |
| Osteomyelitis | 0 (0) | 1 (0.4) | 1 (0.3) | 11 (17.2) | 8 (6.3) | 19 (9.9) |
Note: Numbers represent patient numbers with percentages in parentheses unless otherwise indicated.
Total number of patients at each level of care includes patients with solitary and multiple infections.
Table 5.
Characteristics of infectious disease complications during hospitalization among patients admitted through Level IV (Landstuhl Regional Medical Center) (June–August 2009) following traumatic injury
| Finding | |
|---|---|
| Total days of patient observation1 | 6500 |
| Total number of unique ID events2 | 117 |
| Total number of patients with ≥ 1 infection | 62 |
| ID event incidence density rate, # infections/person-days (95% CI); All patients (n=349) | 1.8 per 100 person-days (1.5, 2.2) |
| Patients admitted initially to Level IV ICU (n=94) | 86 ID events/ 292 person-days |
| Incidence density rate (95% CI) | 2.9 per 100 person-days (2.3, 3.6) |
| Patients admitted initially to Level IV ward (n=262) | 31 ID events/ 3518 person-days |
| Incidence density rate (95% CI) | 0.9 per 100 person-days (0.6, 1.3) |
| Incidence density rate ratio – ICU/Ward (95% CI) | 3.3 (2.1, 5.0; p < 0.0001) |
| Number of infections per patient (%) | |
| 1 event | 32 (51.6%) |
| 2 events | 18 (29.0%) |
| 3 events | 8 (12.9%) |
| ≥4 events | 4 (6.5%) |
| Level of care location for infection (%) | |
| Level IV only | 11 (17.7%) |
| Level V only | 44 (71.0%) |
| Both Level IV and V | 7 (11.3%) |
| Time in days to 1st infection1 from initial trauma, median (IQR) | |
| Bloodstream infection | 6.0 (3.0 – 8.0) |
| Skin and soft tissue infections | 12.0 (6.0 – 22.0) |
| Pneumonia | 3.0 (3.0 – 5.0) |
| Osteomyelitis | 15.0 (10.0 – 36.0) |
| Any infection | 7.5 (4.0 – 12.0) |
A patient was followed through the last hospital discharge captured at which point the data was censored. For patients admitted to participating level 5 hospitals this includes the entire inpatient level 5 period; whereas, patients not transferred to a participating level 5 hospital were censored at the time of the level 4 discharge.
ID events met either predefined criteria (n=95; 81.2%) or infection suspicion (not meeting criteria) plus ≥ 5 days directed antimicrobial therapy (n=22; 18.8%).
The most common ID syndrome observed at Level IV was pneumonia in 10.6% of the ICU admissions (none in the patients admitted to the ward). The more common syndromes in the U.S. hospitals were wound infections (SSTI) at 20.3%, bloodstream infections (BSI) at 8.9%, and osteomyelitis at 9.9%. The infection incidence rate combined across both Level IV and V care (Table 5) was approximately 3.5-fold higher for those patients stratified based on the site of initial admission at level 4 (ICU or ward). Among the infections observed during the inpatient period, 19 (14.3%) required ongoing treatment at discharge.
Clinical Microbiology Findings
Hospital-based active colonization surveillance at Level IV documented 75 of 302 patients screened (24.8%) to have positive cultures and of those 15 (20.0%) yielded a MDRO. At U.S. hospitals, 77 of 192 patients screened (40.1%) were found to have positive cultures and of those 29 (37.7%) yielded a MDRO. For all patients screened, the proportion found to have a MDRO was higher at U.S. hospitals compared to Level IV, 15.1 versus 4.9%, respectively. The most common bacteria observed were E. coli [extended spectrum β-lactamase (ESBL) producers] as shown in Table 6. A total of 117 unique ID events were documented among 62 patients. Of these, 89 (76.1%) had ≥ 1 positive microbiological isolate for a total of 172 distinct microorganisms. Coagulase-negative Staphylococcus, most commonly S. epidermidis, was the most common clinical isolate, typically associated with bloodstream infections. E. coli infections, frequently in BSI and wound infections were commonly isolated in both clinical infection cases as well as the colonization work-ups as previously stated.
Table 6.
Common microbial etiologies by selected infectious disease syndrome among patients admitted through Level IV (Landstuhl Regional Medical Center) (June–August 2009) following traumatic injury
| Classification | Isolation frequency – microbial species (%)1 | |
|---|---|---|
| Colonization2 | Level IV (n=302) | Escherichia coli (16.9) |
| Enterobacter spp. (5.0) | ||
| Klebsiella spp. (3.6) | ||
| Acinetobacter calcoaceticus-baumannii complex (2.7) | ||
| Level V (n=192) | Escherichia coli (24.0) | |
| Staphylococcus aureus (11.5) | ||
| Klebsiella spp. (10.4) | ||
| Acinetobacter calcoaceticus-baumannii complex (6.8) | ||
| Enterobacter spp. (4.2) | ||
| Pseudomonas aeruginosa (3.7) | ||
| Unique ID events (n=117)3 Clinical isolate (n=172) distribution across both Level IV and Level V |
Coagulase negative Staphylococcus (15.1) | |
| Enterobacter spp. (9.9) | ||
| Escherichia coli (9.3) | ||
| Pseudomonas aeruginosa (6.4) | ||
| Bacteroides spp. (5.2) | ||
| Klebsiella spp. (5.2) | ||
| Acinetobacter calcoaceticus-baumannii complex (5.2) | ||
| Staphylococcus aureus (4.7) | ||
| Candida spp. (4.7) | ||
Relative distribution frequency (%) among microbiological evaluations for either colonization surveillance or clinical indication (adjusts for duplicates on a per patient basis).
Colonization – based on infection control hospital policy for active screening for colonization [most commonly entails a groin swab (Level IV and V) combined with external nares swab for MRSA culture and/or MRSA PCR (Level V only)]
A total of 117 unique ID events were documented among 62 patients. Of these, 89 (76.1%) unique ID events had had ≥ 1 positive microbiological isolate for a total of 172 distinct microorganisms.
Post-hospitalization follow-up
During the follow-up period to date, a total of 28 incident infections meeting criteria were observed with the following distribution: sepsis (without focal infection) - 1, BSI – 2, wound/SSTI – 13, osteomyelitis – 5, pneumonia – 1, and miscellaneous – 6 (3 with sinusitis, 2 with urinary tract infections, and 1 with C. difficile infection). A total of 18 of the 81 patients (22.2%), having at least one follow-up assessment, were documented to have an incident infection after the initial U.S hospitalization.
Discussion
As of July 23, 2010, there have been 13,978 and 3,812 U.S. servicemembers wounded in action and not returned to duty within 72 hours in support of Operation Iraqi Freedom and Operation Enduring Freedom (Afghanistan), respectively since the beginning of combat operations.21 These conflicts have witnessed dramatic improvements in combat casualty care and patient survival.5, 10, 11 Concurrently, reports of multidrug-resistant infections present challenges to infection control and clinical management,13 as these patients transit multiple diverse levels of care including forward surgical hospitals, during aeromedical evacuations, Landstuhl Regional Medical Center, tertiary care centers in the United States, and frequently into the Veterans Administration health care system.22, 23 The complex nature of polytrauma in many of these cases6, 7 complicates care increasing the risk of infections at individual hospitals caring for these patients. Research efforts8, 24, 25 coupled with policy guidance13 have led to better understanding and greater system-based standardization of practices directed at the nosocomial reservoir for these MDR infections. The TIDOS project is the first prospective evaluation of infectious disease complications and subsequent outcomes using predefined standardized methodology combined with analysis of clinical management, surgical and medical care (i.e. antimicrobial therapy), and clinical microbiology results across levels of care, medical facilities, and outpatient follow-up. This initial report provides quantitative estimates for incident infections among these trauma patients in the first 3-month period of the project, highlighting the common occurrence of these complications particularly as the patients move to higher levels of care (5.1% at Level IV vs. 26.6% at Level V), as well as the common occurrence of incident infections post-hospitalizations (22% of patients).
The wounding patterns in modern warfare26 and specifically in the current conflicts have been well-described.27 The predominance of severe extremity injury, often related to blast injuries from explosive devices, with extensive soft tissue and bone loss, places these patients at significant risk of complicated soft tissue infections and osteomyelitis. On average, as seen in these data, certain infections arise later (wound/SSTI and osteomyelitis around 12–15 days) as compared to pneumonia (around 3 days) which may lend further insight into understanding potential reservoirs of infection and possible implications for approaches to prevent late-onset infections. In addition, many of these infections require ongoing management at discharge (approx. 15%) further impacting patient quality of life. In this early report of the project, incident infections in 22% of patients, most commonly soft tissue infections and osteomyelitis, documented after initial U.S. hospitalization highlight the necessity to actively survey for these late-onset outcomes and evaluate risk factors. Prior research has identified factors related to the characteristics of trauma and the early indicators of severity that provide predictive value for estimating infectious complications.4, 15, 28, 29 Trauma-specific factors previously reported to increase infection risk include, but are not limited to, requirement for massive blood transfusion,28, 29 Injury Severity Score (ISS) > 15,30 penetrating abdominal trauma,28 open, grossly contaminated comminuted fractures,6 and retained orthopedic hardware/invasive medical devices. Ascertainment of risks and assessment of various treatment strategies should be assessed in the context of both short- and long-term complications particularly in an era of increasing exposure to MDRO in healthcare environments. For example, reports have documented increases in infection rates among a cohort of patients with tibial fractures managed with tibial nailing as the period of observation was increased, as would be expected given the subacute nature of many of these infections.7 A recent report demonstrates encouraging findings supportive of temporary external fixation of type III tibia fractures without late onset of infectious complication in a 2-year follow-up period at one treatment facility.31 This report highlights analyses that could be replicated across military facilities through the TIDOS data. Future analyses of TIDOS data, particularly extending evaluations into the VA healthcare system, will assist in informing best practices.
This report provides a cross-sectional view of a period of time in the summer and early fall of 2009. During this timeframe there were notable occurrences external to the project impacting the findings. Of most importance, the spring of 2009 saw the shift, which continues to date, of much higher proportion of casualties occurring in Afghanistan than Iraq, 65.4% of overall patients wounded in support of OEF. The mechanism of injury and wounding patterns seen in these patients do not differ from previous reports where most injuries occurred in Iraq. In regards to infection risk and clinical management, recent Department of Defense efforts in 2008/2009 to promote standardization of practice patterns for infection control in theater as well as guidance for surgical and antimicrobial therapy for infection prevention have been promulgated.12 It is expected that these efforts have contributed to a reduction in infection rates; however, since there is no similarly well-characterized data to compare against this is uncertain. Previous efforts to estimate infection rates have included analysis of the Joint Theater Trauma Registry using captured data on International Classification of Diseases (ICD-9) codes.32 This report covered a period from 2003–2006 although the lack of complete records restricted the analysis and almost all patients had been injured in Iraq. In this analysis, approximately one third of the patients were found to have an ICD-9 code indicating an infectious complication, most commonly involving wound followed by lung infections, with gram-negative bacteria, when a pathogen was reported, accounting for 47% of reports. The lack of additional clinical information for these codes or verification of fulfilling predefined criteria makes direct comparison with data reported in this analysis difficult. The observed cumulative infection incidence of 26.6% for patients followed through Level 5 care approximates what has been previously reported. Increase in incident infections as patients move across echelons of care is also consistent with the higher rates of infection that have been reported in single center studies in the United States or among British military.23, 33, 34 The predominance of gram-negative infections reported in this study is also consistent with previous observations.23 Notably, the lower rates of Acinetobacter calcoaceticus-baumannii complex infections, frequently MDR, compared to prior reports of high rates of colonization25 and infection22 may be related to increased infection control vigilance although this is not certain. High levels of other multidrug-resistant organisms observed in this report, primarily E. coli [extended spectrum β-lactamase (ESBL) producers], detected through active surveillance for colonization as well as in clinical isolates emphasize the need for ongoing surveillance and development of improved measures for prevention and clinical management.
In order to generalize the TIDOS findings and provide the correct context to other military or civilian trauma populations, it is necessary to compare the cohort to the overall population of patients who are admitted through Level IV following traumatic injury. There are branch points along the path prior to the first opportunity to enroll a patient in the TIDOS cohort that restrict broad sampling of the overall trauma patient population. TIDOS enrollment occurs prior to discharge from an U.S. military hospital which is the first feasible time to interact with a large proportion of these severely traumatized patients and/or their families. As evident through these data, patients returning to duty as well as patients transferred to U.S. military hospitals not participating in the TIDOS project differ significantly (i.e. less severe injury and lower levels of interventions suggestive of patients requiring less intense care), from patients transferred to a participating site. This is not surprising since the participating sites are centers with unique expertise in surgical subspecialty or burn center care and are expected to receive the most seriously injured patients. At a participating site, the opportunity to interact with a patient or their family may be limited by a very brief inpatient stay if the patient is quickly moved to ambulatory management or, on the other extreme, a seriously injured patient where potential to interact with family members may be restricted or deemed not appropriate. Upon review of the data, the TIDOS cohort population appears to be have more extensive injury, as well as a more complicated and prolonged hospital course. In addition, the high rate of Veterans Administration registration (40%) amongst the cohort participants at only one year further supports the data that this sample represent, in general, a more severely injured subset who are likely at higher risk for infectious complications. As the study continues to enroll, now exceeding 725 participants, it is expected that the cohort will provide adequate representation of the population permitting generalization to broader military and civilian trauma patient populations; however, most important it will include substantial numbers of patients experiencing complex combat trauma for analysis which are otherwise not available in the civilian trauma literature.
Limitations of the study include the dependence upon medical record review which at times has variable quality on documentation of symptoms, signs, or additional findings that comprise criteria required in determining if an infectious disease event meets prospectively defined criteria. Medical record review, akin to similar approaches used in hospital-based infection control practice, is however a major advance over limited information available through ICD-9 coded data. Despite this inherent limitation, the TIDOS project provides the most comprehensive assessment of infectious complications following traumatic injury to date within the Department of Defense and emphasizes the significant impact infectious complications have on wounded military personnel. The successful project initiation, along with the JTTR supplemental ID module, will provide unique insights into short and long term infectious complications of combat trauma in order to develop future strategies for improving prevention and treatment of infections.
Acknowledgements
We thank the active duty personnel for participating in this study. We are indebted to the IDCRP TIDOS study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, statisticians, and administrative support personnel for their tireless hours to ensure the success of this project.
Role of the Funding Source. Support for this work (IDCRP-024) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed through the Uniformed Services University. This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072 and the Department of the Navy under the Wounded, Ill, and Injured Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the DoD or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
I/We certify that all individuals who qualify as authors have been listed; each has participated in the conception and design of this work, the analysis of data (when applicable), the writing of the document, and the approval of the submission of this version; that the document represents valid work; that if we used information derived from another source, we obtained all necessary approvals to use it and made appropriate acknowledgements in the document; and that each takes public responsibility for it. Nothing in the presentation implies any Federal/DOD/DON endorsement.
Authors acknowledge that research protocol (IDCRP-024) received applicable Uniformed Services University Institutional Review Board review and approval.
Conflict of Interest. All authors: No conflict.
References
- 1.Murray CK, Hinkle MK, Yun HC. History of infections associated with combat-related injuries. J Trauma. 2008;64:S221–S231. doi: 10.1097/TA.0b013e318163c40b. [DOI] [PubMed] [Google Scholar]
- 2.Cirillo VJ. Two faces of death: fatalities from disease and combat in America's principal wars, 1775 to present. Perspect Biol Med. 2008;51:121–133. doi: 10.1353/pbm.2008.0005. [DOI] [PubMed] [Google Scholar]
- 3.Gawande A. Casualties of war--military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471–2475. doi: 10.1056/NEJMp048317. [DOI] [PubMed] [Google Scholar]
- 4.Kelly JF, Ritenour AE, McLaughlin DF, et al. Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003–2004 versus 2006. J Trauma. 2008;64:S21–S26. doi: 10.1097/TA.0b013e318160b9fb. discussion S6-7. [DOI] [PubMed] [Google Scholar]
- 5.Eastridge BJ, Jenkins D, Flaherty S, Schiller H, Holcomb JB. Trauma system development in a theater of war: Experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2006;61:1366–1372. doi: 10.1097/01.ta.0000245894.78941.90. discussion 72-3. [DOI] [PubMed] [Google Scholar]
- 6.Covey DC, Aaron RK, Born CT, et al. Orthopaedic war injuries: from combat casualty care to definitive treatment: a current review of clinical advances, basic science, and research opportunities. Instr Course Lect. 2008;57:65–86. [PubMed] [Google Scholar]
- 7.Hayda RA, Mazurek MT, Powell IvET, et al. From Iraq back to Iraq: modern combat orthopaedic care. Instr Course Lect. 2008;57:87–99. [PubMed] [Google Scholar]
- 8.Scott P, Deye G, Srinivasan A, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44:1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 9.Kallen AJ, Hidron AI, Patel J, Srinivasan A. Multidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006–2008. Infect Control Hosp Epidemiol. 2010;31:528–531. doi: 10.1086/652152. [DOI] [PubMed] [Google Scholar]
- 10.Eastridge BJ, Costanzo G, Jenkins D, et al. Impact of joint theater trauma system initiatives on battlefield injury outcomes. Am J Surg. 2009;198:852–857. doi: 10.1016/j.amjsurg.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Eastridge BJ, Wade CE, Spott MA, et al. Utilizing a trauma systems approach to benchmark and improve combat casualty care. J Trauma. 2010;69:S5–S9. doi: 10.1097/TA.0b013e3181e421f3. [DOI] [PubMed] [Google Scholar]
- 12.Hospenthal DR, Murray CK, Andersen RC, et al. Guidelines for the prevention of infection after combat-related injuries. J Trauma. 2008;64:S211–S220. doi: 10.1097/TA.0b013e318163c421. [DOI] [PubMed] [Google Scholar]
- 13.Hospenthal DR, Crouch HK, English JF, et al. Response to infection control challenges in the deployed setting: Operations Iraqi and Enduring Freedom. J Trauma. 2010:1–8. doi: 10.1097/TA.0b013e3181e44b3f. [DOI] [PubMed] [Google Scholar]
- 14.Landrum ML, Murray CK. Ventilator associated pneumonia in a military deployed setting: the impact of an aggressive infection control program. J Trauma. 2008;64:S123–S127. doi: 10.1097/TA.0b013e31816086dc. discussion S7-8. [DOI] [PubMed] [Google Scholar]
- 15.Champion HR, Holcomb JB, Lawnick MM, et al. Improved characterization of combat injury. J Trauma. 2010;68:1139–1150. doi: 10.1097/TA.0b013e3181d86a0d. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 17.Horan TCGR. Surveillance of nosocomial infections. In: CG M, editor. Hospital Epidemiology and Infection Control. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 1659–1702. [Google Scholar]
- 18.Division of Healthcare Quality Promotion NCfP, Detection and Control of Infectious Diseases, Centers for Disease Control and Prevention. The National Healthcare Safety Network (NHSN) Manual (Patient Safety Component) - Protocol: Multidrug-resistant Organism (MDRO) and Clostridium difficile-Associated Disease (CDAD) Module. 2008 [Google Scholar]
- 19.Fay MP. Confidence intervals that match Fisher's exact or Blaker's exact tests. Biostatistics. 2010;11:373–374. doi: 10.1093/biostatistics/kxp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta CR, Patel NR. A network algorithm for the exact treatment of the 2xk contingency table. Commun in Statist. 1980;B9:649–664. [Google Scholar]
- 21.U.S. Department of Defense: Operation Iraqi Freedom/Operation Enduring Freedom Casualty Update. [(accessed July 23,2010)]; http://www.defenselink.mil/news/casualty.pdf (Accessed at. [Google Scholar]
- 22.Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002–2004. MMWR Morb Mortal Wkly Rep. 2004;53:1063–1066. [PubMed] [Google Scholar]
- 23.Aronson NE, Sanders JW, Moran KA. In harm's way: infections in deployed American military forces. Clin Infect Dis. 2006;43:1045–1051. doi: 10.1086/507539. [DOI] [PubMed] [Google Scholar]
- 24.Kaspar RL, Griffith ME, Mann PB, et al. Association of bacterial colonization at the time of presentation to a combat support hospital in a combat zone with subsequent 30-day colonization or infection. Mil Med. 2009;174:899–903. doi: 10.7205/milmed-d-04-3908. [DOI] [PubMed] [Google Scholar]
- 25.Weintrob AC, Roediger MP, Barber M, et al. Natural history of colonization with gram-negative multidrug-resistant organisms among hospitalized patients. Infect Control Hosp Epidemiol. 2010;31:330–337. doi: 10.1086/651304. [DOI] [PubMed] [Google Scholar]
- 26.Champion HR, Bellamy RF, Roberts CP, Leppaniemi A. A profile of combat injury. J Trauma. 2003;54:S13–S19. doi: 10.1097/01.TA.0000057151.02906.27. [DOI] [PubMed] [Google Scholar]
- 27.Zouris JM, Walker GJ, Dye J, Galarneau M. Wounding patterns for U.S. Marines and sailors during Operation Iraqi Freedom, major combat phase. Mil Med. 2006;171:246–252. doi: 10.7205/milmed.171.3.246. [DOI] [PubMed] [Google Scholar]
- 28.Petersen K, Riddle MS, Danko JR, et al. Trauma-related infections in battlefield casualties from Iraq. Ann Surg. 2007;245:803–811. doi: 10.1097/01.sla.0000251707.32332.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunne JR, Riddle MS, Danko J, Hayden R, Petersen K. Blood transfusion is associated with infection and increased resource utilization in combat casualties. Am Surg. 2006;72:619–625. discussion 25-6. [PubMed] [Google Scholar]
- 30.Champion HR, Sacco WJ, Copes WS, Gann DS, Gennarelli TA, Flanagan ME. A revision of the Trauma Score. J Trauma. 1989;29:623–629. doi: 10.1097/00005373-198905000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Possley DR, Burns TC, Stinner DJ, et al. Temporary external fixation is safe in a combat environment. J Trauma. 2010;69:S135–S139. doi: 10.1097/TA.0b013e3181e44fcb. [DOI] [PubMed] [Google Scholar]
- 32.Murray CK, Wilkins K, Molter NC, et al. Infections in combat casualties during Operations Iraqi and Enduring Freedom. J Trauma. 2009;66:S138–S144. doi: 10.1097/TA.0b013e31819d894c. [DOI] [PubMed] [Google Scholar]
- 33.Yun HC, Branstetter JG, Murray CK. Osteomyelitis in military personnel wounded in Iraq and Afghanistan. J Trauma. 2008;64:S163–S168. doi: 10.1097/TA.0b013e318160868c. discussion S8. [DOI] [PubMed] [Google Scholar]
- 34.Brown KV, murray CK, Clasper JC. Infectious complications of combat-releated mangled extremity injuries in the British military. J Trauma. 2010;69:S109–S115. doi: 10.1097/TA.0b013e3181e4b33d. [DOI] [PubMed] [Google Scholar]
- 35.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 36.Annane D, Bellissant E, Cavaillon J-M. Septic shock. The Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]