Abstract
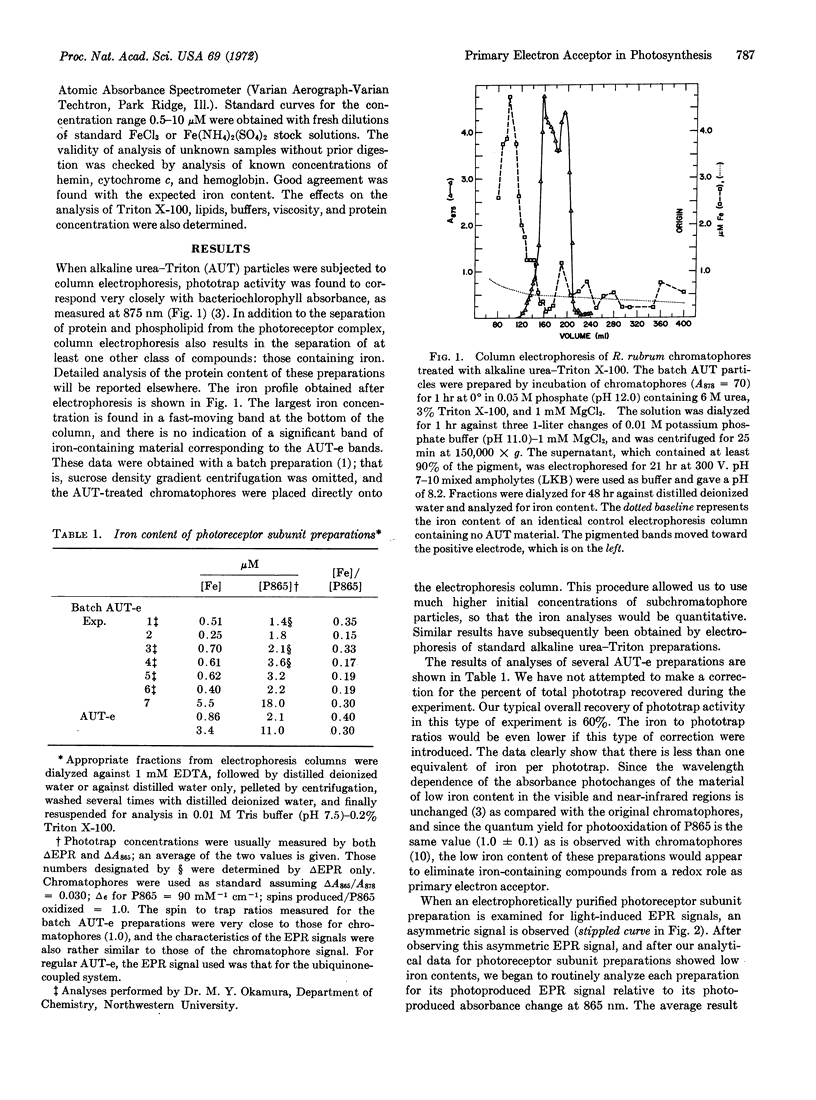
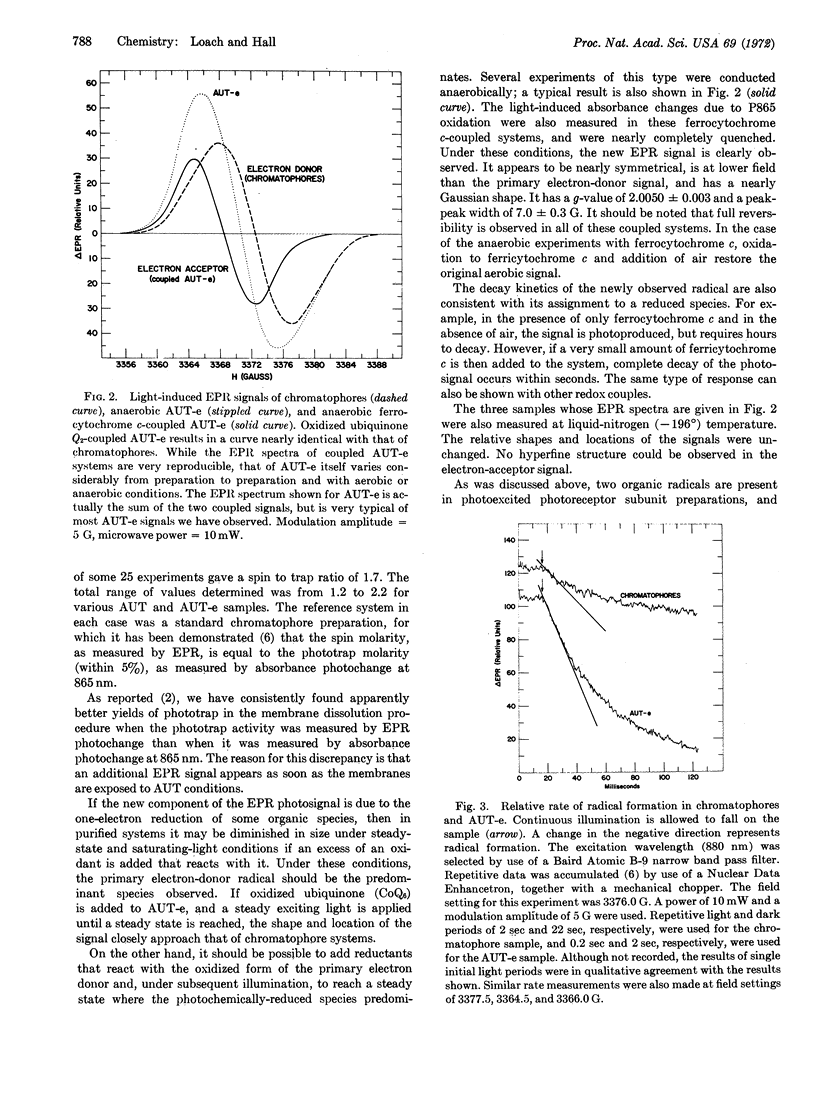
An electrophoretic purification of Rhodospirillum rubrum photoreceptor subunits prepared by alkaline urea-detergent disruption is described. Completely active photoreceptor subunits with less than 0.30 eq of iron (or any other transition metal) per phototrap can routinely be prepared. A new photoproduced electron paramagnetic resonance (EPR) signal has been detected in these preparations; it was shown to be due to a photoreduced species. It has a g-value of 2.0050 ± 0.0003, a peak-peak width of 7.0 ± 0.3 G, and a nearly Gaussian shape. The response of the new signal to microwave power is different from that of the EPR signal of the photoproduced primary electron donor of chromatophores. Quantum yield measurements of spin production show that the new signal is very efficiently formed (ϕ = 0.6) simultaneously with the electron donor radical. No hyperfine structure (down to 0.1 G modulation amplitude) was observed in the new signal, either at room temperature or at the temperature of liquid nitrogen. The possible identity of this molecule is discussed.
Keywords: Rhodospirillum rubrum, photoreceptor, iron-depleted particles, quantum yield, chromatophores
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton J. R., Clayton R. K., Reed D. W. An identification of the radical giving rise to the light-induced electron spin resonance signal in photosynthetic bacteria. Photochem Photobiol. 1969 Mar;9(3):209–218. doi: 10.1111/j.1751-1097.1969.tb07285.x. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. Evidence for the photochemical reduction on coenzyme Q in chromatophores of photosynthetic bacteria. Biochem Biophys Res Commun. 1962 Sep 25;9:49–53. doi: 10.1016/0006-291x(62)90085-2. [DOI] [PubMed] [Google Scholar]
- Commoner B., Heise J. J., Townsend J. LIGHT-INDUCED PARAMAGNETISM IN CHLOROPLASTS. Proc Natl Acad Sci U S A. 1956 Oct;42(10):710–718. doi: 10.1073/pnas.42.10.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer W. A. Low potential titration of the fluorescence yield changes in photosynthetic bacteria. Biochim Biophys Acta. 1969 Sep 16;189(1):54–59. doi: 10.1016/0005-2728(69)90224-2. [DOI] [PubMed] [Google Scholar]
- Cusanovich M. A., Bartsch R. G., Kamen M. D. Light-induced electron transport in Chromatium strain D. II. Light-induced absorbance changes in Chromatium chromatophores. Biochim Biophys Acta. 1968 Feb 12;153(2):397–417. doi: 10.1016/0005-2728(68)90083-2. [DOI] [PubMed] [Google Scholar]
- Dutton P. L. Oxidation-reduction potential dependence of the interaction of cytochromes, bacteriochlorophyll and carotenoids at 77 degrees K in chromatophores of Chromatium D and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1971 Jan 12;226(1):63–80. doi: 10.1016/0005-2728(71)90178-2. [DOI] [PubMed] [Google Scholar]
- Feher G. Some chemical and physical properties of a bacterial reaction center particle and its primary photochemical reactants. Photochem Photobiol. 1971 Sep;14(3):373–387. doi: 10.1111/j.1751-1097.1971.tb06180.x. [DOI] [PubMed] [Google Scholar]
- KUNTZ I. D., Jr, LOACH P. A., CALVIN M. ABSORPTION CHANGES IN BACTERIAL CHROMATOPHORES. Biophys J. 1964 May;4:227–249. doi: 10.1016/s0006-3495(64)86779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl D. H., Townsend J., Commoner B., Crespi H. L., Dougherty R. C., Katz J. J. Effects of isotopic substitution on electron spin resonance signals in photosynthetic organisms. Nature. 1965 Jun 12;206(989):1105–1110. doi: 10.1038/2061105a0. [DOI] [PubMed] [Google Scholar]
- Kohl D. H., Wright J. R., Weissman M. Electron spin resonance studies of free radicals derived from plastoquinone, alpha- and gamma-tocopherol and their relation to free radicals observed in photosynthetic materials. Biochim Biophys Acta. 1969 Aug 5;180(3):536–544. doi: 10.1016/0005-2728(69)90032-2. [DOI] [PubMed] [Google Scholar]
- Loach P. A., Hadsell R. M., Sekura D. L., Stemer A. Quantitative dissolution of the membrane and preparation of photoreceptor subunits from Rhodospirillum rubrum. Biochemistry. 1970 Aug 4;9(16):3127–3135. doi: 10.1021/bi00818a003. [DOI] [PubMed] [Google Scholar]
- Loach P. A. Primary oxidation-reduction changes during photosynthesis in Rhodospirillum rubrum. Biochemistry. 1966 Feb;5(2):592–600. doi: 10.1021/bi00866a028. [DOI] [PubMed] [Google Scholar]
- Loach P. A., Sekura D. L., Hadsell R. M., Stemer A. Quantitative dissolution of the membrane and preparation of photoreceptor subunits from Rhodopseudomonas spheroides. Biochemistry. 1970 Feb 17;9(4):724–733. doi: 10.1021/bi00806a004. [DOI] [PubMed] [Google Scholar]
- Loach P. A., Sekura D. L. Primary photochemistry and electron transport in Rhodospirillum rubrum. Biochemistry. 1968 Jul;7(7):2642–2649. doi: 10.1021/bi00847a029. [DOI] [PubMed] [Google Scholar]
- Loach P. A., Walsh K. Quantum yield for the photoproduced electron paramagnetic resonance signal in chromatophores from Rhodospirillum rubrum. Biochemistry. 1969 May;8(5):1908–1913. doi: 10.1021/bi00833a021. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., FEHER G. A STUDY OF THE PHOTOINDUCED PORPHYRIN FREE RADICAL BY ELECTRON SPIN RESONANCE. Biochim Biophys Acta. 1964 Mar 30;79:430–432. [PubMed] [Google Scholar]
- McElroy J. D., Feher G., Mauzerall D. C. On the nature of the free radical formed during the primary process of bacterial photosynthesis. Biochim Biophys Acta. 1969 Jan 14;172(1):180–183. doi: 10.1016/0005-2728(69)90105-4. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Clayton R. K. The reducing potential of the bacterial photosynthetic reaction center. Photochem Photobiol. 1969 Apr;9(4):395–399. doi: 10.1111/j.1751-1097.1969.tb07305.x. [DOI] [PubMed] [Google Scholar]
- Parson W. W. The role of P870 in bacterial photosynthesis. Biochim Biophys Acta. 1968 Jan 15;153(1):248–259. doi: 10.1016/0005-2728(68)90167-9. [DOI] [PubMed] [Google Scholar]
- Sogo P. B., Pon N. G., Calvin M. PHOTO SPIN RESONANCE IN CHLOROPHYLL-CONTAINING PLANT MATERIAL. Proc Natl Acad Sci U S A. 1957 May 15;43(5):387–393. doi: 10.1073/pnas.43.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLLIN G., GREEN G. Light-induced single electron transfer reactions between chlorophyll a and quinones in solution. I. Some general feature of kinetics and mechanism. Biochim Biophys Acta. 1962 Jul 16;60:524–538. doi: 10.1016/0006-3002(62)90871-5. [DOI] [PubMed] [Google Scholar]



