Abstract
Study Objective:
In this meta-analysis, we compare the short-term efficacy of prazosin vs. IRT on nightmares, sleep quality, and posttraumatic stress symptoms (PTSS).
Methods:
Reference databases were searched for randomized controlled trials using IRT or prazosin for nightmares, sleep disturbance, and/or PTSS. Effect sizes were calculated by subtracting the mean posttest score in the control group from the mean posttest score in the treatment group, and dividing the result by the pooled standard deviation of both groups. Mixed effects models were performed to evaluate effects of treatment characteristics, as well as sample characteristics (veteran vs. civilian) on treatment efficacy.
Results:
Four studies used prazosin, 10 used IRT alone or in combination with another psychological treatment, and 1 included a group receiving prazosin and another group receiving IRT. Overall effect sizes of both treatments were of moderate magnitude for nightmare frequency, sleep quality, and PTSS (p < 0.01). Effect size was not significantly different with type of treatment (psychological vs. pharmacological) on nightmare frequency (p = 0.79), sleep quality (p = 0.65), or PTSS, (p = 0.52). IRT combined with CBT for insomnia showed more improvement in sleep quality compared to prazosin (p = 0.03), IRT alone (p = 0.03), or IRT combined with another psychological intervention, (p < 0.01).
Conclusion:
Although IRT interventions and prazosin yield comparable acute effects for the treatment of nightmares, adding CBT for insomnia to IRT seems to enhance treatment outcomes pertaining to sleep quality and PTSS. More randomized clinical trials with long-term follow-up are warranted.
Commentary:
A commentary on this article appears in this issue on page 9.
Citation:
Seda G, Sanchez-Ortuno MM, Welsh CH, Halbower AC, Edinger JD. Comparative meta-analysis of prazosin and imagery rehearsal therapy for nightmare frequency, sleep quality, and posttraumatic stress. J Clin Sleep Med 2015;11(1):11–22.
Keywords: posttraumatic stress disorder, nightmares, prazosin, imagery rehearsal therapy, cognitive behavior therapy, insomnia
Nightmares are common phenomena occurring in most healthy adults. It has been estimated that around 70% of the general population suffers from occasional nightmares.1 However, when these frightening dreams occur in a recurrent fashion, the nightmare activity may constitute a disorder. In fact, nightmare disorder is listed in both the DSM-5 and in the ICSD-3 as a distinct disorder whose essential features are the presence of repeated negative dreams that lead to awakening, such that the individual becomes fully alert and aware of his or her surroundings.2,3
Nightmares can have an idiopathic origin or may occur after traumatic events.4 Along with other sleep disturbances (e.g., insomnia and poor sleep quality),5 nightmares are a typical symptom of posttraumatic stress disorder (PTSD).6 Indeed, it has been estimated that around 70% of individuals with PTSD report posttraumatic nightmares.7,8 Nonetheless, nightmares are not exclusive to PTSD, since they are also associated with anxiety and depression, among other mental health comorbidities.9,10
BRIEF SUMMARY
Current Knowledge/Study Rationale: Imagery rehearsal therapy (IRT) and prazosin are both used for treatment of nightmares in posttraumatic stress. The main aim of this study was a comparative meta-analysis of each treatment on nightmare frequency, sleep quality, and posttraumatic stress disorder symptoms.
Study Impact: IRT and prazosin had similar effects for nightmare frequency, sleep quality, and posttraumatic stress disorder symptoms; however, adding cognitive-behavior therapy for insomnia to IRT enhanced its effects for improving sleep quality as well as posttraumatic stress disorder symptoms.
Traditionally, nightmares have not been treated directly, but assumed to improve after successful treatment of the primary condition from which they were hypothesized to originate. However, as in the case of PTSD treatment, research has shown that nightmares, and other related sleep disturbances, often persist at clinically significant levels following PTSD treatment.11–13
With this aim, various psychological and pharmacological treatments for chronic nightmares have been proposed and tested thorough recent years. This burgeoning literature led to the Standards of Practice Committee (SPC) of the American Academy of Sleep Medicine to commission a task force to assess the relevant literature on the treatment on nightmares. As a result of that, a Best Practice Guide for the treatment of nightmare disorder in adults was published in 2010.14 This review summarized the different treatment approaches used for nightmares. Among all the treatments reviewed, two modalities, a pharmacological agent named prazosin and a cognitive-behavioral therapy technique known as imagery rehearsal therapy (IRT), emerged as Level A recommendations for the treatment of nightmares in adults with both approaches supported by a substantial amount of high-quality evidence.
Prazosin is a lipid-soluble α-1 adrenergic receptor antagonist that crosses the blood-brain barrier and decreases the sympathetic outflow in the brain. Although it has been approved by the US Food and Drug Administration for the treatment of hyper-tension, it is rarely used today as an antihypertensive agent.15 In contrast, since prazosin arguably blocks the excessive norepinephrine activity, and increased norepinephrine activity is seen patients with PTSD and is associated with heightened arousal and nightmares,16 this medication has been successfully used to treat PTSD-associated nightmares and other PTSD symptoms.
With respect to IRT, while there are some differences across studies regarding the manner in which this technique is applied, the basic components of IRT include asking the patient to select a nightmare, and instructing him or her to write a dream narrative that incorporates a change to some aspect of the selected nightmare. Subsequently, the patient is asked to rehearse daily the new dream while awake. The rationale is that, when the new dream is rehearsed, the new script will become more cognitively dominant than the bothersome nightmare, and eventually, the nightmare will diminish or disappear.17
An important aspect noted in the SPC review paper is that the most frequent type of nightmare addressed in the treatment studies using either prazosin or IRT is the PTSD-associated nightmare. Therefore, and not surprisingly, these treatment studies have also examined whether treatment may have an impact on other sleep-related symptoms commonly seen in PTSD patients, such as poor sleep quality and other types of posttraumatic stress symptoms (PTSS).18,19
After the publication of this influential review paper, a few meta-analyses have been conducted to quantitatively summarize the results of studies testing the treatments for nightmares.20–22 With respect to psychological approaches for the treatment of nightmares, an important aspect noted in those meta-analyses is that treatment protocols using IRT often combine this therapy with another form of psychological intervention. In this line, the meta-analysis conducted by Casement et al. showed that adding cognitive-behavioral treatment for insomnia (CBT-I), although not being superior to IRT alone for reducing nightmare frequency, did result in greater improvement in other parameters of sleep disturbance, such as sleep quality.21 Likewise, the results of the somewhat more restrictive meta-analysis (i.e. including only randomized clinical trials, RCTs) by Augedal et al. showed that multicomponent treatments are not superior than IRT alone at reducing nightmare frequency.20 However, unlike the Casement meta-analysis, Augedal's study did not compare treatment effects on other relevant outcomes that are often included in such studies, such as sleep quality or symptoms of PTSD. Of interest, the Augedal meta-analysis was the only one comparing the effect sizes of studies employing one of the two best-supported treatments for nightmares, IRT and prazosin, concluding that both the psychological and the pharmacological approach were reasonably effective and comparable, both showing medium effect sizes. However, and as noted above, the results of meta-analysis are limited to nightmare indicators and are not extended to other symptoms frequently associated to chronic nightmares, such as poor sleep quality and symptoms of comorbid conditions such as PTSD.
Therefore, in the present meta-analysis, our goal was to add available evidence and complement and extend the findings provided by previous meta-analytic studies by comparing the short-term efficacy of prazosin and IRT interventions not only on nightmares but also on other relevant outcomes, such as sleep quality and/or other PTSS. In addition to comparing the effect sizes for each treatment approach for each outcome measure, we aimed to investigate the effect of sample characteristics (i.e., veteran/military vs. civilian samples) on treatment efficacy. Furthermore, since psychological treatments including IRT usually add other interventions within the treatment protocol (e.g., CBT for insomnia, sleep hygiene education), we wanted to explore whether treatment combinations are associated with enhanced treatment efficacy. Finally, we wanted to compare the treatment efficacy for different IRT delivery formats, such as individual, group, and self-help therapies.
METHODS
Literature Search and Inclusion Criteria
We conducted this meta-analysis using the PRISMA guidelines.23 Studies were identified using 3 search strategies. First, bibliographic databases (Ovid MEDLINE, PsycINFO, and Cochrane Reviews) were searched for studies using IRT or prazosin for treatment of nightmares, sleep quality, and/or symptoms of posttraumatic stress. Searches were conducted using the following terms: imagery, imagery rehearsal therapy, prazosin, furazosin or pratsiol, nightmares, bad dreams, insomnia, sleep quality, sleep disorders, and posttraumatic stress disorder. The search was limited to human studies in English with adults. Studies were included in the meta-analysis if they met the following criteria: (1) treating adult subjects; (2) being a randomized controlled study design using prazosin, IRT, or a cognitive-behavioral treatment with a component of IRT; (3) reporting at least one outcome measure connoting nightmare frequency or sleep disturbance; (4) including sufficient statistical information to calculate the effect size; and (5) written in English.
The initial inquiry identified a total of 93 references. After removing duplicates, 61 articles were screened by reading the abstracts. If no decision was possible after reading an abstract, then we read the full text. Of the articles screened, 11 articles met inclusion criteria and 50 articles were rejected because they were reviews or theoretical articles, single case studies, included adolescent or child treatment samples, were not RCTs, or used treatments other than IRT or prazosin. Reference lists were then examined from articles that met inclusion criteria, recent meta-analyses, and review papers, resulting in 4 additional references identified, resulting in 15 articles that met our inclusion criteria. Disposition of the search hits and articles retrieved is shown in Figure 1.
Figure 1. Flow diagram.
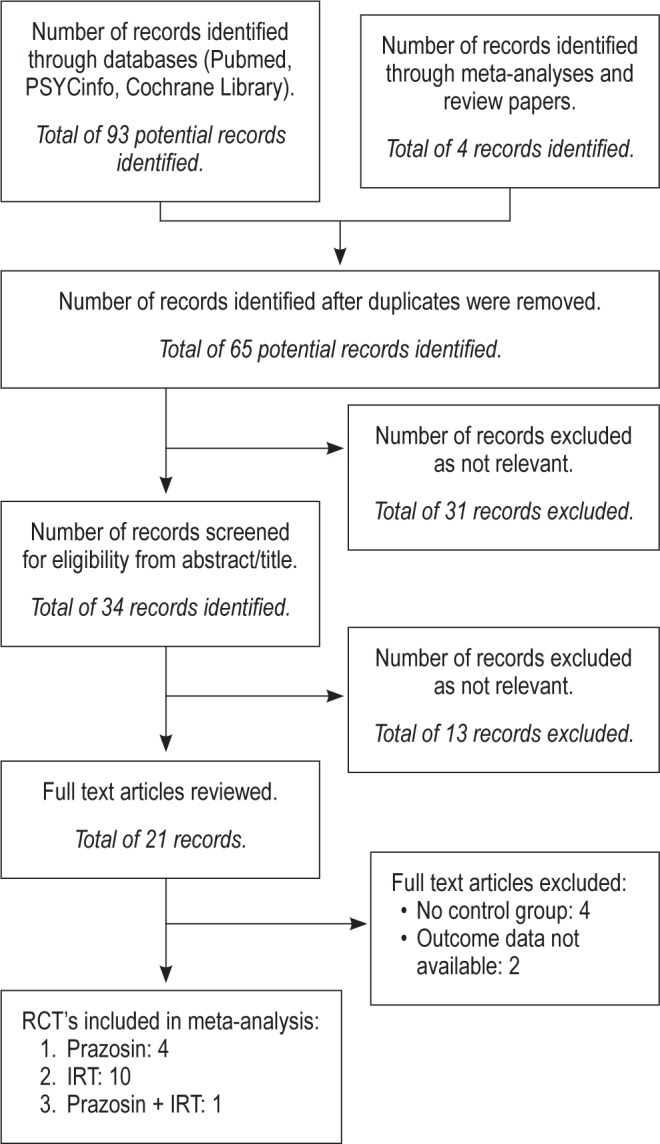
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. RCT, randomized controlled trial; IRT, imagery rehearsal therapy.
Description of Studies
In this meta-analysis, we included a total of 15 RCTs, with 18 contrast groups reporting posttest effects from the treatment group compared to a control group for at least 1 of the 3 treatment outcomes under study—nightmare frequency, sleep quality, and PTSS. Table 1 presents descriptive characteristics for each study included in the meta-analysis, ordered by publication year. Of these, there are 4 pharmacological trials including prazosin,24–27 10 studies including IRT, either alone or combined with another form of psychological intervention,28–37 and one study comprising both IRT and prazosin interventions administered to 2 groups randomly assigned to these treatments.38 All studies were published between 2000 and 2013. The total number of participants included in the meta-analysis (sum of all study participants represented by at least one outcome at posttreatment) was 967. A total of 8 studies included civilian samples, whereas the remaining studies included either military veterans (6 studies) or active-duty military personnel (one study). In terms of the symptom characteristics required for in the trials, the majority of studies required the presence of self-reported nightmares among other symptoms, with the exception of the study by Margolies et al.,37 which required a diagnosis of PTSD combined with insomnia. Across the 13 trials that provided diagnostic information, 90.5% of the participants had a PTSD diagnosis.
Table 1.
Randomized controlled trials on the effects of Prazosin and IRT on nightmares, sleep quality, and posttraumatic stress symptoms.
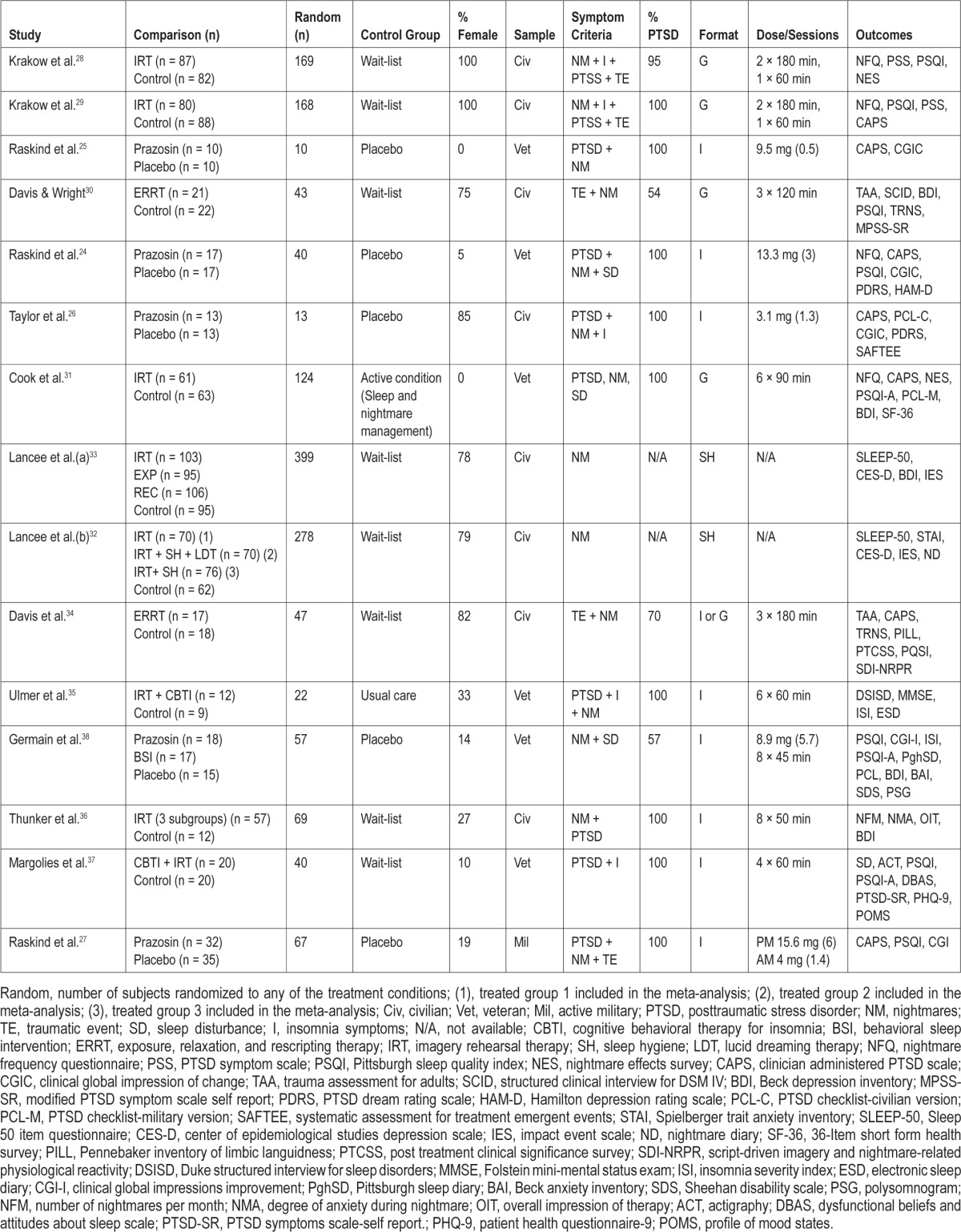
The 5 studies testing prazosin used different medication dosages, ranging from a mean dose of 3.1 mg to a mean dose of 15.6 mg. In 4 of the 5 studies, participants were instructed to take the medication at bedtime. However, in the most recent study by Raskind et al.,27 participants were asked to take a mid-morning as well as a bedtime dose.
Studies including IRT also differed in terms of the components included in the treatment protocol. Whereas 5 studies used IRT as the unique treatment component, 3 studies combined IRT with CBT for insomnia. In these studies, CBT included the major behavioral treatment components, such as stimulus control and sleep restriction therapy. Two studies used a variant of IRT known as exposure, relaxation and rescripting therapy (ERRT). This treatment approach includes sleep hygiene education, altering sleep habits, and progressive muscle relaxation training, combined with rescripting a nightmare and rehearsing the dream. Finally, the study by Lancee et al.33 had 3 intervention groups: a group that received IRT alone, a group receiving IRT combined with sleep hygiene education, and another group receiving IRT combined with lucid dreaming therapy (LDT). The LDT component involved instructing participants to imagine nightmares during the day, while thinking that it is only a dream, and thereby triggering lucidity during the nightmare.
Across the 9 trials delivering the psychological interventions in a face-to-face format, the number of treatment sessions and the length of the sessions varied broadly, ranging from 1 to 8 sessions, and from 45 minutes to 3 hours per session. In 3 studies, the interventions were conducted exclusively in a group format and, in some instances, within the same study, interventions were delivered in both individual and group formats. In 3 other studies the intervention was delivered in an individual format. In 2 trials, the treatment was offered in a self-help book format.
Most of the studies including IRT used participants on a waiting list as the control group. In one study the treated group was compared to a usual care group and another study used an active treatment control, named sleep and nightmare management. Participants included in this control group received psychoeducation about PTSD nightmares and sleep disturbances, as well as elements of standard CBT for insomnia. However, nightmare content was not discussed within this group. In the study by Germain et al.38 the group of participants receiving the psychological intervention was compared to a group of subjects receiving a placebo drug. All the prazosin studies used a medication placebo as the control condition.
Not all of the 15 treatment studies included herein provided relevant data on all 3 outcomes of interest. One study did not report treatment outcomes for nightmare frequency in a way that could be analyzed in this meta-analysis.37 In another study, sleep quality and PTSS were not included as treatment outcomes.36
In studies where there was more than one nightmare measure, we selected the number of nights per week with nightmares as the primary nightmare outcome.30,31,34,37,38 If this information was not available, we then used one of the following: nights with nightmares per month or number of nightmares per week/ month.25,28,29,32,33,35,36 In 3 studies,24,26,27 nightmare outcomes were solely assessed with the item on the Clinician-Administered PTSD Scale (CAPS) “Recurrent distressing dreams.”39 This nightmare-related outcome combines nightmare frequency and nightmare intensity within the same score, which can range from 0 to 8 points. Scores on this item are computed by summing the frequency and intensity of trauma-related distressing dreams. Scores on frequency can range from 0 to 4, a maximum score being computed when frequency is of “daily or almost daily every day.” Likewise, scores on intensity can range from 0 to 4, a maximum score being computed when the nightmare causes “extreme, incapacitating distress, did not return to sleep.” Since none of the 3 studies included in our analyses reported the frequency and severity scores separately, we used the composite nightmare score reported for the CAPS in these studies.
The majority of RCTs included in this meta-analysis used the Pittsburgh Sleep Quality Index (PSQI)40 to evaluate sleep quality, with the exception of the studies by Raskind et al.,24 Taylor et al.,19 and Lancee et al.,32,33 which used other metrics, such as the SLEEP-50 questionnaire41 or the item on the CAPS pertaining to insomnia.42 There was slightly more heterogeneity in the measures used for evaluating PTSS in the studies. The instruments used included the CAPS, the Pittsburgh Sleep Quality Index-Addendum (PSQI-A),43 PTSD Checklist (PCL),44 the Impact of Event Scale-Revised (IES-R),45 or the Clinical Global Impression (CGI) scales.46
Coding
For the analyses, the following variables were extracted from the studies meeting inclusion criteria: the treated and control groups' sample sizes at posttreatment assessment, their post-treatment mean values, and corresponding standard deviations (SD) from the outcomes of interest. When the study provided the standard error of the mean, instead of the SD, the following formula was used to compute the SD: SD = standard error * √n.
For moderation analyses, the following information from each treatment vs. control group comparison was independently coded by 2 of the authors: treatment characteristics (prazosin, IRT alone, IRT combined with CBT for insomnia or IRT combined with other form of psychological therapy), sample characteristics (veterans/military personnel vs. civilians), and treatment format (self-help, group, or individual therapy).
Meta-analyses generally include one effect size per construct per study. In the present meta-analysis, and in line with Casement and Swanson,21 nightmare frequency, sleep quality, and PTSS were considered as separate constructs. Therefore, effect sizes were calculated separately for each of these 3 measures. Treatment effects were based on the first assessment following treatment. For the vast majority of studies included in this meta-analysis, effect sizes were calculated from data obtained from those study participants who completed treatment.
Statistical Analyses
Data were entered and analyzed by the Comprehensive Meta-Analysis software, Version 2. For each outcome measure, effect sizes (Cohen's d) were calculated by subtracting the mean posttest score in the control group from the mean post-test score in the treatment group and dividing the result by the pooled standard deviations of both groups.47
Although it has been suggested that the random effects model is generally a more plausible match than the fixed effect model for computing overall effect sizes across studies,48 we determined the validity of the assumptions for the fixed- or random-effects model on the basis of the Q-statistics. If the Q statistic is sufficiently large (i.e., greater than the critical value at the 0.05 level), then the null hypothesis of homogeneity is rejected in favor of the alternative that the effects sizes were not sampled from a common population. When the Q-statistic is statistically significant, it implies that the effect sizes under study are not homogeneous, and the random-effects model should be applied to compute overall effect sizes across studies.49 Using a random-effects model implies that the true effect could vary from study to study. Since studies will differ in their mixes of participants and in the implementations of interventions, there may be different effect sizes underlying different studies. Therefore, under the random-effects model, large studies are assigned less relative weight and small studies are assigned more relative weight when computing the overall effect size. The random-effect model assumes that a single true effect is common to every study; therefore, the weights assigned to each individual study to compute the overall effect size are mostly dependent on the study size.
To evaluate if there is a moderation effect of type of intervention (psychological versus pharmacological), and sample characteristics (civilians vs. veterans/military personnel) on treatment efficacy, we conducted mixed effects models. A significant moderation effect is indicated when the between groups Q-statistic exceeds the critical value in a χ2 distribution at p < 0.05. Furthermore, using mixed models, we explored whether different variants of the IRT interventions (i.e., IRT alone, IRT combined with CBT for insomnia, and IRT combined with other type of psychological intervention) had a different impact on each of the treatment outcomes under study. Contrast analyses for different formats of treatment delivery (i.e., individual, group, and self-help) were only computed for the studies including IRT.
Finally, potential publication bias was assessed by visual inspection of funnel plots for each treatment outcome, as well as by Egger linear regression method (as implemented in Comprehensive Meta-analysis, Version 2). The funnel plot is based on the fact that precision in estimating the underlying treatment effect will increase as the sample size of component studies increases.49 It consists of a simple diagram of the effect estimates from each study measured against a measure of each study's size or precision. Results from small studies will scatter widely at the bottom of the graph, with the spread narrowing among larger studies. In the absence of publication bias, the studies will be distributed symmetrically about the combined effect size. By contrast, in the presence of bias, the bottom of the plot would tend to show a higher concentration of studies on one side of the mean than the other and, therefore, the plot would be skewed and asymmetrical. This would reflect the fact that smaller studies (which appear toward the bottom) are more likely to be published if they have larger than average effects, which makes them more likely to meet the criterion for statistical significance. Although the funnel plot offers a visual sense of the relationship between effect size and precision, the interpretation of the plot is largely subjective. Therefore, we additionally used the Egger linear regression method to quantify the amount of bias captured by the funnel plot. When the funnel plot and Egger test provided indication of publication bias, we used Duval and Tweedie's trim-and-fill method.50 If there are more small studies on one side of the overall mean than on the other side, the concern is that studies may be missing on the side with fewer studies. The trim-and-fill procedure imputes these missing studies, adds them to the analysis, and then re-computes the summary effect size. That is, trim-and-fill provides an estimate of the overall effect size after the bias has been taken into account.
RESULTS
Effect Sizes and Homogeneity Analyses
Each study's individual posttreatment effect size for nightmares, sleep quality, and PTSS are presented in Figures 2, 3, and 4, respectively. Homogeneity analyses suggested that there was significant heterogeneity in the effect sizes of interventions reporting on nightmare frequency (Q [16] = 29.087, p = 0.02), as well as in the effect sizes of interventions reporting on sleep quality (Q [16] = 40.52, p < 0.01) and PTSS (Q [16] = 43.34, p < 0.01). Therefore, overall effect sizes are based on random effects models.
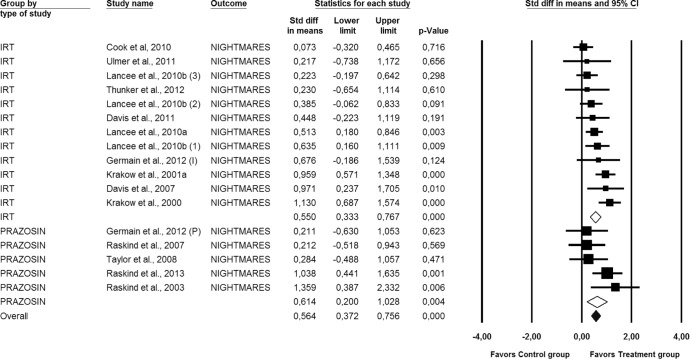
Figure 2. Forest plot presenting the posttreatment total effect sizes and 95% confidence intervals (CI) for Prazosin and IRT on nightmares individually and for all studies combined, calculated with random effects model.
(1) = IRT; (2) = IRT+SH+LDT; (3) = IRT+SH; (I) = IRT; (P) = Prazosin; Std diff, standard difference. For numbers, commas are used as decimal separators.
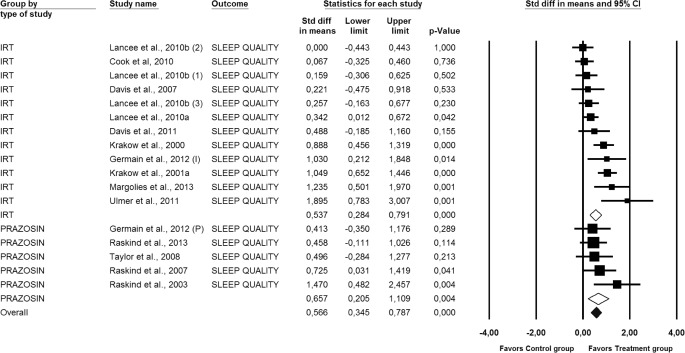
Figure 3. Forest plot presenting the posttreatment total effect sizes and 95% confidence intervals (CI) for Prazosin and IRT on sleep quality individually and for all studies combined, calculated with random effects model.
(1) = IRT; (2) = IRT+SH+LDT; (3) = IRT+SH; (I) = IRT; (P) = Prazosin; Std diff, standard difference. For numbers, commas are used as decimal separators.
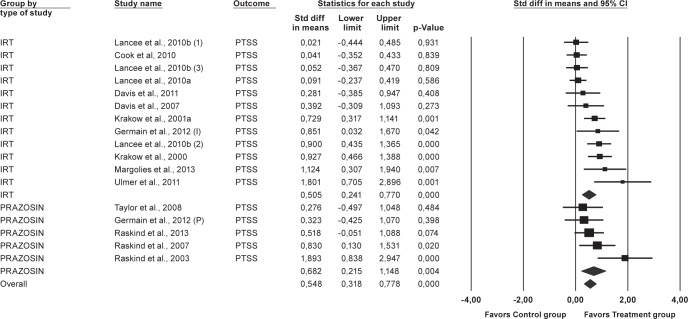
Figure 4. Forest plot presenting the posttreatment total effect sizes and 95% confidence intervals (CI) for Prazosin and IRT on posttraumatic stress symptoms (PTSS) individually and for all studies combined, calculated with random effects model.
(1) = IRT; (2) = IRT+SH+LDT; (3) = IRT+SH; (I) = IRT; (P) = Prazosin; Std diff, standard difference. For numbers, commas are used as decimal separators.
All studies considered, the overall effect size (i.e., the average of the effect sizes of each comparison treatment-control included) for each one of the 3 outcomes under study were statistically significant and of moderate magnitude. Effect sizes of 0.8 can be assumed to be large, while effects of 0.5 are moderate, and effect sizes of 0.20 are small51: for nightmare frequency, the average effect size was 0.56 (95% CI [0.37, 0.76], p < 0.01), for sleep quality, the mean effect size was 0.57 (95% CI [0.34, 0.79], p < 0.01), and for PTSS it was 0.55 (95% CI [0.32, 0.78], p < 0.01).
Figures 2, 3, and 4 also display the combined effect sizes, and their 95% confidence intervals, for interventions including IRT and the combined effect sizes for interventions including prazosin, for each one of the treatment outcomes. As can be seen in each figure, both types of treatments, IRT and prazosin, showed statistically significant mean effect sizes of moderate magnitude.
Contrast Analyses
Prazosin vs. IRT on Nightmares, Perceived Sleep Quality, and PTSS
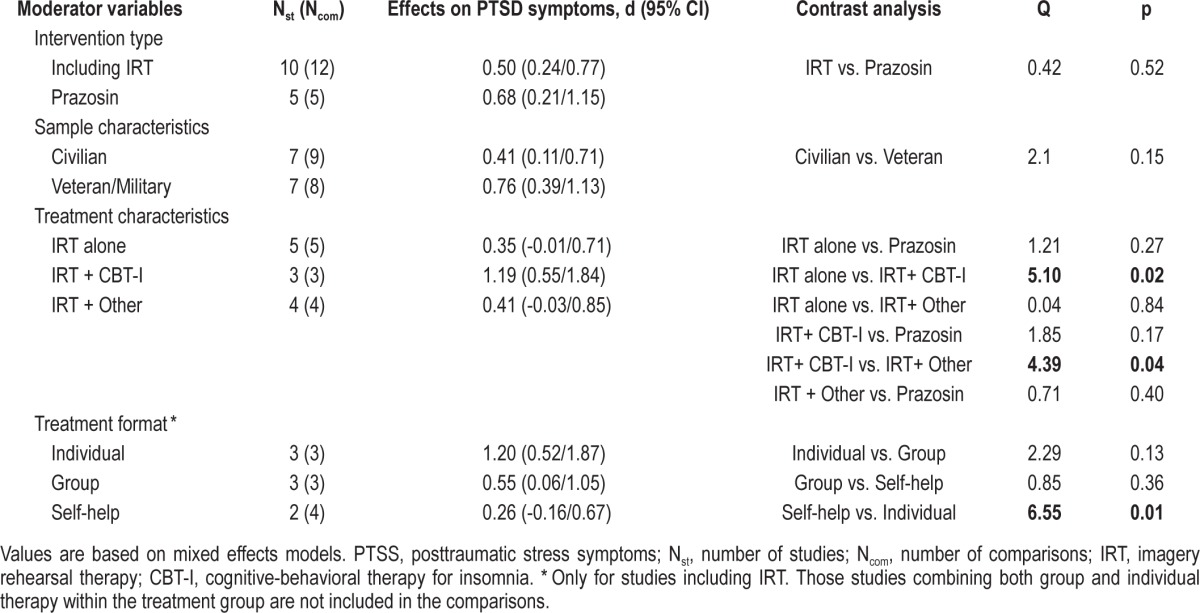
The upper portion of Tables 2, 3, and 4 shows the comparison between the overall effect sizes within study samples that received an IRT intervention and within samples treated with prazosin. Mixed models analyses revealed that the type of treatment (psychological vs. pharmacological) did not affect the effect sizes for nightmare frequency, Q(1) = 0.07; p = 0.79, sleep quality, Q(1) = 0.20; p = 0.65, or PTSS, Q(1) = 0.42; p = 0.52.
Table 2.
Posttreatment mean effect sizes (Cohen's d) and 95% CI for nightmare frequency.
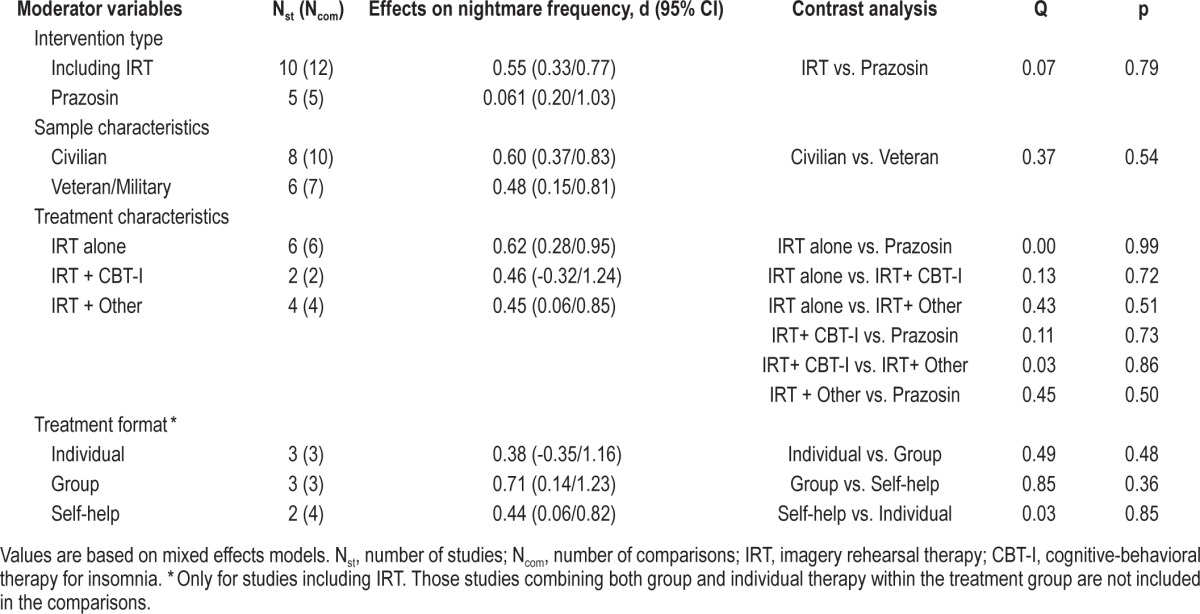
Table 3.
Posttreatment mean effect sizes (Cohen's d) and 95% CI for sleep quality.
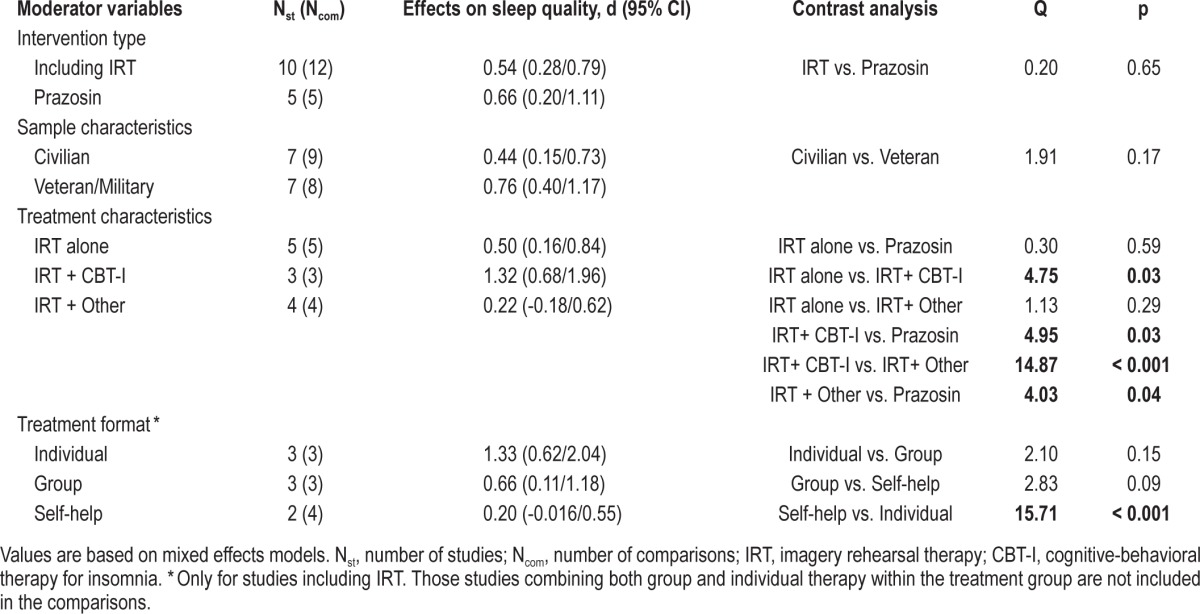
Table 4.
Posttreatment mean effect sizes (Cohen's d) and 95% confidence intervals (CI) for PTSS.
Efficacy of Variations of IRT
In order to explore if different variants of IRT could have a differential impact on treatment outcomes, we divided the IRT interventions into 3 groups: (1) including IRT alone (n = 5), (2) combining IRT and CBT for insomnia (n = 3), and (3) combining IRT with other type of psychological approach (n = 4). Subsequently, we compared these interventions with each other, as well as with the prazosin intervention for each treatment outcome. As can be seen in Table 2, none of these interventions showed superiority over the others to improve nightmare frequency. However, as displayed in Table 3, samples treated with IRT combined with CBT for insomnia showed more improvement in sleep quality than samples treated with prazosin, Q(1) = 4.95; p = 0.03, IRT alone, Q(1) = 4.75; p = 0.03, or IRT combined with another psychological intervention, Q(1) = 14.87; p < 0.01. In a similar fashion, the interventions combining IRT with CBT for insomnia showed statistically significant larger effect sizes for PTSS than interventions using IRT alone, Q(1) = 5.10; p = 0.02, and interventions using IRT combined with other psychological approach, Q(1) = 4.39; p = 0.04, (Table 4). The prazosin studies only showed a statistically significant larger effect size for sleep quality when compared to the effect size of IRT combined with other type of psychological intervention, Q(1) = 4.03; p = 0.04 (Table 3).
Veteran vs. Civilian Samples
Mixed model analyses of population (civilian vs. veteran/ military) as a moderator of treatment outcome revealed that patient population did not have a significant effect on the degree of improvement in nightmare frequency, Q(1) = 0.37; p = 0.54, sleep quality, Q(1) = 1.91; p = 0.17, or PTSS, Q(1) = 2.1; p = 0.15 (Tables 2, 3, and 4).
Individual, Group, or Self-Help IRT Formats
The contrast analyses for different formats of treatment delivery were only computed for the IRT interventions and are displayed in Tables 2, 3, and 4. Among the 12 IRT interventions included herein, 3 were delivered in individual therapy format, 3 in a group format, and 4 in a self-help format. The remaining IRT interventions (n = 2) used a combination of individual and group formats and therefore were not included in this subset of analyses. None of the formats of treatment delivery showed superiority over the others at improving nightmare frequency (Table 2). By contrast, when compared to the self-help format, the individual therapy yielded a statistically significant larger effect size for sleep quality: Q(1) = 15.71; p < 0.01, and for PTSS, Q(1) = 6.55; p = 0.01. The differences between the individual format and the group format and between the group format and the self-help format were not significant (Tables 3 and 4).
Publication Bias
Considering nightmare frequency as treatment outcome, publication bias was undetectable from the funnel plot. This conclusion was supported by the Egger test statistic, t = 0.08; p = 0.93. However, the funnel plots including effect sizes for sleep quality and PTSS showed some asymmetry, suggesting possible publication bias, with smaller studies providing more positive results than larger ones. However, whereas the Egger test was statistically significant (t = 2.84; p = 0.01) for PTSS, that was not the case for sleep quality (t = 2.05; p = 0.06). Under the random effects model, trim-and-fill adjusted overall effect sizes for sleep quality and PTSS were 0.49 (95% CI [0.27, 0.72]), and 0.32 (95% CI [0.08, 0.57]), respectively.
DISCUSSION
The present meta-analysis provides the first systematic comparison of RCTs on the two Level A recommended interventions by the Standards of Practice Committee of the AASM for the treatment of nightmares in adults, extending the comparison of their effects to other relevant outcomes, such as sleep quality and PTSS. Our results indicate that prazosin and interventions including IRT yield overall effect sizes for nightmares, sleep quality and PTSS which are comparable and of moderate magnitude. These findings are in accordance with and extend the results provided by a recent meta-analysis comparing psychological and pharmacological treatments for nightmares.20 Nonetheless, since the RCTs including IRT showed considerable variation regarding the components of the treatment protocol (e.g., some studies combined IRT with CBT for insomnia or with other type of psychological intervention), our subsequent contrast analyses suggest that the subgroup of studies combining IRT with CBT for insomnia performed significantly better at improving overall sleep quality than did studies using prazosin, studies using IRT alone, and studies using IRT combined with other form of psychological intervention.
Perhaps less expected, our contrast analyses also showed that the combination of IRT with CBT for insomnia also yielded better PTSS outcomes when compared with studies using IRT alone or IRT combined with other psychological intervention. This suggests that the sleep regulation promoted by sleep restriction and stimulus control therapy may have an incremental benefit for overall PTSD syndrome, though not for nightmares specifically. This finding contrasts with the results provided by Casement and Swanson in their meta-analysis.21 In their study, combining IRT with CBT for insomnia did not have a significant effect on the degree of improvement in PTSS. However, our meta-analysis included only RCTs, whereas Casement and Swanson were less restrictive at including treatment studies in their analyses, so this may account for our divergent findings.
Of interest, it appears that combining IRT with CBT for insomnia could be as efficacious as PTSD-directed CBT treatments when it comes to reducing PTSS. In fact, a meta-analysis of trauma-focused behavioral treatment versus control group differences reported effect sizes ranging from 0.83 to 1.11.52 These reported effect sizes are not larger than the effect size for PTSS reported herein, 1.19, even though global PTSS are not directly targeted by IRT combined with CBT for insomnia. Nonetheless, in our study, we have not directly compared the efficacy of IRT combined with CBT for insomnia to established treatments for PTSD, so we do not presume to propose the use of IRT combined with CBT for insomnia as a stand-alone intervention for PTSD. Rather, our findings suggest that IRT combined with CBT for insomnia can effectively treat sleep disturbance, and this may result in concurrent reduction of other PTSS. A note of caution, however, needs to be mentioned. The instruments used in the majority of studies to assess PTSS are global instruments that may include both nighttime and daytime symptoms, so one could argue that improvements in PTSS seen in these studies may be attributed solely to better scores on the nighttime symptoms, and that daytime symptoms remained unaltered. However, it also seems plausible that decreasing bad dreams and improving perceived sleep quality may lead to improved daytime energy, which, in turn, facilitates coping with other distress symptoms, thus ameliorating daytime functioning overall.
Yet, the large effect sizes found in the present meta-analysis for this subgroup of studies using IRT combined with CBT for insomnia for the sleep quality and the PTSS outcomes, 1.32 and 1.19, respectively, should be interpreted cautiously, since they are based on a small number of studies. Indeed, only three RCTs used a combination of IRT and CBT for insomnia, and among those, the study by Margolies et al.37 used IRT combined with CBT for insomnia in only about two-thirds of their treated sample. In order to obtain the actual data from the subset of individuals receiving IRT combined with CBT for insomnia, we contacted the first author of the paper. However, Dr. Margolies indicated that, unfortunately, in their study they did not include a measure that tracked IRT adherence, thus they were not able to say with enough accuracy which participants actually completed IRT. Although this is a flaw of the current study, the decision to keep the Margolies paper in this meta-analysis owed to the fact that there is a paucity of RCTs in this field. Furthermore, the majority of individuals included in their treated sample, from which the mean values, standard deviation and sample size were extracted for this meta-analysis, did receive the IRT combined with CBT for insomnia.
With respect to the format of treatment delivery for the interventions including IRT, the results of our contrast analyses favor the individual format over self-help for all three treatment outcomes included herein. Perhaps more surprising, there was no significant difference between the group format and self-help. Although it is generally assumed that face-to-face therapy is superior to self-help treatment, the lack of statistical significance might be due to the small number of studies. Actually, in only two studies, conducted by the same research group,32,33 the IRT intervention was delivered in a self-help format. Furthermore, in these studies the exclusion criteria were high scores on posttraumatic complaints or currently in treatment for PTSD. Therefore, the milder severity of PTSS in individuals included in the self-help studies, compared to individuals receiving treatment in the group format (the majority of those having a PTSD disorder diagnosis), could partially explain why the group format did not outperform the self-help format in terms of symptom reduction. Arguably, more studies with direct comparisons among different types of treatment delivery are needed.
Another question we explored in this meta-analysis was whether military trauma nightmares and PTSS could be more resistant to treatment than civilian trauma nightmares and PTSS, as it has been suggested elsewhere.53 However, we did not find differences in treatment effect sizes for civilian and military/veteran samples across the diverse range of treatment outcomes included herein.
A variable of crucial importance in treatment studies is sustained efficacy over time. A treatment that produces an initial response, or a response that holds for one month after termination, may or may not be an efficacious treatment for a disorder such as nightmares or PTSD, which tend to be longstanding. In this study, we focused on treatment outcomes immediately after the treatment was completed, and both prazosin and IRT interventions seemed to be efficacious However, little is known about the symptom recurrence upon cessation of prazosin, since follow-up assessments are not provided in the RCTs on prazosin. Hence, we were not able to compare both treatments at follow-up assessments. In this regard, the meta-analysis by Augedal et al.21 does provide some indication (based on follow-up data available from three studies) that the effects from psychological interventions for nightmares may last from 4 to 24 weeks. This contention is further supported by the meta-analysis by Hansen et al.,23 including both RCTs and uncontrolled studies of IRT and exposure-based interventions for nightmares.
Of note, the majority of IRT studies included herein used participants on a waiting list as controls. This is a very different scenario from the prazosin trials, wherein the control group received placebo pills and were required to meet with study staff for titration visits. Therefore, it could be argued that an intervention study in which the control group does not receive placebo and is simply on a waiting list, would show a larger effect size. This is obviously a limitation of our comparisons. However, in an attempt to explore this hypothesis, we computed the mean effect size for the comparisons including an active control group (see Table 1: n = 3 IRT comparisons and n = 5 prazosin comparisons, total n = 8) and the mean effect size for the studies including a wait-list control (n = 9), for each one of the three treatment outcomes under study. Our contrast analyses indicated that the mean effect sizes of the studies with a wait-list control group for nightmares, sleep quality, and PTSS were not significantly larger than the mean effect sizes of comparisons including an active control group, Q(1) = 0.74, p = 0.39; Q(1) = 0.55, p = 0.46; and Q(1) = 0.61, p = 0.44, respectively. Despite these preliminary findings, more RCTs with active control groups are needed to analyze the influence of therapeutic attention in psychological interventions.
Admittedly, this meta-analysis has other limitations as well. Since we were interested in reexamining conclusions drawn from the published literature, we did not attempt to address the “file drawer” problem by tracking down unpublished studies. Even so, we did explore the potential presence of publication bias using a funnel plot and the Egger test statistic. Although publication bias was undetectable for the nightmare outcome, the funnel plots for the sleep quality outcome and PTSS outcome did show some indication of publication bias, as well as did the Egger test statistic for PTSS. The mean effect sizes were therefore calculated again while imputing missing studies by the trim-and-fill procedure of Duval and Tweedie. The adjusted effect sizes for sleep quality and PTSS only diverged to a limited extent from the effect sizes originally obtained in this study. Furthermore, the adjusted effect sizes were still statistically significant. As such, we conclude that publication bias is not a major issue in this study. Finally, although it could be argued that we should have coded the included studies according to study quality, it should be noted that all studies included in our analyses were RCTs. Therefore, one may assume that the variance with concern to study quality was limited.
We have also noticed that the studies used different outcome measures to evaluate the effectiveness of treatment, so consistency in nightmare metrics, as well as in other relevant outcomes, would improve the ability to compare results among trials, as has been suggested elsewhere.14 For this meta-analysis, we choose primarily a nightmare frequency outcome (number of nights with nightmares or number of nightmares per night), as this is the most common measure used in studies, both for inclusion of participants in the trial21 and for assessing treatment effects.22 However, three of the studies included herein reported nightmare outcomes using scores on the nightmare item of the CAPS. Although this item includes information about the frequency of the distressing dreams, it also inquires about the intensity of those. In order to ascertain whether these slightly different outcomes differed significantly in terms of effect sizes, we conducted a post hoc contrast analysis comparing studies in which a pure frequency nightmare outcome was employed (n = 14 treatment vs. control group comparisons) and studies in which a combination of frequency and intensity of nightmare was used (n = 3 treatment vs. control group comparisons). Our mixed effect analysis indicated that these two types of nightmare outcomes did not show statistically significant different effect sizes: nightmare frequency, 0.52 (95% CI [0.32, 0.72], p < 0.01), vs. nightmare frequency and intensity, 0.87 (95% CI [0.35, 1.38], p < 0.01), Q(1) = 1.55, p = 0.21. Finally, the manner in which IRT was applied in the studies also differed among research groups. Future research would benefit from the publication of detailed treatment manuals to standardize treatment delivery.
Despite the limitations noted, the results from this meta-analysis provide some evidence for preferring the combination of IRT with CBT for insomnia over prazosin to improve overall sleep quality. Moreover, our findings highlight the beneficial effects of sleep regulation provided by CBT for insomnia, combined with IRT, on PTSS. More controlled studies including head to head prazosin and IRT comparisons, as well as studies using multimodal treatments (i.e., combination of medication and psychotherapy) and psychological treatment dismantling studies, are warranted.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. Dr. Sánchez-Ortuño was supported by a research fellowship award from Fundación Séneca, Murcia, Spain. The opinions expressed herein are those of the authors and do not necessarily reflect those of the Department of the Navy, Department of Defense, the U.S. Government, or any of its agencies.
REFERENCES
- 1.Hublin C, Kaprio J, Partinen M, Kosenkenvou M. Nightmares: familial aggregation and association with psychiatric disorders in nationwide twin cohort. Am J Med Genet. 1999;88:329–36. doi: 10.1002/(sici)1096-8628(19990820)88:4<329::aid-ajmg8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 3.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 4.Ross JR, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146:697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- 5.Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155:929–33. doi: 10.1176/ajp.155.7.929. [DOI] [PubMed] [Google Scholar]
- 6.Wittmann L, Schredl M, Kramer M. Dreaming in posttraumatic stress disorder: a critical review of phenomenology, psychophysiology and treatment. Psychother Psychosom. 2007;76:25–39. doi: 10.1159/000096362. [DOI] [PubMed] [Google Scholar]
- 7.Spoormarker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature. Sleep Med Rev. 2008;12:169–84. doi: 10.1016/j.smrv.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Leskin GA, Woodward SH, Young HE, Sheikh JI. Effects of comorbid diagnoses on sleep disturbance in PTSD. J Psychiatr Res. 2002;36:449–52. doi: 10.1016/s0022-3956(02)00025-0. [DOI] [PubMed] [Google Scholar]
- 9.Köthe M, Pietrowsky R. Behavioral effects of nightmares and their correlations to personality patterns. Dreaming. 2001;11:43–52. [Google Scholar]
- 10.Berquier A, Ashton R. Characteristics of the frequent nightmare sufferer. J Abnorm Psychol. 1992;101:246–50. doi: 10.1037//0021-843x.101.2.246. [DOI] [PubMed] [Google Scholar]
- 11.Belleville G, Guay S, Marchand A. Persistence of sleep disturbances following cognitive-behavior therapy for posttraumatic stress disorder. J Psychosom Res. 2011;70:318–27. doi: 10.1016/j.jpsychores.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Zayfert C, DeViva JC. Residual insomnia following cognitive behavioral therapy for PTSD. J Trauma Stress. 2004;17:69–73. doi: 10.1023/B:JOTS.0000014679.31799.e7. [DOI] [PubMed] [Google Scholar]
- 13.Germain A. Sleep disturbances in posttraumatic stress disorder. Psychiatr Ann. 2009;39:335–41. doi: 10.3928/00485713-20160125-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6:389–401. [PMC free article] [PubMed] [Google Scholar]
- 15.Kung S, Espinel Z, Lapid MI. Treatment of nightmares with prazosin: a systemic review. Mayo Clin Proc. 2012;87:890–900. doi: 10.1016/j.mayocp.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geracioti TD, Jr, Baker DG, Ekhator NN, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–30. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- 17.Krakow B, Zadra A. Imagery rehearsal therapy: principles and practice. Sleep Med Clin. 2010;5:289–98. [Google Scholar]
- 18.Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–3. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 19.Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63:629–32. doi: 10.1016/j.biopsych.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Augedal AW, Hansen KS, Kronhaug CR, Harvey AG, Pallesen S. Randomized controlled trials of psychological and pharmacological treatments for nightmares: a meta-analysis. Sleep Med Rev. 2013;17:143–52. doi: 10.1016/j.smrv.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Casement MD, Swanson LM. A meta-analysis of imagery rehearsal for post-trauma nightmares: effects on nightmare frequency, sleep quality, and post-traumatic stress. Clin Psychol Rev. 2012;32:566–74. doi: 10.1016/j.cpr.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen K, Höfling V, Kröner-Borowik T, Stangier U, Steil R. Efficacy of psychological interventions aiming to reduce chronic nightmares: a meta-analysis. Clin Psych Rev. 2013;33:146–55. doi: 10.1016/j.cpr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 24.Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–3. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 25.Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928–34. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 26.Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63:629–32. doi: 10.1016/j.biopsych.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170:1003–10. doi: 10.1176/appi.ajp.2013.12081133. [DOI] [PubMed] [Google Scholar]
- 28.Krakow B, Hollifield M, Schrader R, et al. A controlled study of imagery rehearsal for chronic nightmares in sexual assault survivors with PTSD: a preliminary report. J Trauma Stress. 2000;13:589–609. doi: 10.1023/A:1007854015481. [DOI] [PubMed] [Google Scholar]
- 29.Krakow B, Hollifield M, Johnston L, et al. Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: a randomized controlled trial. JAMA. 2001;286:537–45. doi: 10.1001/jama.286.5.537. [DOI] [PubMed] [Google Scholar]
- 30.Davis JL, Wright DC. Randomized clinical trial for treatment of chronic nightmares in trauma-exposed adults. J Trauma Stress. 2007;20:123–33. doi: 10.1002/jts.20199. [DOI] [PubMed] [Google Scholar]
- 31.Cook JM, Harb GC, Gehrman PR, Cary MS, Gamble GM, Forbes D, Ross RJ. Imagery rehearsal for posttraumatic nightmares: a randomized controlled trial. J Trauma Stress. 2010;23:553–63. doi: 10.1002/jts.20569. [DOI] [PubMed] [Google Scholar]
- 32.Lancee J, Spoormaker VI, van den Bout J. Cognitive-behavioral self-help treatment for nightmares: a randomized controlled trial. Psychother Psychosom. 2010;79:371–7. doi: 10.1159/000320894. [DOI] [PubMed] [Google Scholar]
- 33.Lancee J, van den Bout J, Spoormaker VI. Expanding self-help imagery rehearsal therapy for nightmares with sleep hygiene and lucid dreaming: a waiting-list controlled trial. Int J Dream Res. 2010;3:111–20. [Google Scholar]
- 34.Davis JL, Rhudy JL, Pruiksma KE, Byrd P, Williams AE, McCabe KM, Bartley EJ. Physiological predictors of response to exposure, relaxation, and rescripting therapy for chronic nightmares in a randomized clinical trial. J Clin Sleep Med. 2011;7:622–31. doi: 10.5664/jcsm.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulmer CS, Edinger JD, Calhoun PS. A multi-component cognitive-behavioral intervention for sleep disturbance in veterans with PTSD: a pilot study. J Clin Sleep Med. 2011;7:57–68. [PMC free article] [PubMed] [Google Scholar]
- 36.Thünker J, Pietrowsky R. Effectiveness of a manualized imagery rehearsal therapy for patients suffering from nightmare disorders with and without a comorbidity of depression or PTSD. Behav Res Ther. 2012;50:558–64. doi: 10.1016/j.brat.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Margolies SO, Rybarcyk B, Vrana SR, et al. Efficacy of cognitive-behavioral treatment for insomnia and nightmares in Afghanistan and Iraq veterans with PTSD. J Clin Psychol. 2013;69:1026–42. doi: 10.1002/jclp.21970. [DOI] [PubMed] [Google Scholar]
- 38.Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US Military Veterans. J Psychosom Res. 2012;72:89–96. doi: 10.1016/j.jpsychores.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blake D, Weathers F, Nagy L, et al. Boston, MA: National Center for Posttraumatic Stress Disorder; 1990. Clinician-Administered PTSD Scale (CAPS) pp. 1–9. [Google Scholar]
- 40.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 41.Spoormaker VI, Verbeek I, van den Bout J, Klip EC. Initial validation of the SLEEP-50 questionnaire. Behav Sleep Med. 2005;3:227–46. doi: 10.1207/s15402010bsm0304_4. [DOI] [PubMed] [Google Scholar]
- 42.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 43.Germain A, Hall M, Krakow B, Katherine Shear M, Buysse DJ. A brief sleep scale for posttraumatic stress disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. J Anxiety Disord. 2005;19:233–44. doi: 10.1016/j.janxdis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behav Res Ther. 1996;34:669–73. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 45.Weiss DS, Marmar CR. The Impact of Event Scale-Revised. In: Wilson JP, Keane TM, editors. Assessing Psychological Trauma and PTSD: A Practitioner's Handbook. New York: Guilford Press; 1997. [Google Scholar]
- 46.Guy W. Rockville, MD: U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. ECDEU Assessment Manual for Psychopharmacology—Revised (DHEW Publ No ADM 76-338) pp. 218–22. [Google Scholar]
- 47.Robinson LA, Berman JS, Neimeyer RA. Psychotherapy for the treatment of depression: a comprehensive review of controlled outcome research. Psychol Bull. 1990;108:30–49. doi: 10.1037/0033-2909.108.1.30. [DOI] [PubMed] [Google Scholar]
- 48.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester: John Wiley & Sons; 2009. [Google Scholar]
- 49.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferguson CJ, Brannick MT. Publication bias in psychological science: prevalence, methods for identifying and controlling, and implications for the use of meta-analyses. Psychol Methods. 2012;17:120–8. doi: 10.1037/a0024445. [DOI] [PubMed] [Google Scholar]
- 51.Lipsey MW, Wilson DB. The efficacy of psychological, educational, and behavioral treatment. Confirmation from meta-analysis. Am Psychol. 1993;48:1181–209. doi: 10.1037//0003-066x.48.12.1181. [DOI] [PubMed] [Google Scholar]
- 52.Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 2005;162:214–27. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- 53.Prigerson HG, Maciejewski PK, Rosenheck RA. Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis. 2001;189:99–108. doi: 10.1097/00005053-200102000-00005. [DOI] [PubMed] [Google Scholar]