Abstract
Objective:
The purpose of this systematic review is to evaluate the diagnostic value of biological markers (exhaled breath condensate, blood, salivary and urinary) in the diagnosis of OSA in comparison to the gold standard of nocturnal PSG.
Methods:
Studies that differentiated OSA from controls based on PSG results, without age restriction, were eligible for inclusion. The sample of selected studies could include studies in obese patients and with known cardiac disease. A detailed individual search strategy for each of the following bibliographic databases was developed: Cochrane, EMBASE, MEDLINE, PubMed, and LILACS. The references cited in these articles were also crosschecked and a partial grey literature search was undertaken using Google Scholar. The methodology of selected studies was evaluated using the 14-item Quality Assessment Tool for Diagnostic Accuracy Studies.
Results:
After a two-step selection process, nine articles were identified and subjected to qualitative and quantitative analyses. Among them, only one study conducted in children and one in adults found biomarkers that exhibit sufficiently satisfactory diagnostic accuracy that enables application as a diagnostic method for OSA.
Conclusion:
Kallikrein-1, uromodulin, urocotin-3, and orosomucoid-1 when combined have enough accuracy to be an OSA diagnostic test in children. IL-6 and IL-10 plasma levels have potential to be good biomarkers in identifying or excluding the presence of OSA in adults.
Citation:
De Luca Canto G, Pachêco-Pereira C, Aydinoz S, Major PW, Flores-Mir C, Gozal D. Diagnostic capability of biological markers in assessment of obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med 2015;11(1):27–36.
Keywords: biological markers, diagnosis, sleep apnea syndromes, review
Obstructive sleep apnea (OSA) has become widely recognized as a potential cause of significant morbidity in both children and adults.1,2 OSA symptoms include habitual snoring and reporting of disturbed unrefreshing sleep, frequently accompanied by excessive daytime sleepiness, and daytime neurobehavioral problems.3 The increasing understanding, awareness and familiarity with OSA has resulted in an ever expanding spectrum of OSA-associated morbidities that encompasses not only the central nervous system (cognitive, mood disturbances, and behavioral deficits), but affects also many other organ systems, ultimately imposing substantial increases in healthcare costs, as well as adverse outcomes.4–7
Among the prototypic risk factors associated with OSA, adenotonsillar hypertrophy, obesity, craniofacial and anatomical anomalies, and neuromuscular disorders, seemingly interact to a greater or lesser extent among patients, leading to the putative assumption that multiple clinical phenotypes exist and potentially merit divergent therapeutic approaches better tailored at the constellation of pathophysiological mechanisms leading to OSA in these clinical clusters.3 The prevalence of OSA is markedly variable both during childhood (1% to 5%) and during adulthood (4% to 15%), with major contributions of age, gender, and ethnicity.1,8–11 However, it is clear that independently of whether we consider the lowest or the highest estimated prevalence reported for any population, OSA is a frequent condition that imposes a high degree of disease burden, thereby requiring timely diagnosis and effective treatment.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The purpose of this systematic review was to evaluate the diagnostic properties of markers in biological samples, such as in exhaled breath condensate, blood, saliva, and urine, and compare their predictive characteristics to the gold standard in the diagnosis of OSA—nocturnal PSG.
Study Impact: A substantial number of studies have been published in the literature in the quest for diagnostic biomarkers of OSA in both children and adults; however, most of the explored approaches do not identify definitive biomarkers, and only a small number of candidates appears promising and merits further research.
An overnight in-laboratory polysomnographic evaluation (PSG) remains the gold standard diagnostic method for OSA at any age.3,12 Unfortunately, overnight PSGs are onerous, labor-intensive, may impose substantial inconvenience to the child and caretakers, and are variably accessible around the world. Waiting time between referral for evaluation to diagnosis may commonly take 3–6 months across the United States and even longer elsewhere.13 Although the PSG is employed as the gold standard for diagnosing the vast majority of sleep disorders, the relative complexity of PSG application and the inherent costs associated with PSG has spurred the quest for alternative diagnostic methods.13 Among these, simple approaches such as questionnaires with or without medical history and physical examination, audiotaping, videotaping, pulse oximetry, abbreviated polysomnography (aPSG), home-based polygraphy, or multichannel recordings have all been assessed, albeit with variable success.12,14–18 However, among the alternative diagnostic tools, special interest has recently centered on the identification of biomarkers.
A biomarker is a “biological molecule found in blood, other body fluids, or tissues that is a sign of a normal or abnormal processes, or of a condition or disease.”13 Gene expression arrays have revealed significant and reproducible changes in a restricted number of genes that could enable discriminatory ability in the recognition of OSA. Similarly, a number of serum and urinary proteins have been identified that display favorable significant receiver-operator properties towards the diagnosis of OSA.13 Provided that acceptable sensitivity and specificity are achieved, a unique set of disease biomarkers would enable greatly simplified, user-friendly, and context-relevant approaches to the diagnosis of OSA in the future.19 Over the last 14 years, a substantial number of studies have tackled the identification of an ideal biomarker for OSA, and although, there is still no simple and useful disease marker panel for OSA available, considerable progress has been accomplished and merits critical review and scrutiny.19 Therefore the purpose of this systematic review was to critically evaluate the diagnostic properties of markers in biological samples, such as in exhaled breath condensate (EBC), blood, saliva, and urine, and compare their predictive characteristics to the gold standard—nocturnal PSG. We further aimed to formulate potential future exploratory research directions aiming at advancing this promising area of clinical translation in sleep medicine.
METHODS
This systematic review was done adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses PRISMA Checklist.20
Diagnostic Terminology
All terms that mean obstructive sleep apnea (OSA), including sleep disordered breathing (SDB), sleep-related breathing disorder (SRBD) and obstructive sleep apnea syndrome (OSAS) were standardized as OSA.
Protocol and Registration
The systematic review protocol was registered at the international prospective register of systematic reviews (PROSPERO). The number of register is CRD42014007427.
Study Design
A systematic review of human studies that evaluated the diagnostic value of biological markers (blood, EBC, salivary, and urinary) in the diagnosis of OSA was undertaken.
Eligibility Criteria
Inclusion Criteria
Studies that differentiated the OSA group from controls based on full PSG results, without age restriction, were eligible for inclusion. The sample of selected studies could include studies in obese patients and in those with known cardiac disease.
Retained articles included only those studies whose primary objective was to identify biomarkers in subjects with OSA confirmed by overnight PSG. Only studies in English, Portuguese and Spanish languages were considered.
Exclusion Criteria
Reviews, letters, conference abstracts, and personal opinions were not considered.
Studies using daytime PSG, home-based PSG or multichannel polygraphic recordings were also excluded. Studies using biomarkers to detect the presence of OSA-associated morbidities (cognitive or behavioral deficits, excessive daytime sleepiness, cardiovascular or metabolic end-organ dysfunction) were excluded. In addition, studies in which the clinical cohort included craniofacial, genetic syndromes, neuromuscular diseases, or patients with a primary disease for which OSA prevalence is being investigated, such as patients with kidney disease or rheumatologic conditions were also discarded. In phase 2, we excluded studies that did not report sensitivity and specificity or in which the data presented did not enable these assessments to be extrapolated.
Information Sources
Detailed individual search strategies for each of the following bibliographic databases were developed: Cochrane, EMBASE, MEDLINE, PubMed, and LILACS. A partial grey literature search was taken using Google Scholar. The end search date was January 3, 2014, and an updated search was completed on March 20, 2014, across all databases. The references cited in the selected articles were also checked for any incremental references that could have been inadvertently omitted during the electronic database searches.
Search
Appropriate truncation and word combinations were selected and adapted for each database search (see Appendix 1). All references were managed by reference manager software (RefWorks-COS, ProQuest, Bethesda, MD), and duplicate hits were removed.
Study Selection
The selection was completed in 2 phases.
In phase 1, two reviewers independently reviewed the titles and abstracts of all identified electronic database citations (GDL and CPP). The following criteria were applied to select studies in phase 1: studies with an objective of identifying biomarkers in subjects with OSA confirmed by full overnight PSG. A third author (SA) was involved when disagreements emerged among the 2 initial evaluators. Any studies that did not fulfill the inclusion criteria were discarded.
In phase 2, the following selection criteria were applied to the full articles to confirm their eligibility: only studies that reported sensitivity and specificity or in which the data presented enabled these diagnostic assessments to be extrapolated were selected. The same 2 reviewers (GDL and CPP) independently participated in phase 2. The reference list of all included articles was critically assessed by one examiner (GDL). The articles that were selected were then read by both examiners (GDL and CPP). Any disagreement in either phase was resolved by discussion and mutual agreement among the 3 reviewers (GDL, CPP, SA). A fourth author with extensive experience in sleep medicine and biomarker discovery (DG) was involved when controversy arose in the process of reaching a final decision. Final selection was always based on the full-text of the publication.
Data Collection Process
One author (GDL) collected the required information from the selected articles. A second author (CPP) crosschecked all the collected information and confirmed its accuracy. Again, any disagreement in either phase was resolved by discussion and mutual agreement among the 3 reviewers (GDL, CPP, SA). The fourth author was involved as required, to enable formulation of the final decision (DG).
Data Items
For all of the included studies the following information was recorded: author(s), year of publication, country, sample size, age, type of biomarkers, apnea hypopnea index used to define OSA from the PSG, name of biomarkers, and results (including sensitivity and specificity). If the required data were not complete, attempts were made to contact the authors to retrieve the missing information.
Risk of Bias in Individual Studies
The methodology of selected studies was evaluated using the 14-item Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS).21 Two reviewers (GDL and CPP) scored each item as “yes,” “no,” or “unclear” and assessed independently the quality of each included study. Disagreement between the 2 reviewers was resolved by a third reviewer (CFM).
Summary Measures
Sensitivity and specificity of biomarkers as diagnostic tests against PSG were considered as the main outcomes.
Synthesis of Results
The diagnostic capability of the identified biomarkers against PSG was combined through a meta-analysis following the appropriate Cochrane guidelines.22 Review Manager 5.2 (Rev-Man 5.2, The Nordic Cochrane Centre, Copenhagen, Denmark) was used to constructed receiver operating characteristic (ROC) graphs and forest plots as part of the meta-analysis. Some of the required data were calculated by the authors.
Risk of Bias across Studies
To decrease the heterogeneity, the studies were separated in 3 groups according to age (children or adults) and biomarker characteristics (single or combined biomarkers).
Additional Analyses
Additional analysis was performed using positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+), negative likelihood Ratio (LR-), diagnostic odds ratio (DOR), and Youden's Index. The cutoff values used to interpret these data are presented in Appendix 2.
RESULTS
Study Selection
A flowchart describing the process of identification, inclusion, and exclusion of studies is shown in Figure 1. A total of 141 articles were retrieved during phase 1 selection. Thereafter, 132 studies were excluded due to different reasons (see Appendix 3). Only 9 articles were finally included in the qualitative and quantitative synthesis. Eight of those23–31 were initially identified from the main electronic search; only one31 was directly received from expert sources.
Figure 1. Flow diagram of literature search and selection criteria.
Adapted from PRISMA.
Study Characteristics
From the 9 selected studies, 4 were conducted in children23,24,30,31 and 5 in adults.25–29 The studies in children were conducted in 2 different countries: Hungary23 and United States.24,30,31 The following PSG-based criteria were used for OSA: AHI ≥ 1/h TST,23 AHI ≥ 2/h TST,31 AHI > 2/h TST,24 AHI > 5/h TST.30 Two of these studies tested urinary biomarkers against PSG,24,31 one tested blood-based biomarkers,30 and one evaluated EBC.23 The sample size ranged from 28 to 120 subjects.23,24 A summary of the study descriptive characteristics can be found in Table 1.
Table 1.
Summary of study descriptive characteristics of included studies (children).

The studies conducted in adults were conducted in Brazil,25 China,28 Germany,29 Thailand,26 and Turkey.27 The following definitions for OSA were used: AHI > 5/h TST,29 AHI ≥ 5/h TST,26–28 and AHI ≥ 15/h TST.25 All of the studies25,27–29 evaluated blood biomarkers. One of the studies appraised both blood and EBC.26 The sample size ranged from 6328 to 1,02125 participants. A summary of the study descriptive characteristics can be found in Table 2.
Table 2.
Summary of study descriptive characteristics of included studies (adults).

Risk of Bias within Studies
The studies were very homogeneous—all had high methodological quality, even though none of the studies fulfilled all methodological quality criteria. In 8 studies, the QUADAS21 criteria were fulfilled in 78.6%. In another study,25 QUADAS criteria were met in 85.7% (Appendix 4).
Results of Individual Studies
Although the studies used different types of biomarkers and reported different sensitivity and specificity all 9 articles concluded that biomarkers had the capacity to correctly classify OSA and non-OSA subjects.
Synthesis of Results
To improve our interpretation of results, the studies were clustered in 3 groups, according the sample and the index test: using only one biomarker in children or adults, and using combined bio-markers in children. Diagnostic tables were constructed using the data extracted from each article (Tables 3, 4, 5). In these tables, all accuracy measurements (sensitivity, specificity, PPV, NPV, LR+, LR-, DOR, and Younden's Index) are presented. Some studies25–27 provided more than one accuracy measurement. Therefore those findings are reported twice in the same table. From the 4 studies conducted in children, a total of 258 subjects were assessed. From the 5 studies conducted in adults, 1,458 subjects were evaluated. The total sample for this meta-analysis was 1,716 subjects.
Table 3.
Diagnostic test accuracy (children).
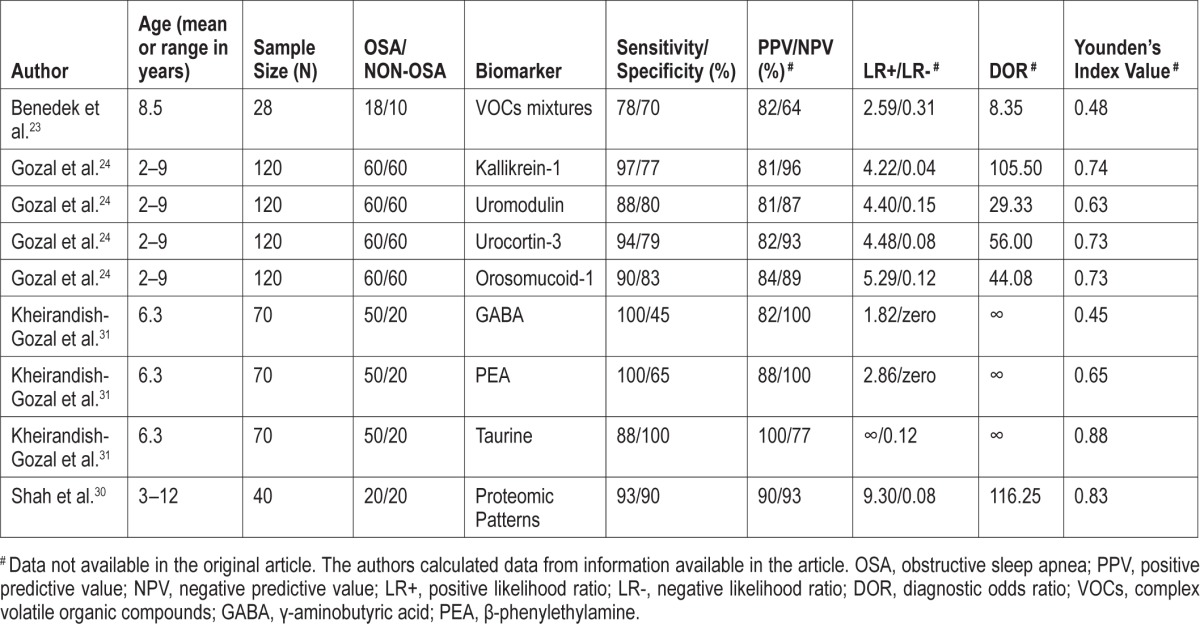
Table 4.
Measurements for combined biomarkers (children).

Table 5.
Diagnostic test accuracy (adults).

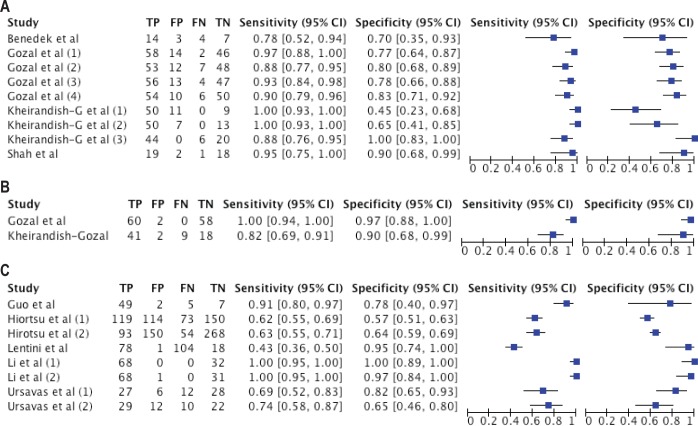
The diagnostic accuracy (sensitivity, specificity, and 95% confidence interval) of the studies included in a meta-analysis is shown in Figures 2 and 3. The sensitivity and specificity for different selected studies varied substantially from 43% to 100%, and from 45% to 100%, respectively. Only 5 studies reported excellent sensitivity: Li et al. (100%),26 Gozal et al. (95%),24 Shah et al. (93%),30 Guo et al. (91%),28 and Kheirandish-Gozal et al. (82%).31 From these 5 studies, only Gozal et al.24 and Li et al.26 also reported excellent specificity (both 97%).
Figure 2. Forest plot with diagnostic test accuracy (sensitivity, specificity, and 95% confidence interval) of each study.
(A) Studies in children that analyzed each biomarker individually. (B) Studies in children that combined three or four biomarkers in one analysis. (C) Studies in adults. TP, true positive; FP, false positive; FN, false negative; TN, true negative.
Figure 3. Receiver operating characteristic (ROC) curves for each group.

(A) Studies in children that analyzed each biomarker individually. (B) Studies in children that combined three or four biomarkers in one analysis. (C) Studies in adults.
Risk of Bias across Studies
The main methodological limitations of the studies were related to poor reporting for items 1 (Was the spectrum of patients representative of the patients who will receive the test in clinical practice?), and for items 10 and 11 (blind interpretation of the reference and index test). The complete item list analyzed is presented in Appendix 3.
Additional Analysis
Only one pediatric24 and one adult study26 reported LR values considered excellent DTA. Thioredoxin (TRX),28 kallikrein-1,24 uromodulin,24 urocortin-3,24 orosomucoid-1,24 proteomic patters,30 and urinary neurotransmitters31 had accuracy enough to be an acceptable diagnostic test.
Regarding PPV values, taurine,31 the combined biomarkers tested by Gozal et al.24 had the highest PPV values among pediatric studies (100% and 97%). In studies conducted in adults interleukin-6 (IL-6),26 creatine phosphokinase (CK),29 interleukin-10 (IL-10),26 and TRX28 had the highest PPV values.
The combined biomarkers tested by Gozal et al.24, y-amino-butyric acid,32 and phenylethylamine (PEA)31 had the highest NPV (100%) in pediatric studies, while IL-6 and IL-1026 had the highest NPV in adults ones (100%).
The combined biomarkers tested by Gozal et al.24 and the 2 biomarkers tested by Li et al.26 reported excellent Youden's Index (0.97, 1.00, 0.97).
Five studies24,26,28,30,31 reported the highest diagnostic odds ratio (DOR). The results reported when the biomarkers were combined in Gozal et al.24 and Kheirandish-Gozal et al.31 showed better accuracy results than when they were tested individually (DTA measurements are presented in Tables 3 and 4).
In summary, only the biomarkers tested by Gozal et al.24 satisfied the criteria required for an excellent diagnostic test in children. Kheirandish-Gozal et al.31 and Shah et al.30 satisfied the criteria for acceptable diagnostic test in children.
In adults, the study conducted by Li et al.26 that tested IL-6 and IL-10 could be considered an excellent diagnostic test.
DISCUSSION
Summary of Evidence
This systematic review investigated the available evidence on the diagnostic capability of biomarkers for the diagnosis of OSA. The actual gold standard for OSA diagnosis, i.e., the overnight PSG, has several important limitations: (a) it is potentially stressful, (b) requires sleep outside the home environment, (c) may not be widely available; and (d) is expensive.33 Therefore, development of simple, cheap, and reliable diagnostic tools that would at least permit large scale screening of at-risk populations, and enable accurate identification of the subjects with definitive disease or with definitive absence of disease would potentially revolutionize the field.13 This urgent need to find an ideal bio-marker for OSA could explain the large number of studies about this topic published since 2000. We found a large number of studies in phase 1 screening process. Unfortunately, 106 studies were excluded because they did not report sensitivity and specificity. Without these values it is impossible to properly assess the real diagnostic capability of any alternative test. Brockman et al.33 emphasizes that the lack of important information in DTA publications is sobering, as clear guidelines for reporting validity measures of alternative exploratory diagnostic methods were published in 2003, and encourage future authors of DTA studies to follow these recommendations.
Before we analyze our results, it is important emphasize that there was wide variation in the OSA diagnostic criteria employed by the pediatric studies. The AHI was the most frequently used diagnostic PSG measure of OSA severity. However, the use of AHI was associated with two major limitations. Firstly, the clinically accepted consensus for the cutoff AHI value for either the presence or absence of OSA remains unresolved. Secondly, no widely accepted agreement has been reached regarding whether children with PSG-based AHI values between the “normal cutoff” and 5/h TST should undergo surgical adenotonsillectomy.19 Based on these considerations, it becomes apparent that the definitive diagnosis of OSA solely based on the low-end spectrum of the PSG-based measures (e.g., AHI, RDI, OAHI) is difficult if not impossible. Similar, albeit less vague overlap exists among adult patients, even if the PSG criteria for the presence of OSA have been more firmly established and accepted around the world.34
Although we found only nine eligible studies, this meta-analysis is informative, because by combining the available data it increases the sample size to 258 children and 1,458 adults. The results of meta-analysis showed that five studies24,26,28–31 provide acceptable metrics enabling identification of those who really suffered from OSA (true positive), while two of them24,26 performed well for identification of those who did not have OSA (true-negative). The LR values confirm that both had excellent DTA. Similarly, PPV and NPV values showed that five studies24,26,28,29,31 performed acceptably in identifying OSA subjects. Two studies24,26 were also good in identifying OSA and non-OSA subjects, thereby concurring with sensitivity and specificity numbers.
Also, the combined biomarker approaches tested by Gozal et al.24 and the two biomarkers tested by Li et al.26 reported excellent Youden's Index (0.97, 1.00, 0.97), the latter indicative of high accuracy.22
The DOR for three pediatric24,30,31 and two adult studies26,28 indicate that the biomarkers tested in children (combined kallikrein-1, uromodulin, urocotin-3, orosomucoid-1, proteomic patterns, and urinary neurotransmitters) and in adults (IL-6, IL-10, TRX) had better discriminatory test performance. Is it important to emphasize that the results reported when the bio-markers were combined in Gozal et al.24 and Kheirandish-Gozal et al.31 showed better accuracy measurements than when the biomarkers tested in these studies were analyzed individually (Table 3).
In summary, only the putative biomarkers tested in Gozal et al.24 satisfied the required criteria for an excellent diagnostic test in children. Gozal et al.24 investigated urinary biomarkers in 60 OSA patients, 30 primary snorers, and 30 healthy controls in order to identify urinary protein clusters that were highly sensitive and specific for OSA. They found that unique sets of proteins were either increased or decreased in the urine of OSA children, and that their combined ROC curve analysis using four candidate proteins simultaneously provided a near-perfect DTA (close to 100%). Another useful set of different biomarkers was subsequently identified by Kheirandish-Gozal et al.,31 who examined urinary neurotransmitters in 50 OSA and 20 controls. They reported an overnight increase in epinephrine and norepinephrine levels in children with OSA, while taurine levels were decreased. Using combinatorial approaches and cutoff values for overnight changes of these four neurotransmitters enabled a good prediction of OSA. Also, Shah et al.30 evaluated the proteomic patterns of 20 children with OSA and of 20 children with habitual primary snoring but no evidence of OSA using surface-enhanced laser desorption/ionization time of flight mass spectrometry. The proteomic patterns were capable of diagnosing OSA with 93% sensitivity and 90% specificity. However, their methodological approaches did not allow for identification of the actual candidates, such that this work remains a proof of principle rather than provide yet other defined biomarker candidates.
In adults, the study conducted by Li et al.,26 which tested IL-6 and IL-10, could be considered an excellent diagnostic test. The study aimed to identify the best biomarker, either single or in combination, with best cost-effectiveness ratio. The authors analyzed 8-isoprostane, IL-6, TNF-α, and IL-10 in the EBC and serum of OSA, non-OSA, and healthy smoking subjects. These investigators reported that levels, in both EBC and serum, differed significantly across the four biomarkers tested.
Overall Assessment
A previous review focused on different pediatric OSA diagnostic tests33 have identified several approaches that putatively provide either acceptable or excellent DTA in the prediction of OSA. These tests have included sleep lab-based polygraphy, anterior rhinomanometry, and urinary biomarkers. However, the authors33 stated that there was still insufficient evidence to recommend any of these alternative tests to PSG for diagnosis of pediatric OSA.
The current systematic meta-analysis indicates that although all selected articles concluded that biomarkers could be useful to reliably diagnose OSA, not all approaches can actually be used as viable or definitive biomarkers. Only the combination of kallikrein-1, uromodulin, urocortin-3, and orosomucoid-1 displayed sufficient accuracy to be considered an OSA diagnostic test in children. In contrast, the combination of urinary neurotransmitters31 and of the serum proteomic patterns30 displayed acceptable accuracy to serve as a screening test in children. In adults, IL-6 and IL-10 show favorable potential to become a good biomarker to identify OSA and non-OSA subjects.
Limitations
Except for one study,25 the other studies used a sample from sleep center or subjects with OSA symptoms. This can affect the prevalence, which can bias the sensitivity and specificity of the biomarker-based test. Thus, we do not know if the tests would respond similarly when applied to the general population. Other identified limitations in the published studies were: lack of a masked interpretation of the reference and index test and no clear information regarding how many investigators analyzed the test data or if their techniques were calibrated. Finally, 106 potential biomarkers studies had to be excluded due to lack of DTA values, suggesting that if such DTA assessments were available, the present conclusions could be markedly affected, further reinforcing the need for standardized reporting of predictive DTA values. Notwithstanding such considerations, the current findings are encouraging toward the implementation of biomarkers in the diagnosis of OSA, and prompted us to perform a relatively simplistic financial cost analysis of potential savings embedded in such a clinically based approach. For example, assuming that a combinatorial multiple biomarker-based assay would be required, and estimating that the global cost of such assay would amount to one-fourth of the cost of a PSG, then application of the biomarker-based approach would be economically advantageous if < 25% of the biomarker test results would be equivocal, thereby necessitating a PSG. Similar models can be implemented using various cost estimates, with obviously, more favorable “equivocal result” rates still being financially viable if the assay costs are lower.
CONCLUSIONS
Kallikrein-1, uromodulin, urocortin-3, and orosomucoid-1 have enough accuracy to be used as an OSA diagnostic test in children when used in combination.
Plasma IL-6 and IL-10 levels are potentially promising to become a good biomarker aiming to identify adult individuals with and without OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Gozal is supported in part by NIH grants RO1 HL-65270 and P50 HL-107160. The authors have indicated no financial conflicts of interest. This research was performed at the Department of Dentistry, University of Alberta, Canada.
Appendix 1—Search.
Appendix 1.
Search.
Appendix 2
Appendix 2.

REFERENCES
- 1.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–35. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 2.Brockmann PE, Schaefer C, Poets A, Poets CF, Urschitz MS. Diagnosis of obstructive sleep apnea in children: a systematic review. Sleep Med Rev. 2013;17:331–40. doi: 10.1016/j.smrv.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Deeks JJ, Bossuyt P, Gatsonis C, editors. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration. 2010. Available from: http://srdta.cochrane.org/
Appendix 3—Excluded articles and reasons for exclusion.
Appendix 3.
Excluded articles and reasons for exclusion.


REFERENCES
- 1.Aihara K, Oga T, Chihara Y, et al. Analysis of systemic and airway inflammation in obstructive sleep apnea. Sleep Breath. 2013;17:597–604. doi: 10.1007/s11325-012-0726-y. [DOI] [PubMed] [Google Scholar]
- 2.Akinnusi ME, Laporta R, El-Solh AA. Lectin-like oxidized low-density lipoprotein receptor-1 modulates endothelial apoptosis in obstructive sleep apnea. Chest. 2011;140:1503–10. doi: 10.1378/chest.11-0302. [DOI] [PubMed] [Google Scholar]
- 3.Alzoghaibi MA, Bahammam AS. Lipid peroxides, superoxide dismutase and circulating IL-8 and GCP-2 in patients with severe obstructive sleep apnea: a pilot study. Sleep Breath. 2005;9:119–26. doi: 10.1007/s11325-005-0022-1. [DOI] [PubMed] [Google Scholar]
- 4.Antonopoulou S, Loukides S, Papatheodorou G, Roussos C, Alchanatis M. Airway inflammation in obstructive sleep apnea: is leptin the missing link? Respir Med. 2008;102:1399–405. doi: 10.1016/j.rmed.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Arias MA, Garcia-Rio F, Alonso-Fernandez A, et al. CPAP decreases plasma levels of soluble tumour necrosis factor-alpha receptor 1 in obstructive sleep apnoea. Eur Respir J. 2008;32:1009–15. doi: 10.1183/09031936.00007008. [DOI] [PubMed] [Google Scholar]
- 6.Bhushan B, Khalyfa A, Spruyt K, et al. Fatty-acid binding protein 4 gene polymorphisms and plasma levels in children with obstructive sleep apnea. Sleep Med. 2011;12:666–71. doi: 10.1016/j.sleep.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braga CW, Martinez D, Wofchuk S, Portela LV, Souza DO. S100B and NSE serum levels in obstructive sleep apnea syndrome. Sleep Med. 2006;7:431–5. doi: 10.1016/j.sleep.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Bratel T, Wennlund A, Carlstrom K. Pituitary reactivity, androgens and catecholamines in obstructive sleep apnoea. Effects of continuous positive airway pressure treatment (CPAP) Respir Med. 1999;93:1–7. doi: 10.1016/s0954-6111(99)90068-9. [DOI] [PubMed] [Google Scholar]
- 9.Burioka N, Miyata M, Fukuoka Y, Endo M, Shimizu E. Day-night variations of serum interleukin-6 in patients with severe obstructive sleep apnea syndrome before and after continuous positive airway pressure (CPAP) Chronobiol Int. 2008;25:827–34. doi: 10.1080/07420520802384101. [DOI] [PubMed] [Google Scholar]
- 10.Calvin AD, Somers VK, Steensma DP, et al. Advanced heart failure and nocturnal hypoxaemia due to central sleep apnoea are associated with increased serum erythropoietin. Eur J Heart Fail. 2010;12:354–9. doi: 10.1093/eurjhf/hfq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122:1162–7. doi: 10.1378/chest.122.4.1162. [DOI] [PubMed] [Google Scholar]
- 12.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124:1386–92. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- 13.Chin K, Nakamura T, Shimizu K, et al. Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. Am J Med. 2000;109:562–7. doi: 10.1016/s0002-9343(00)00580-5. [DOI] [PubMed] [Google Scholar]
- 14.Cholidou KG, Kostakis ID, Manali ED, et al. Calprotectin: a protein related to cardiovascular risk in adult patients with obstructive sleep apnea. Cytokine. 2013;61:917–23. doi: 10.1016/j.cyto.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Christou K, Moulas AN, Pastaka C, Gourgoulianis KI. Antioxidant capacity in obstructive sleep apnea patients. Sleep Med. 2003;4:225–8. doi: 10.1016/s1389-9457(02)00253-8. [DOI] [PubMed] [Google Scholar]
- 16.Chung F, Chau E, Yang Y, Liao P, Hall R, Mokhlesi B. Serum bicarbonate level improves specificity of STOP-Bang screening for obstructive sleep apnea. Chest. 2013;143:1284–93. doi: 10.1378/chest.12-1132. [DOI] [PubMed] [Google Scholar]
- 17.Cintra F, Tufik S, D'Almeida V, et al. Cysteine: a potential biomarker for obstructive sleep apnea. Chest. 2011;139:246–52. doi: 10.1378/chest.10-0667. [DOI] [PubMed] [Google Scholar]
- 18.Cofta S, Wysocka E, Dziegielewska-Gesiak S, et al. Plasma selectins in patients with obstructive sleep apnea. Adv Exp Med Biol. 2013;756:113–9. doi: 10.1007/978-94-007-4549-0_15. [DOI] [PubMed] [Google Scholar]
- 19.Constantinidis J, Ereliadis S, Angouridakis N, Konstantinidis I, Vital V, Angouridaki C. Cytokine changes after surgical treatment of obstructive sleep apnoea syndrome. Eur Arch Otorhinolaryngol. 2008;265:1275–9. doi: 10.1007/s00405-008-0627-7. [DOI] [PubMed] [Google Scholar]
- 20.Culla B, Guida G, Brussino L, et al. Increased oral nitric oxide in obstructive sleep apnoea. Respir Med. 2010;104:316–20. doi: 10.1016/j.rmed.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Deboer MD, Mendoza JP, Liu L, Ford G, Yu PL, Gaston BM. Increased systemic inflammation overnight correlates with insulin resistance among children evaluated for obstructive sleep apnea. Sleep Breath. 2012;16:349–54. doi: 10.1007/s11325-011-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duru S, Hikmet Firat I, Colak N, Ginis Z, Delibasi T, Ardic S. Serum S100B protein: a useful marker in obstructive sleep apnea syndrome. Neurol Neurochir Pol. 2012;46:450–5. doi: 10.5114/ninp.2012.31355. [DOI] [PubMed] [Google Scholar]
- 23.El-Solh AA, Mador MJ, Sikka P, Dhillon RS, Amsterdam D, Grant BJ. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest. 2002;121:1541–7. doi: 10.1378/chest.121.5.1541. [DOI] [PubMed] [Google Scholar]
- 24.Feng X, Li P, Zhou C, Jia X, Kang J. Elevated levels of serum chemerin in patients with obstructive sleep apnea syndrome. Biomarkers. 2012;17:248–53. doi: 10.3109/1354750X.2012.658864. [DOI] [PubMed] [Google Scholar]
- 25.Ferrarini A, Ruperez FJ, Erazo M, et al. Fingerprinting-based metabolomic approach with LC-MS to sleep apnea and hypopnea syndrome: a pilot study. Electrophoresis. 2013;34:2873–81. doi: 10.1002/elps.201300081. [DOI] [PubMed] [Google Scholar]
- 26.Goldbart AD, Krishna J, Li RC, Serpero LD, Gozal D. Inflammatory mediators in exhaled breath condensate of children with obstructive sleep apnea syndrome. Chest. 2006;130:143–8. doi: 10.1378/chest.130.1.143. [DOI] [PubMed] [Google Scholar]
- 27.Gozal D, Lipton AJ, Jones KL. Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep. 2002;25:59–65. doi: 10.1093/sleep/25.1.59. [DOI] [PubMed] [Google Scholar]
- 28.Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176:188–93. doi: 10.1164/rccm.200610-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9:254–9. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gozal D, Serpero LD, Kheirandish-Gozal L, Capdevila OS, Khalyfa A, Tauman R. Sleep measures and morning plasma TNF-alpha levels in children with sleep-disordered breathing. Sleep. 2010;33:319–25. doi: 10.1093/sleep/33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Molero-Ramirez H, Tan HL, Bandla HP. Circulating adropin concentrations in pediatric obstructive sleep apnea: potential relevance to endothelial function. J Pediatr. 2013;163:1122–6. doi: 10.1016/j.jpeds.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep. 2004;27:1507–11. doi: 10.1093/sleep/27.8.1507. [DOI] [PubMed] [Google Scholar]
- 33.Guven SF, Turkkani MH, Ciftci B, Ciftci TU, Erdogan Y. The relationship between high-sensitivity C-reactive protein levels and the severity of obstructive sleep apnea. Sleep Breath. 2012;16:217–21. doi: 10.1007/s11325-011-0492-2. [DOI] [PubMed] [Google Scholar]
- 34.Hira HS, Shukla A, Kaur A, Kapoor S. Serum uric acid and lactate levels among patients with obstructive sleep apnea syndrome: which is a better marker of hypoxemia? Ann Saudi Med. 2012;32:37–42. doi: 10.5144/0256-4947.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Htoo AK, Greenberg H, Tongia S, et al. Activation of nuclear factor kappaB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath. 2006;10:43–50. doi: 10.1007/s11325-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 36.Imagawa S, Yamaguchi Y, Ogawa K, et al. Interleukin-6 and tumor necrosis factor-alpha in patients with obstructive sleep apnea-hypopnea syndrome. Respiration. 2004;71:24–9. doi: 10.1159/000075645. [DOI] [PubMed] [Google Scholar]
- 37.Jurado-Gamez B, Fernandez-Marin MC, Gomez-Chaparro JL, et al. Relationship of oxidative stress and endothelial dysfunction in sleep apnoea. Eur Respir J. 2011;37:873–9. doi: 10.1183/09031936.00027910. [DOI] [PubMed] [Google Scholar]
- 38.Jurado-Gamez B, Cabrera CB, Ballesteros LC, et al. Association of cellular adhesion molecules and oxidative stress with endothelial function in obstructive sleep apnea. Intern Med. 2012;51:363–8. doi: 10.2169/internalmedicine.51.6571. [DOI] [PubMed] [Google Scholar]
- 39.Kaditis AG, Alexopoulos EI, Damani E, et al. Obstructive sleep-disordered breathing and fasting insulin levels in nonobese children. Pediatr Pulmonol. 2005;40:515–23. doi: 10.1002/ppul.20306. [DOI] [PubMed] [Google Scholar]
- 40.Kaditis AG, Alexopoulos EI, Kalampouka E, et al. Nocturnal change of circulating intercellular adhesion molecule 1 levels in children with snoring. Sleep Breath. 2007;11:267–74. doi: 10.1007/s11325-007-0117-y. [DOI] [PubMed] [Google Scholar]
- 41.Kaditis AG, Alexopoulos E, Chaidas K, et al. Urine concentrations of cysteinyl leukotrienes in children with obstructive sleep-disordered breathing. Chest. 2009;135:1496–501. doi: 10.1378/chest.08-2295. [DOI] [PubMed] [Google Scholar]
- 42.Kaditis AG, Alexopoulos EI, Damani E, et al. Urine levels of catecholamines in Greek children with obstructive sleep-disordered breathing. Pediatr Pulmonol. 2009;44:38–45. doi: 10.1002/ppul.20916. [DOI] [PubMed] [Google Scholar]
- 43.Kanbay A, Kokturk O, Ciftci TU, Tavil Y, Bukan N. Comparison of serum adiponectin and tumor necrosis factor-alpha levels between patients with and without obstructive sleep apnea syndrome. Respiration. 2008;76:324–30. doi: 10.1159/000134010. [DOI] [PubMed] [Google Scholar]
- 44.Khalyfa A, Gharib SA, Kim J, et al. Peripheral blood leukocyte gene expression patterns and metabolic parameters in habitually snoring and non-snoring children with normal polysomnographic findings. Sleep. 2011;34:153–60. doi: 10.1093/sleep/34.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khalyfa A, Kheirandish-Gozal L, Capdevila OS, Bhattacharjee R, Gozal D. Macrophage migration inhibitory factor gene polymorphisms and plasma levels in children with obstructive sleep apnea. Pediatr Pulmonol. 2012;47:1001–11. doi: 10.1002/ppul.22560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kheirandish-Gozal L, Peris E, Wang Y, et al. Lipopolysaccharide-binding protein plasma levels in children: effects of obstructive sleep apnea and obesity. J Clin Endocrinol Metab. 2014;99:656–63. doi: 10.1210/jc.2013-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kheirandish-Gozal L, Capdevila OS, Tauman R, Gozal D. Plasma C-reactive protein in nonobese children with obstructive sleep apnea before and after adenotonsillectomy. J Clin Sleep Med. 2006;2:301–4. [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J, Bhattacharjee R, Dayyat E, et al. Increased cellular proliferation and inflammatory cytokines in tonsils derived from children with obstructive sleep apnea. Pediatr Res. 2009;66:423–8. doi: 10.1203/PDR.0b013e3181b453e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim J, Bhattacharjee R, Snow AB, Capdevila OS, Kheirandish-Gozal L, Gozal D. Myeloid-related protein 8/14 levels in children with obstructive sleep apnoea. Eur Respir J. 2010;35:843–50. doi: 10.1183/09031936.00075409. [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Bhattacharjee R, Khalyfa A, et al. DNA methylation in inflammatory genes among children with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:330–8. doi: 10.1164/rccm.201106-1026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, Gozal D, Bhattacharjee R, Kheirandish-Gozal L. TREM-1 and pentraxin-3 plasma levels and their association with obstructive sleep apnea, obesity, and endothelial function in children. Sleep. 2013;36:923–31. doi: 10.5665/sleep.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kishida K, Funahashi T, Shimomura I. Adiponectin as a routine clinical biomarker. Best practice & research. Clin Endocrinol Metab. 2014;28:119–30. doi: 10.1016/j.beem.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Kohler M, Stoewhas AC, Ayers L, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184:1192–9. doi: 10.1164/rccm.201106-0964OC. [DOI] [PubMed] [Google Scholar]
- 54.Krishna J, Shah ZA, Merchant M, Klein JB, Gozal D. Urinary protein expression patterns in children with sleep-disordered breathing: preliminary findings. Sleep Med. 2006;7:221–7. doi: 10.1016/j.sleep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Kuramoto E, Kinami S, Ishida Y, Shiotani H, Nishimura Y. Continuous positive nasal airway pressure decreases levels of serum amyloid A and improves autonomic function in obstructive sleep apnea syndrome. Int J Cardiol. 2009;135:338–45. doi: 10.1016/j.ijcard.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 56.Kurt OK, Yildiz N. The importance of laboratory parameters in patients with obstructive sleep apnea syndrome. Blood Coagul Fibrinolysis. 2013;24:371–4. doi: 10.1097/MBC.0b013e32835d53d4. [DOI] [PubMed] [Google Scholar]
- 57.Ladesich JB, Pottala JV, Romaker A, Harris WS. Membrane level of omega-3 docosahexaenoic acid is associated with severity of obstructive sleep apnea. J Clin Sleep Med. 2011;7:391–6. doi: 10.5664/JCSM.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lam JC, Yan CS, Lai AY, et al. Determinants of daytime blood pressure in relation to obstructive sleep apnea in men. Lung. 2009;187:291–8. doi: 10.1007/s00408-009-9161-7. [DOI] [PubMed] [Google Scholar]
- 59.Larkin EK, Rosen CL, Kirchner HL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–84. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 60.Lavie L, Kraiczi H, Hefetz A, et al. Plasma vascular endothelial growth factor in sleep apnea syndrome: effects of nasal continuous positive air pressure treatment. Am J Crit Care Med. 2002;165:1624–8. doi: 10.1164/rccm.20110-040OC. [DOI] [PubMed] [Google Scholar]
- 61.Lederer DJ, Jelic S, Basner RC, Ishizaka A, Bhattacharya J. Circulating KL-6, a biomarker of lung injury, in obstructive sleep apnoea. Eur Respir J. 2009;33:793–6. doi: 10.1183/09031936.00150708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee LA, Chen NH, Huang CG, Lin SW, Fang TJ, Li HY. Patients with severe obstructive sleep apnea syndrome and elevated high-sensitivity C-reactive protein need priority treatment. Otolaryngol Head Neck Surg. 2010;143:72–7. doi: 10.1016/j.otohns.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Lee SD, Ju G, Choi JA, Kim JW, Yoon IY. The association of oxidative stress with central obesity in obstructive sleep apnea. Sleep Breath. 2012;16:511–7. doi: 10.1007/s11325-011-0536-7. [DOI] [PubMed] [Google Scholar]
- 64.Li AM, Chan MH, Chan DF, et al. Insulin and obstructive sleep apnea in obese Chinese children. Pediatr Pulmonol. 2006;41:1175–81. doi: 10.1002/ppul.20508. [DOI] [PubMed] [Google Scholar]
- 65.Li Y, Chongsuvivatwong V, Geater A, Liu A. Are biomarker levels a good follow-up tool for evaluating obstructive sleep apnea syndrome treatments? Respiration. 2008;76:317–23. doi: 10.1159/000119542. [DOI] [PubMed] [Google Scholar]
- 66.Li AM, Chan MH, Yin J, et al. C-reactive protein in children with obstructive sleep apnea and the effects of treatment. Pediatr Pulmonol. 2008;43:34–40. doi: 10.1002/ppul.20732. [DOI] [PubMed] [Google Scholar]
- 67.Li AM, Ng C, Ng SK, et al. Adipokines in children with obstructive sleep apnea and the effects of treatment. Chest. 2010;137:529–35. doi: 10.1378/chest.09-2153. [DOI] [PubMed] [Google Scholar]
- 68.Lin QC, Xie HS, Liu XJ, Zhou JL, Zhao JM. [Relationship between obstructive sleep apnea-hypopnea syndrome and high sensitivity C-reactive protein in non-obese subjects] Zhonghua Yi Xue Za Zhi. 2013;93:2355–8. [PubMed] [Google Scholar]
- 69.Loubaki L, Jacques E, Semlali A, Biardel S, Chakir J, Series F. Tumor necrosis factor-alpha expression in uvular tissues differs between snorers and apneic patients. Chest. 2008;134:911–8. doi: 10.1378/chest.08-0886. [DOI] [PubMed] [Google Scholar]
- 70.Lui MM, Lam JC, Mak HK, et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135:950–6. doi: 10.1378/chest.08-1798. [DOI] [PubMed] [Google Scholar]
- 71.Makino S, Handa H, Suzukawa K, et al. Obstructive sleep apnoea syndrome, plasma adiponectin levels, and insulin resistance. Clin Endocrinol. 2006;64:12–9. doi: 10.1111/j.1365-2265.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- 72.Malakasioti G, Alexopoulos E, Befani C, et al. Oxidative stress and inflammatory markers in the exhaled breath condensate of children with OSA. Sleep Breath. 2012;16:703–8. doi: 10.1007/s11325-011-0560-7. [DOI] [PubMed] [Google Scholar]
- 73.Mancuso M, Bonanni E, LoGerfo A, et al. Oxidative stress biomarkers in patients with untreated obstructive sleep apnea syndrome. Sleep Med. 2012;13:632–6. doi: 10.1016/j.sleep.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 74.Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med. 2006;166:1725–31. doi: 10.1001/archinte.166.16.1725. [DOI] [PubMed] [Google Scholar]
- 75.Montgomery-Downs HE, Krishna J, Roberts LJ, 2nd, Gozal D. Urinary F2-isoprostane metabolite levels in children with sleep-disordered breathing. Sleep Breath. 2006;10:211–5. doi: 10.1007/s11325-006-0079-5. [DOI] [PubMed] [Google Scholar]
- 76.Murase K, Mori K, Yoshimura C, et al. Association between plasma neutrophil gelatinase associated lipocalin level and obstructive sleep apnea or nocturnal intermittent hypoxia. PloS One. 2013;8:e54184. doi: 10.1371/journal.pone.0054184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Norman D, Bardwell WA, Arosemena F, et al. Serum aminotransferase levels are associated with markers of hypoxia in patients with obstructive sleep apnea. Sleep. 2008;31:121–6. doi: 10.1093/sleep/31.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ntalapascha M, Makris D, Kyparos A, et al. Oxidative stress in patients with obstructive sleep apnea syndrome. Sleep Breath. 2013;17:549–55. doi: 10.1007/s11325-012-0718-y. [DOI] [PubMed] [Google Scholar]
- 79.O'Brien LM, Serpero LD, Tauman R, Gozal D. Plasma adhesion molecules in children with sleep-disordered breathing. Chest. 2006;129:947–53. doi: 10.1378/chest.129.4.947. [DOI] [PubMed] [Google Scholar]
- 80.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol. 2003;94:179–84. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- 81.Osorio RS, Ayappa I, Mantua J, et al. The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer's disease in cognitively normal elderly individuals. Neurobiol Aging. 2014;35:1318–24. doi: 10.1016/j.neurobiolaging.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oyama J, Yamamoto H, Maeda T, Ito A, Node K, Makino N. Continuous positive airway pressure therapy improves vascular dysfunction and decreases oxidative stress in patients with the metabolic syndrome and obstructive sleep apnea syndrome. Clin Cardiol. 2012;35:231–6. doi: 10.1002/clc.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ozben S, Guvenc TS, Huseyinoglu N, et al. Low serum copeptin levels in patients with obstructive sleep apnea. Sleep Breath. 2013;17:1187–92. doi: 10.1007/s11325-013-0822-7. [DOI] [PubMed] [Google Scholar]
- 84.Pallayova M, Steele KE, Magnuson TH, et al. Sleep apnea predicts distinct alterations in glucose homeostasis and biomarkers in obese adults with normal and impaired glucose metabolism. Cardiovasc Diabetol. 2010;9:83. doi: 10.1186/1475-2840-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pallayova M, Steele KE, Magnuson TH, et al. Sleep apnea determines soluble TNF-alpha receptor 2 response to massive weight loss. Obes Surg. 2011;21:1413–23. doi: 10.1007/s11695-011-0359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Papaioannou I, Twigg GL, Kemp M, et al. Melatonin concentration as a marker of the circadian phase in patients with obstructive sleep apnoea. Sleep Med. 2012;13:167–71. doi: 10.1016/j.sleep.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 87.Park CS, Guilleminault C, Hwang SH, Jeong JH, Park DS, Maeng JH. Correlation of salivary cortisol level with obstructive sleep apnea syndrome in pediatric subjects. Sleep Med. 2013;14:978–84. doi: 10.1016/j.sleep.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 88.Patacchioli FR, Tabarrini A, Ghiciuc CM, et al. Salivary biomarkers of obstructive sleep apnea syndrome in children. Pediatr Pulmonol. 2014;49:1145–52. doi: 10.1002/ppul.22972. [DOI] [PubMed] [Google Scholar]
- 89.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peled N, Shitrit D, Bendayan D, Peled E, Kramer MR. Association of elevated levels of vascular endothelial growth factor in obstructive sleep apnea syndrome with patient age rather than with obstructive sleep apnea syndrome severity. Respiration. 2007;74:50–5. doi: 10.1159/000095675. [DOI] [PubMed] [Google Scholar]
- 91.Petrosyan M, Perraki E, Simoes D, et al. Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breath. 2008;12:207–15. doi: 10.1007/s11325-007-0160-8. [DOI] [PubMed] [Google Scholar]
- 92.Phillips CL, Yang Q, Williams A, et al. The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea. J Sleep Res. 2007;16:217–25. doi: 10.1111/j.1365-2869.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 93.Pinto P, Barbara C, Montserrat JM, et al. Effects of CPAP on nitrate and norepinephrine levels in severe and mild-moderate sleep apnea. BMC Pulm Med. 2013;13:13. doi: 10.1186/1471-2466-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Przybylowski T, Bielicki P, Kumor M, et al. [Exhaled nitric oxide in patients with obstructive sleep apnea syndrome] Pneumonol Alergol Pol. 2006;74:21–5. [PubMed] [Google Scholar]
- 95.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep. 2007;30:29–34. doi: 10.1093/sleep/30.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roche F, Gaspoz JM, Pichot V, et al. Association between C-reactive protein and unrecognised sleep-disordered breathing in the elderly. Eur Respir J. 2009;33:797–803. doi: 10.1183/09031936.00023208. [DOI] [PubMed] [Google Scholar]
- 97.Rubinsztajn R, Kumor M, Byskiniewicz K, Chazan R. [The influence of 3 weeks therapy with continuous positive airway pressure on serum leptin and homocysteine concentration in patients with obstructive sleep apnea syndrome] Pneumonol Alergol Pol. 2006;74:63–7. [PubMed] [Google Scholar]
- 98.Ryan S, Nolan GM, Hannigan E, Cunningham S, Taylor C, McNicholas WT. Cardiovascular risk markers in obstructive sleep apnoea syndrome and correlation with obesity. Thorax. 2007;62:509–14. doi: 10.1136/thx.2006.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salord N, Gasa M, Mayos M, et al. Impact of OSA on biological markers in morbid obesity and metabolic syndrome. J Clin Sleep Med. 2014;10:263–70. doi: 10.5664/jcsm.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med. 2002;165:67–70. doi: 10.1164/ajrccm.165.1.2101062. [DOI] [PubMed] [Google Scholar]
- 101.Shamsuzzaman AS. Elevated C-Reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–64. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 102.Shi YK, Chen JX, Huang Y, Li AY. Serum S100A12 levels are associated with the presence and severity of obstructive sleep apnea syndrome in male patients. Sleep Breath. 2014;18:269–74. doi: 10.1007/s11325-013-0876-6. [DOI] [PubMed] [Google Scholar]
- 103.Simiakakis M, Kapsimalis F, Chaligiannis E, Loukides S, Sitaras N, Alchanatis M. Lack of effect of sleep apnea on oxidative stress in obstructive sleep apnea syndrome (OSAS) patients. PloS One. 2012;7:e39172. doi: 10.1371/journal.pone.0039172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sokucu SN, Karasulu L, Dalar L, Seyhan EC, Altin S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med. 2012;8:521–5. doi: 10.5664/jcsm.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Staats R, Stoll P, Zingler D, Virchow JC, Lommatzsch M. Regulation of brain-derived neurotrophic factor (BDNF) during sleep apnoea treatment. Thorax. 2005;60:688–92. doi: 10.1136/thx.2004.038208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stefanini Dde O, Barros EL, Stefanini R, Pradella-Hallinan ML, Pignatari SS, Fujita RR. Comparing the clinical profile of non obese children with sleep apnea and snoring. Braz J Otorhinolaryngol. 2012;78:22–6. doi: 10.5935/1808-8694.20120004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steiropoulos P, Papanas N, Nena E, et al. Inflammatory markers in middle-aged obese subjects: does obstructive sleep apnea syndrome play a role? Mediators Inflamm. 2010;2010:675320. doi: 10.1155/2010/675320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sukegawa M, Noda A, Sugiura T, et al. Assessment of continuous positive airway pressure treatment in obstructive sleep apnea syndrome using 24-hour urinary catecholamines. Clin Cardiol. 2005;28:519–22. doi: 10.1002/clc.4960281106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Svensson M, Venge P, Janson C, Lindberg E. Relationship between sleep-disordered breathing and markers of systemic inflammation in women from the general population. J Sleep Res. 2012;21:147–54. doi: 10.1111/j.1365-2869.2011.00946.x. [DOI] [PubMed] [Google Scholar]
- 110.Takahashi K, Chin K, Nakamura H, et al. Plasma thioredoxin, a novel oxidative stress marker, in patients with obstructive sleep apnea before and after nasal continuous positive airway pressure. Antioxid Redox Signal. 2008;10:715–26. doi: 10.1089/ars.2007.1949. [DOI] [PubMed] [Google Scholar]
- 111.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics. 2004;113:e564–9. doi: 10.1542/peds.113.6.e564. [DOI] [PubMed] [Google Scholar]
- 112.Tauman R, Serpero LD, Capdevila OS, et al. Adipokines in children with sleep disordered breathing. Sleep. 2007;30:443–9. doi: 10.1093/sleep/30.4.443. [DOI] [PubMed] [Google Scholar]
- 113.Ting H, Lo HS, Chang SY, et al. Post- to pre-overnight sleep systolic blood pressures are associated with sleep respiratory disturbance, pro-inflammatory state and metabolic situation in patients with sleep-disordered breathing. Sleep Med. 2009;10:720–5. doi: 10.1016/j.sleep.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 114.Tual-Chalot S, Gagnadoux F, Trzepizur W, Priou P, Andriantsitohaina R, Martinez MC. Circulating microparticles from obstructive sleep apnea syndrome patients induce endothelin-mediated angiogenesis. Biochim Biophys Acta. 2014;1842:202–7. doi: 10.1016/j.bbadis.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 115.Ucar ZZ, Taymaz Z, Erbaycu AE, Kirakli C, Tuksavul F, Guclu SZ. Nocturnal hypoxia and arterial lactate levels in sleep-related breathing disorders. South Med J. 2009;102:693–700. doi: 10.1097/SMJ.0b013e3181a93897. [DOI] [PubMed] [Google Scholar]
- 116.Uysal A, Liendo C, McCarty DE, et al. Nocturnal hypoxemia biomarker predicts sleepiness in patients with severe obstructive sleep apnea. Sleep Breath. 2014;18:77–84. doi: 10.1007/s11325-013-0851-2. [DOI] [PubMed] [Google Scholar]
- 117.Van Hoorenbeeck K, Franckx H, Debode P, et al. Weight loss and sleep-disordered breathing in childhood obesity: effects on inflammation and uric acid. Obesity. 2012;20:172–7. doi: 10.1038/oby.2011.282. [DOI] [PubMed] [Google Scholar]
- 118.Vavougios G, Pastaka C, Tsilioni I, et al. The DJ-1 protein as a candidate biomarker in obstructive sleep apnea syndrome. Sleep Breath. 2014 Feb 15; doi: 10.1007/s11325-014-0952-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 119.Villa MP, Supino MC, Fedeli S, et al. Urinary concentration of 8-isoprostane as marker of severity of pediatric OSAS. Sleep Breath. 2014 Jan 17; doi: 10.1007/s11325-013-0934-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 120.Wang YN, Yang Y, Luo YQ, Chen LL. [Effects of nasal continuous positive airway pressure short-term treatment on C-reactive protein and intercellular adhesion molecule-1 in patients with obstructive sleep apnea-hypopnea syndrome] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2005;30:666–9. [PubMed] [Google Scholar]
- 121.Wang Y, Wang JJ, Zhao MQ, Liu SM, Li YZ. Changes of serum brain-derived neurotrophic factor in children with obstructive sleep apnoea-hypopnoea syndrome following adenotonsillectomy. J Int Med Res. 2010;38:1942–51. doi: 10.1177/147323001003800607. [DOI] [PubMed] [Google Scholar]
- 122.Wang W, Redline S, Khoo MC. Autonomic markers of impaired glucose metabolism: effects of sleep-disordered breathing. J Diabetes Sci Technol. 2012;6:1159–71. doi: 10.1177/193229681200600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Q, Feng X, Zhou C, Li P, Kang J. Decreased levels of serum omentin-1 in patients with obstructive sleep apnoea syndrome. Ann Clin Biochem. 2013;50:230–5. doi: 10.1177/0004563212473275. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y, Li Y, Chen P, Luo Y, Yang Y, Yang Y. Elevated fractalkine in patients with obstructive sleep apnea hypopnea syndrome. Sleep Breath. 2013;17:203–8. doi: 10.1007/s11325-012-0674-6. [DOI] [PubMed] [Google Scholar]
- 125.Wang X, Xing GH. Serum YKL-40 concentrations are elevated and correlated with disease severity in patients with obstructive sleep apnea syndrome. Scand J Clin Lab Invest. 2014;74:74–8. doi: 10.3109/00365513.2013.859726. [DOI] [PubMed] [Google Scholar]
- 126.Yamauchi M, Nakano H, Maekawa J, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127:1674–9. doi: 10.1378/chest.127.5.1674. [DOI] [PubMed] [Google Scholar]
- 127.Ye J, Liu H, Li Y, Liu X, Zhu JM. Increased serum levels of C-reactive protein and matrix metalloproteinase-9 in obstructive sleep apnea syndrome. Chinese Med J. 2007;120:1482–6. [PubMed] [Google Scholar]
- 128.Ye L, Ma GH, Chen L, et al. Quantification of circulating cell-free DNA in the serum of patients with obstructive sleep apnea-hypopnea syndrome. Lung. 2010;188:469–74. doi: 10.1007/s00408-010-9253-4. [DOI] [PubMed] [Google Scholar]
- 129.Yokoe T. Elevated levels of C-Reactive Protein and Interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 130.Zamarron C, Ricoy J, Riveiro A, Gude F. Plasminogen activator inhibitor-1 in obstructive sleep apnea patients with and without hypertension. Lung. 2008;186:151–6. doi: 10.1007/s00408-008-9076-8. [DOI] [PubMed] [Google Scholar]
- 131.Zamarron C, Riveiro A, Gude F. Circulating levels of vascular endothelial markers in obstructive sleep apnoea syndrome. Effects of nasal continuous positive airway pressure. Arch Med Sci. 2011;7:1023–8. doi: 10.5114/aoms.2011.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang XB, Lin QC, Deng CS, Chen GP, Cai ZM, Chen H. Elevated serum cystatin C in severe OSA younger men without complications. Sleep Breath. 2013;17:235–41. doi: 10.1007/s11325-012-0678-2. [DOI] [PubMed] [Google Scholar]
Appendix 4—QUADAS criteria fulfilled.
Appendix 4.
QUADAS criteria fulfilled.

REFERENCES
- 1.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–52. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jean-Louis G, Zizi F, Brown D, Ogedegbe G, Borer J, McFarlane S. Obstructive sleep apnea and cardiovascular disease: evidence and underlying mechanisms. Minerva Pneumol. 2009;48:277–93. [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–84. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 4.Ali SS, Oni ET, Warraich HJ, et al. Systematic review on noninvasive assessment of subclinical cardiovascular disease in obstructive sleep apnea: new kid on the block! Sleep Med Rev. 2014;18:379–91. doi: 10.1016/j.smrv.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012;141:1601–10. doi: 10.1378/chest.11-2214. [DOI] [PubMed] [Google Scholar]
- 6.Tan HL, Gozal D, Kheirandish-Gozal L. Obstructive sleep apnea in children: a critical update. Nat Sci Sleep. 2013;5:109–23. doi: 10.2147/NSS.S51907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62:569–76. doi: 10.1016/j.jacc.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32:731–6. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamagishi K, Ohira T, Nakano H, et al. Cross-cultural comparison of the sleep-disordered breathing prevalence among Americans and Japanese. Eur Respir J. 2010;36:379–84. doi: 10.1183/09031936.00118609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ralls FM, Grigg-Damberger M. Roles of gender, age, race/ethnicity, and residential socioeconomics in obstructive sleep apnea syndromes. Curr Opin Pulm Med. 2012;18:568–73. doi: 10.1097/MCP.0b013e328358be05. [DOI] [PubMed] [Google Scholar]
- 11.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32:731–6. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spruyt K, Gozal D. Screening of pediatric sleep-disordered breathing: a proposed unbiased discriminative set of questions using clinical severity scales. Chest. 2012;142:1508–15. doi: 10.1378/chest.11-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gozal D. Serum, urine, and breath-related biomarkers in the diagnosis of obstructive sleep apnea in children: is it for real? Curr Opin Pulm Med. 2012;18:561–7. doi: 10.1097/MCP.0b013e328358be2d. [DOI] [PubMed] [Google Scholar]
- 14.Lamm C, Mandeli J, Kattan M. Evaluation of home audiotapes as an abbreviated test for obstructive sleep apnea syndrome (OSAS) in children. Pediatr Pulmonol. 1999;27:267–72. doi: 10.1002/(sici)1099-0496(199904)27:4<267::aid-ppul7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Sivan Y, Kornecki A, Schonfeld T. Screening obstructive sleep apnoea syndrome by home videotape recording in children. Eur Respir J. 1996;9:2127–31. doi: 10.1183/09031936.96.09102127. [DOI] [PubMed] [Google Scholar]
- 16.Brouillette RT, Morielli A, Leimanis A, Waters KA, Luciano R, Ducharme FM. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics. 2000;105:405–12. doi: 10.1542/peds.105.2.405. [DOI] [PubMed] [Google Scholar]
- 17.Finn D, McNally P. Estimated population prevalence of obstructive sleep apnoea in a community of German third graders. Eur Respir J. 2011;37:975. doi: 10.1183/09031936.00185610. author reply 75–6. [DOI] [PubMed] [Google Scholar]
- 18.Andreu AL, Chiner E, Sancho-Chust JN, et al. Effect of an ambulatory diagnostic and treatment programme in patients with sleep apnoea. Eur Respir J. 2012;39:305–12. doi: 10.1183/09031936.00013311. [DOI] [PubMed] [Google Scholar]
- 19.Wong TK. The search on an ideal disease marker for childhood obstructive sleep apnea syndrome. Sleep. 2011;34:133–4. doi: 10.1093/sleep/34.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deeks JJ, Bossuyt P, Gatsonis C, editors. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration. 2010. Available from: http://srdta.cochrane.org/
- 23.Benedek P, Lazar Z, Bikov A, Kunos L, Katona G, Horvath I. Exhaled biomarker pattern is altered in children with obstructive sleep apnoea syndrome. Int J Pediatr Otorhinolaryngol. 2013;77:1244–7. doi: 10.1016/j.ijporl.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Gozal D, Jortani S, Snow AB, et al. Two-dimensional differential in-gel electrophoresis proteomic approaches reveal urine candidate biomarkers in pediatric obstructive sleep apnea. Am J Respir Crit Care Med. 2009;180:1253–61. doi: 10.1164/rccm.200905-0765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirotsu C, Tufik S, Guindalini C, Mazzotti DR, Bittencourt LR, Andersen ML. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PloS One. 2013;8:e66891. doi: 10.1371/journal.pone.0066891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Chongsuvivatwong V, Geater A, Liu A. Exhaled breath condensate cytokine level as a diagnostic tool for obstructive sleep apnea syndrome. Sleep Med. 2009;10:95–103. doi: 10.1016/j.sleep.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Ursavas A, Karadag M, Rodoplu E, Yilmaztepe A, Oral HB, Gozu RO. Circulating ICAM-1 and VCAM-1 levels in patients with obstructive sleep apnea syndrome. Respiration. 2007;74:525–32. doi: 10.1159/000097770. [DOI] [PubMed] [Google Scholar]
- 28.Guo Q, Wang Y, Li QY, Li M, Wan HY. Levels of thioredoxin are related to the severity of obstructive sleep apnea: based on oxidative stress concept. Sleep Breath. 2013;17:311–6. doi: 10.1007/s11325-012-0692-4. [DOI] [PubMed] [Google Scholar]
- 29.Lentini S, Manka R, Scholtyssek S, Stoffel-Wagner B, Luderitz B, Tasci S. Creatine phosphokinase elevation in obstructive sleep apnea syndrome: an unknown association? Chest. 2006;129:88–94. doi: 10.1378/chest.129.1.88. [DOI] [PubMed] [Google Scholar]
- 30.Shah ZA, Jortani SA, Tauman R, Valdes R, Jr, Gozal D. Serum proteomic patterns associated with sleep-disordered breathing in children. Pediatr Res. 2006;59:466–70. doi: 10.1203/01.pdr.0000198817.35627.fc. [DOI] [PubMed] [Google Scholar]
- 31.Kheirandish-Gozal L, McManus CJ, Kellermann GH, Samiei A, Gozal D. Urinary neurotransmitters are selectively altered in children with obstructive sleep apnea and predict cognitive morbidity. Chest. 2013;143:1576–83. doi: 10.1378/chest.12-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takagi T, Morser J, Gabazza EC, et al. The coagulation and protein C pathways in patients with sleep apnea. Lung. 2009;187:209–13. doi: 10.1007/s00408-009-9152-8. [DOI] [PubMed] [Google Scholar]
- 33.Brockmann PE, Schaefer C, Poets A, Poets CF, Urschitz MS. Diagnosis of obstructive sleep apnea in children: a systematic review. Sleep Med Rev. 2013;17:331–40. doi: 10.1016/j.smrv.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Nerfeldt P, Aoki F, Friberg D. Polygraphy vs. polysomnography: missing osas in symptomatic snorers--a reminder for clinicians. Sleep Breath. 2014;18:297–303. doi: 10.1007/s11325-013-0884-6. [DOI] [PubMed] [Google Scholar]