Abstract
Study Objectives:
The impact of hospitalization on sleep in late-life is underexplored. The current study examined patterns of sleep quality before, during, and following hospitalization, investigated predictors of sleep quality patterns, and examined predictors of classification discordance between two suggested clinical cutoffs used to demarcate poor/good sleep.
Methods:
This study included older adults (n = 163; mean age 79.7 ± 6.9 years, 31% female) undergoing inpatient post-acute rehabilitation. Upon admission to inpatient post-acute rehabilitation, patients completed the Pittsburgh Sleep Quality Index (PSQI) retrospectively regarding their sleep prior to hospitalization. They subsequently completed the PSQI at discharge, and 3 months, 6 months, 9 months, and 1 year post discharge. Patient demographic and clinical characteristics (pain, depression, cognition, comorbidity) were collected upon admission.
Results:
Using latent class analysis methods, older adults could be classified into (1) Consistently Good Sleepers and (2) Chronically Poor Sleepers based on patterns of self-reported sleep quality pre-illness, during, and up to 1 year following inpatient rehabilitation. This pattern was maintained regardless of the clinical cutoff employed (> 5 or > 8). Logistic regression analyses indicated that higher pain and depressive symptoms were consistently associated with an increased likelihood of being classified as a chronic poor sleeper. While there was substantial classification discordance based on clinical cutoff employed, no significant predictors of this discordance emerged.
Conclusions:
Clinicians should exercise caution in assessing sleep quality in inpatient settings. Alterations in the cutoffs employed may result in discordant clinical classifications of older adults. Pain and depression warrant detailed considerations when working with older adults on inpatient units when poor sleep is a concern.
Citation:
Dzierzewski JM, Mitchell M, Rodriguez JC, Fung CH, Jouldjian S, Alessi CA, Martin JL. Patterns and predictors of sleep quality before, during, and after hospitalization in older adults. J Clin Sleep Med 2015;11(1):45–51.
Keywords: sleep, hospitalization, aging, latent class analysis, clinical cutoffs
With the exception of descriptive investigations on sleep during acute hospital stays1,2 and our prior work on the longitudinal patterns of changes in sleep quality among older adults after acute health events,3 little is known regarding the impact of acute health changes and hospitalization on sleep in late-life. However, it is known that as an individual increases in age sleep complaints and sleep disorders become a common phenomenon.4,5 In addition, long-term changes in health status, pain symptoms, and cognitive impairment are factors with known negative associations with sleep disturbances in older adults.6–12 This paper aims to extend previous work and examine patterns and predictors of sleep quality throughout the hospitalization process (i.e., from pre-admission through 1 year post-discharge follow-up).
It is critical to better understand the sleep of older adults during acute health changes and hospitalization, as sleep in these settings has been found to be of particularly poor quality, with increased daytime sleeping and poor nighttime sleep.13,14 Additionally, poor sleep in hospitalized older adults has been associated with less functional improvement during and for up to 3 months after discharge, increased mortality risk within one year, and decreased cognitive functioning following discharge.12–14 While these findings suggest that disturbed sleep represents an important independent risk factor for poor outcomes among older adults recovering from acute health events and hospitalizations, questions remain concerning (1) how sleep quality may change throughout the course of an acute health event and/ or hospitalization, and (2) the optimal methods to assess sleep disturbances in older adults during this process so interventions can be effectively targeted.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Health factors are suspected to play a role in sleep changes with the aging process. However, little is known regarding the impact of acute health changes and hospitalization on sleep in older adults.
Study Impact: Sleep quality does not appear to significantly change throughout the hospitalization process; however, slight alterations in clinical cutoffs used to demarcate poor sleep can lead to drastic changes in classification status. Caution is suggested for clinicians working with hospitalized elders using questionnaires to identify patients with poor sleep.
The Pittsburgh Sleep Quality Index (PSQI) represents a potentially useful questionnaire to monitor sleep disturbances in older adults as they move through the healthcare continuum. However, strategies for using the PSQI to monitor sleep changes in older adults undergoing hospitalization have not been explored. Authors have suggested clinical threshold cutoffs to distinguish between “disturbed” and “normal” sleep. The most common cutoff, which was suggested in the original validation study, is a score greater than 5 representing poor sleep.15 However, other authors have suggested a higher cutoff score (> 8) for poor sleep for individuals with physical comorbidities.16 We recently examined the pattern of sleep quality before, during, and after inpatient post-acute rehabilitation using latent class analysis (LCA) with PSQI scores used in a continuous fashion, and not scored dichotomously as is common clinical practice.3
Here we extend our prior work and examine the PSQI as it is commonly used clinically, with threshold cutoffs using two different scores to indicate poor sleep (> 5 and > 8), examine predictors of classification, and examine predictors of discrepancies in classification based on the cutoffs employed. Using LCA, we first examined patterns of poor sleep based on a PSQI > 5.15 We then examined predictors of class membership to determine whether clinical information gathered during post-acute rehabilitation would predict class membership. In a second analysis, we repeated these steps using a PSQI > 8 cutoff score.16 We specifically hypothesized that age, comorbidity burden, cognitive functioning, pain symptoms, depression, and reason for rehabilitation admission (orthopedic versus all other) would be significant predictors of membership in classes with good versus poor self-reported sleep quality with the PSQI > 5 cutoff; while age and comorbidity burden would no longer be significant predictors of class membership with the PSQI > 8 cutoff, since that cutoff was identified for use with medically ill patients. Lastly, if we observed substantial discrepancy in classification between the PSQI > 5 and PSQI > 8 cutoffs, we planned to examine whether clinical variables would predict a patient's likelihood of discordant classification. No hypothesis was made regarding predictors of classification discordance between PSQI > 5 and PSQI > 8 cutoffs, as this aim was exploratory in nature.
METHODS
Participants
A detailed description of the study methods and participants have been published previously.3,13 Briefly, participants were drawn from a prospective cohort study of older post-acute rehabilitation patients. A total of 245 participants aged ≥ 65 years provided written informed consent and had their self-reported sleep quality assessed across 6 measurement occasions via the PSQI. Exclusion criteria included: residence in a nursing home prior to hospital admission, severe medical illness (e.g., end of life care), behavioral disturbance (e.g., dementia with severe agitation), or inability to communicate verbally in English. One hundred sixty-three individuals (67% of the total sample) provided ≥ 1 PSQI score during the post-discharge follow-up period (at 3-, 6-, 9-, or 12-month follow-up) and were included in the analyzed sample. Research methods were reviewed and approved by the Veterans Administration Greater Los Angeles Healthcare System Institutional Review Board.
Procedures
Post-acute rehabilitation offers an optimal setting to investigate the impact of health on sleep in old age, as these settings are commonly used in the US healthcare system, and operate with the explicit goal of improving functional status and facilitating a return to independent living. All patients over age 65 admitted for rehabilitation services (i.e., for physical, occupational or kineseotherapy; n = 996) at 2 post-acute care sites (a freestanding, for-profit, community nursing home focused on short-term rehabilitation, and an inpatient rehabilitation unit within a Veterans Administration Medical Center) were approached for screening and consent immediately following admission. Following enrollment, a baseline assessment interview was completed. The interview was completed in 2 sessions. The first interview session was completed immediately following enrollment. The second interview session was completed one week later, or immediately preceding discharge if the inpatient post-acute rehabilitation stay was < 7 days (n = 6). The following information was gathered through a structured medical record review by a trained research nurse following discharge: medications taken during rehabilitation, reason for rehabilitation (orthopedic rehabilitation versus all others), length of acute inpatient rehabilitation stay, and medical record information needed for completion of the comorbidity measure.
Long-term follow-up assessments were conducted at 3-, 6-, 9-, and 12-months from the date of admission to the rehabilitation facility. The 3-, 6-, and 9-month follow-up assessments were conducted in-person at the research participant's residence, while the 12-month follow-up assessment was conducted by telephone. If in-person interviews could not be completed because the participant had moved out of the area after discharge or preferred not to have a research associate in their residence, the assessment was conducted by telephone. Thirty-three percent of all 3-, 6-, and 9-month follow-up interviews were conducted by telephone.
Sleep Measures
Self-reported sleep quality was repeatedly assessed with the PSQI.15 The PSQI contains 19 items (score range 0–21) and assesses overall sleep quality. The PSQI is a well-validated, commonly-used self-report measure of sleep quality used to aid in the assessment of sleep disturbance.15 The PSQI has been used in a variety of populations, including: children, college students, older adults, physically healthy and unhealthy individuals, and those with and without disturbed sleep.15,17–25 Additionally, the PSQI has been used in a multitude of settings, including out-patient clinics and inpatient wards, specialty sleep clinics, and primary care offices.15,26,27
At study enrollment (shortly following inpatient acute rehabilitation admission), participants completed the PSQI regarding their sleep for the 1-month period “before their recent illness.” This pre-illness PSQI was intended to describe their sleep quality prior to the events that led to hospitalization and ultimately the post-acute inpatient rehabilitation stay. The PSQI was then administered one week later, in an attempt to assess their sleep during the post-acute rehabilitation (7-day PSQI). At 3-, 6-, 9-, and 12-months following rehabilitation admission, participants were contacted and the PSQI was repeated (either in person or by telephone) to assess sleep quality over the preceding week.
Other Measures
Age, gender, ethnicity, and reason for post-acute inpatient rehabilitation admission were obtained for each participant from the transferring hospital discharge records and rehabilitation facility medical records. Global cognitive functioning was assessed with the Mini-Mental State Examination (MMSE), a 20-item measure in which higher scores suggest better cognitive functioning (score range = 0–30), during the rehabilitation stay.28 Patients who scored < 15 on the MMSE were excluded from the study, as many of the study measures have not been validated with patients with severely impaired cognitive functioning. Depressive symptoms experienced during the rehabilitation stay were quantified with the 15-item version of the Geriatric Depression Scale (GDS-15).29 The GDS-15 is a well validated, self-report measure of geriatric depression (score range = 0–15, higher score indicate more depressive symptoms).29 Illness severity and comorbidity burden during acute inpatient rehabilitation was computed with the Cumulative Illness Rating Scale for Geriatrics (CIRS-G).30,31 This measure was completed by an experienced research registered nurse, using data gathered during a brief physical examination by a study physician and abstracted medical record data. The CIRSG quantifies comorbidity through rating of 14 body systems (heart, respiratory, liver, etc.) on a continuum from no impairment to extremely severe impairment.30,31 The experience of pain during the rehabilitation stay was assessed with a modified version of the Geriatric Pain Measure [(GPM) score range 0–29; higher scores indicate worse pain].32
Statistical Analysis
To evaluate patterns of sleep quality over time, we developed two series of LCA models. A LCA model is akin to a factor analysis, in that both seek to use observed variables to identify underlying latent variables. Where a factor analysis seeks to identify continuous underlying factors, LCA seeks to identify latent nominal categories (classes).33 In this study, LCA was used to identify different latent classes of sleep quality observed at 6 time points: (1) pre-illness, assessed on admission to rehabilitation, (2) during rehabilitation, assessed one week after admission, (3) 3-month follow-up, (4) 6-month follow-up, (5) 9-month follow-up, and (6) 12-month follow-up. The first series of LCA models used PSQI score > 5 as the demarcation between good and poor sleep. The second series of LCA models used PSQI score > 8 as the demarcation between good and poor sleep.
We then tested logistic regression models predicting membership in each of the classes for each of the PSQI cutoff scores. The models included age, CIRS-G score, MMSE score, GPM score, GDS-15 score, and reason for admission (orthopedic vs. all others) as predictors of class membership. For all statistical tests, a p-value < 0.05 was considered statistically signifi-cant. LCA were performed using Mplus (version 7), utilizing Maximum Likelihood estimation, which uses all available data. Logistic regressions were conducted using Stata Version 13.1.
RESULTS
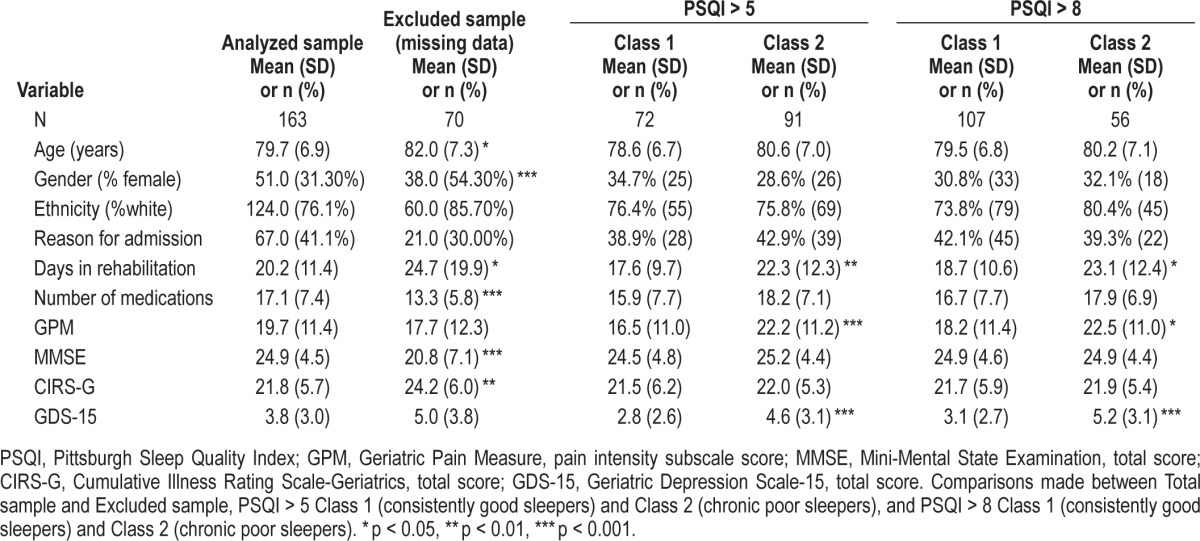
Nine hundred sixty-six patients over 65 years of age were admitted for rehabilitation during the study period. The final sample was composed of 244 patients (28 patients refused initial screening, 106 experienced early discharge/death, 24 did not receive rehabilitation, 8 were identified more than 7 days into their rehabilitation stay, 15 were too ill or behaviorally disturbed, 11 had resided in a nursing home prior to admission, 37 had difficulty communicating in English, and 492 refused study enrollment). We previously reported that, other than an expected gender difference (43.0% vs. 96.6% men at facilities A and B, respectively), there were few differences between the two study sites.13 T-tests comparing participants retained in the current analysis (n = 163, mean age 79.7 ± 6.9 years) with those excluded due to absence of post-discharge follow-up data (n = 70) revealed no differences in pre-illness PSQI (p = 0.948) or 7-day PSQI scores (p = 0.401). As such, we proceeded with our planned analysis for the combined sample from both sites with confidence that results would not be biased by systematically missing data. Overall, 67% of participants provided PSQI data at the 3-month follow-up or later. There were no differences in missing follow-up observations across the 2 latent classes. Refer to Table 1 for demographic and clinical characteristics of participants.
Table 1.
Characteristics of the overall sample at baseline (n = 163), excluded sample (n = 70), and of study participants in each of the 2 classes based on each of the PSQI cutoff scores (> 5 and > 8).
Patterns of Sleep Quality
We first examined patterns of sleep quality based on a PSQI > 5 cutoff. A score of 1 was assigned if the patient had a PSQI score > 5 (associated with poor sleep quality), and a score of 0 was assigned if the patient had a PSQI score ≤ 5 (associated with good sleep quality). A latent class analysis was then performed on these dichotomous PSQI scores (where 1 indicated poor sleep). Using the bootstrap likelihood ratio test,34 the latent class analysis indicated that a 2-class solution fit significantly better than a 1-class solution (p < 0.001); however, a 3-class solution was not significantly better than a 2-class solution (p = 0.124). The 2-class model also was superior in terms of BIC (965.1 and 985.9 for the 2- and 3-class models, respectively, where smaller BIC values reflect better model fit). As a result, the 2-class solution was adopted.
In the 2-class model, Class 1 was typified by having a low probability of poor sleep (PSQI > 5) and Class 2 was typified by having a high probability of poor sleep. The largest proportion (56%) of participants fell into Class 2 (chronic poor sleep), while 44% of participants fell into Class 1 (consistently good sleep). In terms of the classification quality of the 2-class LCA model, the entropy of the 2-class model was 0.711. Each participant was assigned to the class in which they had the highest probability of membership. The mean probability of classification into the correct class was 0.931 for Class 1 and 0.921 for Class 2, suggesting that members of each class were likely to be accurately classified (i.e., as the probability approaches 1, the probability of incorrect classification diminishes).
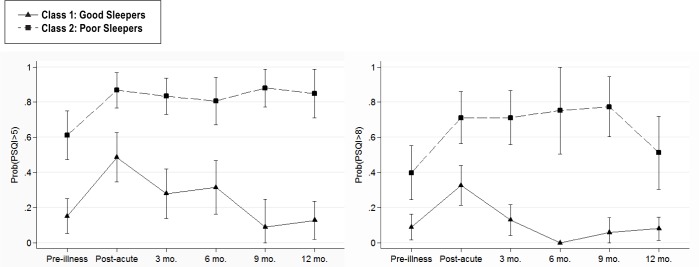
We next examined patterns of sleep quality based on PSQI > 8 cutoff. The above LCA was repeated using a PSQI score > 8 as the demarcation between good and poor sleep. The latent class analysis again indicated that a 2-class solution fit significantly better than a 1-class solution (p < 0.001); however, a 3-class solution was not significantly better than a 2-class solution (p = 0.264). The 2-class model also was superior in terms of BIC (847.2 and 870.8 for the 2- and 3-class models, respectively, where smaller BIC values reflect better model fit). As a result, the 2-class solution was adopted. Again, Class 1 was typified by having a low probability of poor sleep (PSQI > 8) and Class 2 was typified by having a high probability of poor sleep. The largest proportion (66%) of participants fell into Class 1 (consistently good sleep), while 34% of participants fell into Class 2 (chronic poor sleep). The entropy of the 2-class model was 0.736. The mean probability of classification into the correct class was 0.913 for Class 1 and 0.941 for Class 2. Figure 1 shows the pattern of PSQI scores across the 2 classes (for both PSQI > 5 cutoff and PSQI > 8 cutoff), showing separate lines for each class.
Figure 1. Probability of PSQI scores above suggested clinical cutoffs (PSQI > 5 left panel; PSQI > 8 right panel) at each time point (pre-illness, during post-acute rehabilitation, and 3-, 6-, 9- and 12-months follow-up).
Predictors of Sleep Quality
Based on the results of the LCA utilizing PSQI > 5 cutoff, participants were assigned to a class based on their modal probability of class membership, and the class membership was treated as an observed variable. This resulted in a dummy variable with a code of 1 for those belonging to Class 1 (good sleepers) and a code of 0 for those belonging to Class 2 (poor sleepers). Logistic regression analysis was performed predicting class membership from age, CIRS-G score, MMSE score, GPM score, GDS-15 score, and reason for admission (orthopedic vs. non-orthopedic) [χ2(6) = 29.94; p < 0.001]. The model results are shown in Table 2. Older age was associated with a lower likelihood of being a good sleeper (OR = 0.94, p = 0.016). In addition, higher GPM scores and higher GDS-15 scores were associated with a lower likelihood of being a good sleeper (OR = 0.96, p < 0.007; OR = 0.81, p = 0.001, respectively).
Table 2.
Logistic regressions predicting class membership based on (1) PSQI > 5, (2) PSQI > 8, and (3) classification discordance.
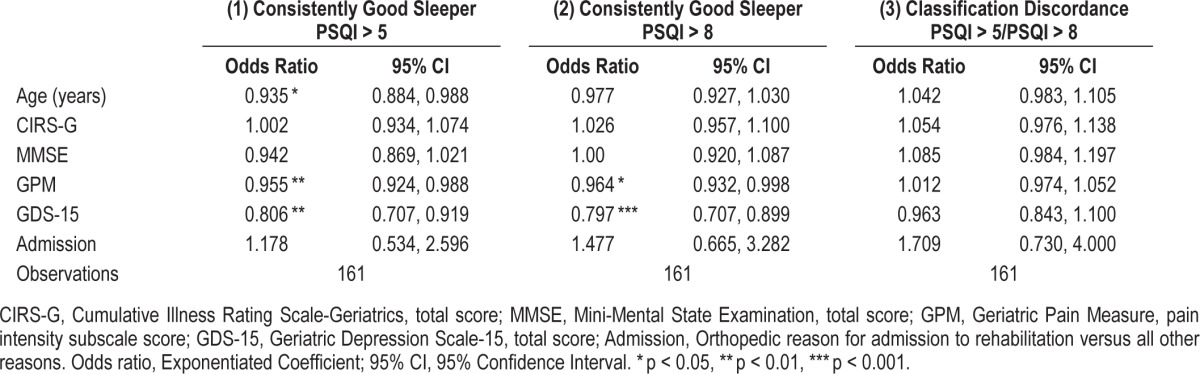
Based on the results of the LCA utilizing the PSQI > 8 cutoff, participants were assigned to a class based on their modal probability of class membership, and class membership was treated as an observed variable. Again, a logistic regression analysis was performed predicting class membership from age, CIRS-G score, MMSE score, GPM score, GDS-15 score, and reason for admission (orthopedic vs. non-orthopedic). The overall contribution of all of the predictors was significant [χ2(6) = 22.82, p < 0.001]. The model results are shown in Table 2. Higher GPM scores and higher GDS-15 scores were associated with a lower likelihood of being a good sleeper using this cutoff score (OR = 0.96, p = 0.039; OR = 0.80, p < 0.001, respectively).
Classification Discordance Based on PSQI Cutoff
The discordance in classification of the two latent class analyses was assessed by cross-tabulating class membership. Just over 75% of participants (77.3%) were classified in a concordant fashion across models as being consistently good sleepers or chronic poor sleepers. A total of 107 patients were classified as good sleepers according to the PSQI > 8 cutoff. Of those, 71 were concordantly classified with the PSQI > 5 cutoff as good sleepers. There were 36 participants that were discordantly classified (i.e., classified differently depending on which PSQI cutoff was used). Refer to Table 1 for demographic and clinical characteristics of participants classified as consistently good sleepers and chronic poor sleepers based on PSQI > 5 and PSQI > 8 cutoffs.
A logistic regression analysis was performed with “discordant” as the outcome variable (1 representing being classified as chronic poor sleeper by the PSQI > 5 cutoff but being classified as a consistently good sleeper by the PSQI > 8 cutoff). The predictors entered into the logistic regression included: age, CIRSG comorbidity score, MMSE score, GPM pain score, GDS-15 depression score, and reason for admission (orthopedic vs. non-orthopedic). The overall contribution of all of the predictors was not significant [χ2(6) = 8.29, p = 0.218]. The model results are shown in Table 2. Discordant classification was not significantly associated with any of the included predictor variables.
DISCUSSION
The results of these analyses revealed two distinct patterns of sleep quality before, during, and for one year following post-acute rehabilitation in older adults: (1) consistently good sleepers, and (2) chronic poor sleepers. Pain and depressive symptoms were consistently associated with classification in the chronic poor sleep group. While roughly half of patients were considered consistently poor sleepers based on a PSQI > 5 cutoff, this number dropped to approximately a third when the cutoff score was changed to PSQI > 8. No significant predictors of this classification discordance were discovered.
Older age, more pain, and more depression all were related to increased odds of being classified as a chronic poor sleeper based on a PSQI > 5 cutoff. However, when class membership was predicted based on a PSQI > 8 cutoff, only more pain and more depression were associated with increased odds of being classified as a chronic poor sleeper. As such, pain and mood symptoms appear to be the most robust predictors of whether older adults will experience consistently good versus chronically poor sleep throughout the course of an acute health event and through the recovery period.
The relationship between chronically poor sleep and pain may be partially explained by the known association between poor sleep and pain in healthy adults.9,10 Depressive symptoms were also a significant predictor of class membership indicating consistently good versus chronic poor sleep quality. Given the literature suggesting strong relationships between depressive symptoms and sleep disturbance in late life,35 this significant association is not surprising. As depressive symptoms, pain, and sleep disturbances are all associated with one another,36 these variables may interact and exacerbate one another. Additional investigation into the interrelationships among pain, depressed mood, and sleep, especially in at-risk populations, is needed.
Older age was predictive of membership in the chronic poor sleeper group when the PSQI > 5 cutoff was used. However, this variable failed to maintain significance when the cutoff was changed to PSQI > 8. As the higher PSQI cutoff has been recommended in individuals with physical comorbidities,16 and as increased age is related to an increase in physical complications, it is not surprising age was no longer predictive of group membership with the higher PSQI cutoff score. Future research should further examine the potential utility of graded clinical PSQI cutoffs based on patient age.
Interestingly, we did not observe an association between comorbidity burden and sleep classification. Studies demonstrating an increased prevalence of poor sleep in late life have often times cited poor physical health as the primary explanatory variable.6,8 The current investigation utilized a gold standard assessment of comorbidity burden that is quantified via a medical record review and physical examination. This variable was not predictive of membership in the chronic poor sleep class, regardless of PSQI cutoff. Poor physical health appears to be a less important predictor of chronically poor sleep than either depression or pain symptoms in a sample of medically ill older adults.
While we observed substantial classification discordance between PSQI > 5 and PSQI > 8, we were not able to identify significant predictors of this discordance. This may have been due to reduced power to detect significant associations, as the number of older patients discordantly classified was only 36 (i.e., those classified as poor sleepers based on PSQ > 5 cutoff and classified as good sleepers based on PSQI > 8 cutoff). Alternatively, perhaps we did not measure pertinent variables to classification discordance. Future investigations would be wise to assess for a wide range of clinical characteristics in order to more fully examine predictors of classification discordance between recommended clinical cutoffs for PSQI.
There are several limitations to the current investigation. We could not examine covariation of sleep classification with predictor variables due to a lack of repeated assessments of some covariates. Additionally, while the PSQI was retrospectively assessed pre-illness, this was not the case for many of the other variables (e.g., depression). Temporal differences in assessment could have impacted the observed associations. Another limitation regards the nature of the sleep data. While self-report sleep does not completely overlap with objective measures of sleep, it is self-reported sleep quality that often precipitates treatment seeking and determines whether a treatment can be deemed successful. Some older adults with MMSE scores below traditional cutoffs suggestive of impaired cognitive functioning (MMSE < 24) were included in the sample, and while it is possible that inclusion of these older patients may have altered the observed patterns of sleep, follow-up analysis (not reported) excluding participants with an MMSE < 24 revealed no changes in the patterns of obtained results or predictors of classification between those with normal versus low MMSE scores. Lastly, while two different suggested PSQI cutoffs were examined, we were unable to examine the precision of the various cutoffs. Without corroborating evidence of objective sleep disturbance, we were unable to determine the most appropriate PSQI cutoffs to use with older adults before, during, and after an acute medical problem. Future investigations should include multiple indicators of sleep health to aid in evaluation of PSQI characteristics.
Clinically there are several important implications of the current study. Both pain and depressive symptoms were associated with increased odds of being classified as a chronic poor sleeper. There is evidence that both pain and depressive symptoms can be effectively managed in late-life.37,38 Additionally, recent evidence suggests that pain and sleep disturbance and depression and sleep disturbance can be successfully treated simultaneously in outpatient settings.39,40 Adaptation of these interventions for inpatient settings should be explored. Given the availability of promising treatment approaches, early screening for pain or depressive symptoms and sleep disturbances in older rehabilitation patients should be implemented. Such a practice could potentially prevent the development or sustainment of poor sleep quality among older adults, which may have downstream positive effects on outcomes of rehabilitation and quality-of-life among older patients. Lastly, given the large impact of minor changes in the PSQI cutoff, care is needed in using the PSQI to screen and evaluate older patients for disturbed sleep.
LCA methodology proved a useful technique for examining the patterns of PSQI-rated sleep quality before, during, and after inpatient rehabilitation in older adults. Regardless of the PSQI cutoff employed, a two-class model indicating consistent good sleep and chronic poor sleep emerged. The identified classes provided a useful framework for testing potential predictors of sleep health in older adults experiencing an acute health event. Pain and depression emerged as robust predictors of membership in the chronic poor sleep group. Questions remain regarding the nature of sleep changes before, during, and after hospitalization in older adults. We previously examined the PSQI, scored continuously, throughout the course of the rehabilitation process and found a 4-class model.3 Both the PSQI > 5 and PSQI > 8 cutoffs yielded a very similar pattern of classifications as the 4-class solution with respect to those with consistently good sleep and chronically poor sleep; however, the 4-class solution based on continuous PSQI differed by further distinguishing those patients who transitioned from good to poor sleep and those who transitioned from poor to good sleep during the study time period. While the PSQI, scored in a binary fashion, provided a means to examine the patterns and predictors of sleep change in response to acute inpatient rehabilitation, the overall depiction was very granular. Perhaps the PSQI, traditionally scored, may not be a very sensitive measure to changes before, during, or after rehabilitation services in older adults. Future research should examine more sensitive ways to track sleep changes in older adults undergoing rehabilitation services.
DISCLOSURE STATEMENT
This work was performed at the University of California, Los Angeles and the VA Greater Los Angeles Healthcare System. This work was supported by UCLA Claude Pepper Older Americans Independence Center by NIA (5P30AG028748 & AG-10415), NIH/NCATS UCLA CTSI (UL1TR000124), NIA K23 AG028452, VA Health Services Research & Development IIR-01-053-1, IIR 04-321-2, and AIA-03-047, VA Advanced Geriatrics Fellowship Program, and VA Greater Los Angeles Healthcare System Geriatric Research, Education and Clinical Center. CHF was supported by National Institute on Aging (K23AG045937). The content is solely the responsibility of the authors and not necessarily the responsibility of the funding agencies (VA or NIH). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Mr. Sergio Martinez, Ms. Terry Vandenberg, Ms. Christina Kurtz, and Ms. Karen Josephson for their assistance with the project. We thank the participating facilities and their staff.
REFERENCES
- 1.Isaia G, Corsinovi L, Bo M, et al. Insomnia among hospitalized elderly patients: prevalence, clinical characteristics and risk factors. Arch Gerontol Geriatr. 2011;52:133–7. doi: 10.1016/j.archger.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Drouot X, Cabello B, d'Ortho MP, Brochard L. Sleep in the intensive care unit. Sleep Med Rev. 2008;12:391–403. doi: 10.1016/j.smrv.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Martin JL, Dzierzewski JM, Mitchell M, Fung CH, Jouldjian S, Alessi CA. Patterns of sleep quality during and after postacute rehabiliation in older adults: a latent class analysis approach. J Sleep Res. 2013;22:640–7. doi: 10.1111/jsr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiological study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 5.Dzierzewski JM, O'Brien EM, Kay D, McCrae CS. Tackling sleeplessness: psychological treatment options for insomnia in older adults. Nat Sci Sleep. 2010;2:47–61. doi: 10.2147/NSS.S7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the American Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Foley DJ, Vitiello MV, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15:344–50. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 8.Vitiello MV, Moe KE, Prinz PN. Sleep complaints cosegregate with illness in older adults: clinical research informed by and informing epidemiological studies of sleep. J Psychosom Res. 2002;53:555–9. doi: 10.1016/s0022-3999(02)00435-x. [DOI] [PubMed] [Google Scholar]
- 9.Dzierzewski JM, Williams JM, Roditi D, et al. Daily variations in nighttime sleep and subjective morning pain in older adults with insomnia; Evidence of covariation over time. J Am Geriatr Soc. 2010;58:925–30. doi: 10.1111/j.1532-5415.2010.02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 12.Dzierzewski JM, Fung CH, Jouldjian S, Alessi CA, Irwin MR, Martin JL. Decrease in daytime sleeping is associated with improvement in cognition after hospital discharge in older adults. J Am Geriatr Soc. 2014;62:47–53. doi: 10.1111/jgs.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alessi CA, Martin JL, Webber AP, et al. More daytime sleeping predicts less functional recovery among older people undergoing inpatient post-acute rehabilitation. Sleep. 2008;31:1291–300. [PMC free article] [PubMed] [Google Scholar]
- 14.Martin JL, Fiorentino L, Jouldjian S, Mitchell M, Josephson KR, Alessi CA. Poor self-reported sleep quality predicts mortality within one year of inpatient post-acute rehabilitation among older adults. Sleep. 2011;134:1715–21. doi: 10.5665/sleep.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CFI, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 17.Ucer O, Gumus B. Quantifying subjective assessment of sleep quality, quality of life and depressed mood in children with enuresis. World J Urol. 2014;32:239–43. doi: 10.1007/s00345-013-1193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda T, Nagai T, Kato-Nishimura K, Mohri I, Taniike M. Sleep problems in physically disabled children and burden on caregivers. Brain Dev. 2012;34:223–9. doi: 10.1016/j.braindev.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Cheuk YS, Wong WS. The effect of optimism on depression: the mediating and moderating role of insomnia. J Health Psychol. 2011;16:1251–8. doi: 10.1177/1359105311407366. [DOI] [PubMed] [Google Scholar]
- 20.Clegg-Kraynok MM, McBean AL, Montgomery-Downs HE. Sleep quality and characteristics of college students who use prescription psychostimulants nonmedically. Sleep Med. 2011;12:598–602. doi: 10.1016/j.sleep.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Beaudreau SA, Spira AP, Stewart A, et al. Validation of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older black and white women. Sleep Med. 2012;13:36–42. doi: 10.1016/j.sleep.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spira AP, Beaudreau SA, Stone KL, et al. Reliability and validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older men. J Gerontol A Biol Sci Med Sci. 2012;67A:433–9. doi: 10.1093/gerona/glr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomfohr LM, Schweizer CA, Dimsdale JE, Loreda JS. Psychometric characteristics of the Pittsburgh Sleep Quality Index in English speaking non-Hispanic whites and English and Spanish speaking Hispanics of Mexican descent. J Clin Sleep Med. 2013;15:61–6. doi: 10.5664/jcsm.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saint Martin M, Sforza E, Barthelemy JC, Thomas-Anterion C, Roche F. Sleep perception in non-insomniac healthy elderly: a three-year longitudinal study. Rejuvenation Res. 2014;17:11–8. doi: 10.1089/rej.2013.1457. [DOI] [PubMed] [Google Scholar]
- 25.Lai YC, Huang MC, Chen HC, et al. Familiality and clinical outcomes of sleep disturbances in major depressive and bipolar disorders. J Psychosom Res. 2014;76:61–7. doi: 10.1016/j.jpsychores.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Bush AL, Armento MEA, Weiss BJ, et al. The Pittsburgh Sleep Quality Index in older primary care patients with generalized anxiety disorder: psychometrics and outcomes following cognitive behavioral therapy. Psychiatry Res. 2012;199:24–30. doi: 10.1016/j.psychres.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarlata S, Pedone C, Curcio G, et al. Pre-polysomnographic assessment using the Pittsburgh Sleep Quality Index questionnaire is not useful in identifying people at higher risk for obstructive sleep apnea. J Med Screen. 2013;20:220–6. doi: 10.1177/0969141313511591. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–72. [Google Scholar]
- 30.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 31.Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a greiatric residential population. J Am Geriatr Soc. 1995;43:130–7. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 32.Ferrell BA, Stein WM, Beck JC. The Geriatric Pain Measure: validity, reliability and factor analysis. J Am Geriatr Soc. 2000;48:1669–73. doi: 10.1111/j.1532-5415.2000.tb03881.x. [DOI] [PubMed] [Google Scholar]
- 33.Vermunt JK. Latetent class modeling with covariates: two improved three-step approaches. Polit Anal. 2010;18:469. [Google Scholar]
- 34.Nylund KL, Asparouhov T, Muthen B. Deciding on the number of classes in latent class analysis and growth mixture modeling. A Monte Carlo simulation study. Struct Equ Modeling. 2007;14:535–69. [Google Scholar]
- 35.Buysse DJ. Insomnia, depression and aging. Assessing sleep and mood interactions in older adults. Geriatrics. 2004;59:47–51. [PubMed] [Google Scholar]
- 36.Chiu YH, Silman AJ, Macfarlane GJ, et al. Poor sleep and depression are independently associated with a reduced pain threshold. Results of a population based study. Pain. 2012;115:316–21. doi: 10.1016/j.pain.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Abdulla A, Bone M, Adams N, et al. Evidence-based clinical practice guidelines on management of pain in older people. Age Ageing. 2013;42:151–3. doi: 10.1093/ageing/afs199. [DOI] [PubMed] [Google Scholar]
- 38.Scogin F, Welsh D, Hanson A, Stump J, Coates A. Evidence-based psychotherapies for depression in older adults. Clin Psychol Sci Pract. 2005;12:222–37. [Google Scholar]
- 39.Pigeon WR, Moynihan J, Matteson-Rusby S, et al. Comparative effectiveness of CBT interventions for co-morbid chronic pain and insomnia: a pilot study. Behav Res Ther. 2012;50:685–9. doi: 10.1016/j.brat.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manber R, Bernert RA, Suh S, Nowakowski S, Siebern AT, Ong JC. CBT for insomnia in patients with high and low depressive symptom severity: adherence and clinical outcomes. J Clin Sleep Med. 2011;7:645–52. doi: 10.5664/jcsm.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]