Abstract
Background:
Sleep disorders are frequent in chronic kidney disease (CKD). Among them, restless legs syndrome (RLS) may affect up to 60% of patients on dialysis, and it has been related to a poor quality of life and higher cardiovascular risk. Despite its high prevalence in advanced stages of renal disease, RLS frequency in non-dialysis CKD has not been clearly established. The aim of this study was to assess the frequency of RLS in non-dialysis CKD patients (stages 2 to 4) followed in a reference nephrology outpatient clinic.
Methods:
A standardized questionnaire following the international RLS study group diagnostic criteria was self-administered by 110 patients regularly followed in the nephrology clinic. The series comprised 69 men and 41 women, aged 68 ± 13.2 years, with mean serum creatinine of 1.7 ± 0.8 mg/dL. Subsequently, patients classified as probable RLS according to the questionnaire underwent a systematic neurological examination. The presence of peripheral artery disease was evaluated by the ankle-brachial index (ABI).
Results:
The frequency of probable RLS according to the questionnaire results was 21% (17% for men and 27% for women). However, after thorough neurological examination, the diagnosis of RLS was confirmed in only 5 patients. Therefore, the overall definitive RLS frequency was 4.5% (within the prevalence reported for the general population) and was higher among women (9.7% vs 0.2%). In the remaining cases symptoms were due to leg discomfort related with other disorders. Patients with probable and improbable RLS were not significantly different in age, ABI, diabetes, and other comorbid circumstances, except for tricyclic antidepressant prescription, which was more frequent in the probable RLS group (17% vs 2%). Renal function was better in definitive RLS patients than cases classified as probable RLS by the questionnaire but not confirmed after neurological exam.
Conclusions:
Although RLS can represent an early manifestation of CKD, its prevalence seems very close to that reported for the general population. Diagnostic confirmation of RLS dramatically falls after expert examination, raising the question whether, in the study of RLS cohorts, CKD has a potentially causal relationship or is a confounding factor associated with other causes of leg discomfort.
Citation:
Calviño J, Cigarrán S, Lopez LM, Martinez A, Sobrido MJ. Restless legs syndrome in non-dialysis renal patients: is it really that common? J Clin Sleep Med 2015;11(1):57–60.
Keywords: restless legs syndrome, chronic renal failure, prevalence, RLS mimics, IRLSSG questionnaire
Sleep disorders are common among dialysis patients (up to 60%) but may also be frequent in chronic kidney disease (CKD), even before renal replacement therapy.1 Insomnia or insufficient sleep time, excessive daytime sleepiness, restless legs syndrome (RLS), and obstructive sleep apnea are the most common problems.1,2 Since the daily clinical practice of nephrologists is mainly focused on renal and cardiovascular endpoints, sleep complaints in the non-dialyzed population might be under-recognized. Except for severe cases, patients with chronic renal disease may not mention their sleep complaints to the nephrologist if not specifically asked. However, besides affecting quality of life, sleep disorders may further increase cardiovascular morbidity and mortality in the CKD population.3,4
BRIEF SUMMARY
Current Knowledge/Study Rationale: The frequency of restless legs syndrome (RLS) among patients with chronic kidney disease (CKD) is debated and may be overestimated due to co-morbidities and RLS mimics, such as vascular disease, arthritis and peripheral neuropathy. Most investigations of RLS in renal disease have studied dialysis patients. The aim of our study was to address this issue in non dialysis CKD.
Study Impact: The results of our study suggest that the prevalence of RLS in CKD may be similar to that in the general population. Expert neurological evaluation is essential for the confirmation of RLS, while self-administered questionnaires based on the consensus diagnostic criteria can lead to overestimation of the frequency of RLS among patients with kidney diseases.
There is growing interest to improve identification of patients with RLS among CKD since, in addition to a negative impact on sleep and quality of life, it is associated with increased morbidity and mortality.5 RLS is characterized by unpleasant sensations in the legs causing an urge to move them. These symptoms usually arise in the first part of the night and worsen while sitting or resting.6 RLS, which is common in the general population and may dramatically decrease quality of life, can be familial, idiopathic, or associated with a miscellaneous spectrum of disorders, including iron deficiency, Parkinson disease, multiple sclerosis, hypertension, chronic obstructive pulmonary disease, depression, pregnancy, sleep apnea, and CKD.7–12 Alcohol, tobacco, and caffeine consumption also increase the risk of RLS.13 Renal disease, particularly end-stage renal disease (ESRD), is one of the most common disorders associated with RLS.8,11,14 In uremia, RLS has been mostly related to ESRD, with the reported prevalence among dialysis patients being as high as 50%.1–4 However, the prevalence of RLS in non-dialysis CKD patients, as well as its association with the severity of renal dysfunction is still unclear. The aim of this study was to ascertain the prevalence of RLS in patients with CKD (stages 2 to 4) and its relationship with renal function and other parameters.
METHODS
Based on the diagnostic criteria of the International Restless Legs Syndrome Study Group (IRLSSG), we prepared a written questionnaire with the following questions:
Do you experience an imperative need to move your legs accompanied or caused by discomfort or unpleasant sensation difficult to describe?
Does moving your legs (e.g., walking, stretching), at least temporally, relieve these sensations?
Do these unpleasant sensations occur or worsen at rest, lying or sitting or during inactivity, and improve with physical activity?
Are these sensations or urge to move generally worse in the evening or at night than during the day?
Each question matches one of the 4 RLS diagnostic criteria.6 Between March and June 2013, 125 patients (81 men and 44 women) with CKD stages 2 to 4 followed at the nephrology out-patient clinic answered the questionnaire. Subjects under 18 years old, pregnant women, patients on dialysis, and renal transplant patients were not included. Patients with 4 affirmative answers were classified as probable RLS and were then asked about severity through the IRLSSG rating scale standard scale,15 which includes 10 items evaluating both frequency and severity of symptoms over the previous week. A 4-point score was employed, where 0 denoted no symptoms and 4 indicated very severe symptoms. Only the IRLS scores of those patients who were later confirmed to have RLS were considered for subsequent analyses.
Demographic characteristics and comorbid clinical conditions, such as heart failure, chronic obstructive pulmonary disease, depression, and diabetes were recorded. Medications that may exacerbate symptoms including serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, dopamine antagonists (e.g., antiemetics), and antihistamine drugs were also registered. Peripheral artery disease (PAD) was screened by the ankle-brachial index (ABI). Laboratory data including serum creatinine, blood urea nitrogen (BUN), sodium, potassium, uric acid, proteinuria (albumin to creatinine ratio), calcium, phosphorus, parathormone, vitamin D, C-reactive protein, hemoglobin, fo-late, and iron status were obtained from routine blood and urine samples. A neurologist thoroughly examined all the patients with affirmative answer to all 4 questions to rule out conditions that may mimic RLS, such as cramps, neuropathic pain, and postural-induced myoclonus, as well as ruling out other underlying neurological disease responsible for the symptoms.
Statistical Analysis
The commercially available software SPSS (version 15.0) was used for statistical analysis of differences between the patient groups defined as probable or improbable RLS by the RLS questionnaire, as well as the definitive RLS group, as determined by neurological assessment. All results were expressed as mean and standard deviation. Coefficients of skewness and kurtosis were used to evaluate the normal distribution of the data. The χ2 test or Fisher exact test, as appropriate, were used to compare categorical variables. Differences in continuous variables were tested using the Mann-Whitney U-test or Kruskal-Wallis. P-values < 0.05 were considered significant.
RESULTS
Of 125 patients enrolled during the study period, 15 were excluded from further analysis due to incompletely or incorrectly filled-out RLS questionnaire. The final group consisted of 110 patients, (69 men, 41 women) aged 68 ± 13.3 years. Twenty-three (21%) gave 4 affirmative answers to the screening questionnaire and were thus classified as probable RLS. Distribution by age, sex, associated clinical conditions, and medications among subjects with probable and improbable RLS are summarized in Table 1. There were no differences regarding age, diabetes, or comorbid disorders between these 2 groups. The percentage of patients with depression showed a trend to be higher in the probable RLS group, and tricyclic antidepressant therapy was significantly more frequent among probable RLS patients (17% vs 2%). In men the rate of probable RLS was 17% (12 of 69 patients), whereas in women the frequency was significantly higher (27%, 11 of 41 cases). No differences were found in renal function, parathyroid, vitamin D, calcium/phosphate metabolism, and lipid profile between groups (Table 2).
Table 1.
Patient characteristics according to probability of RLS.
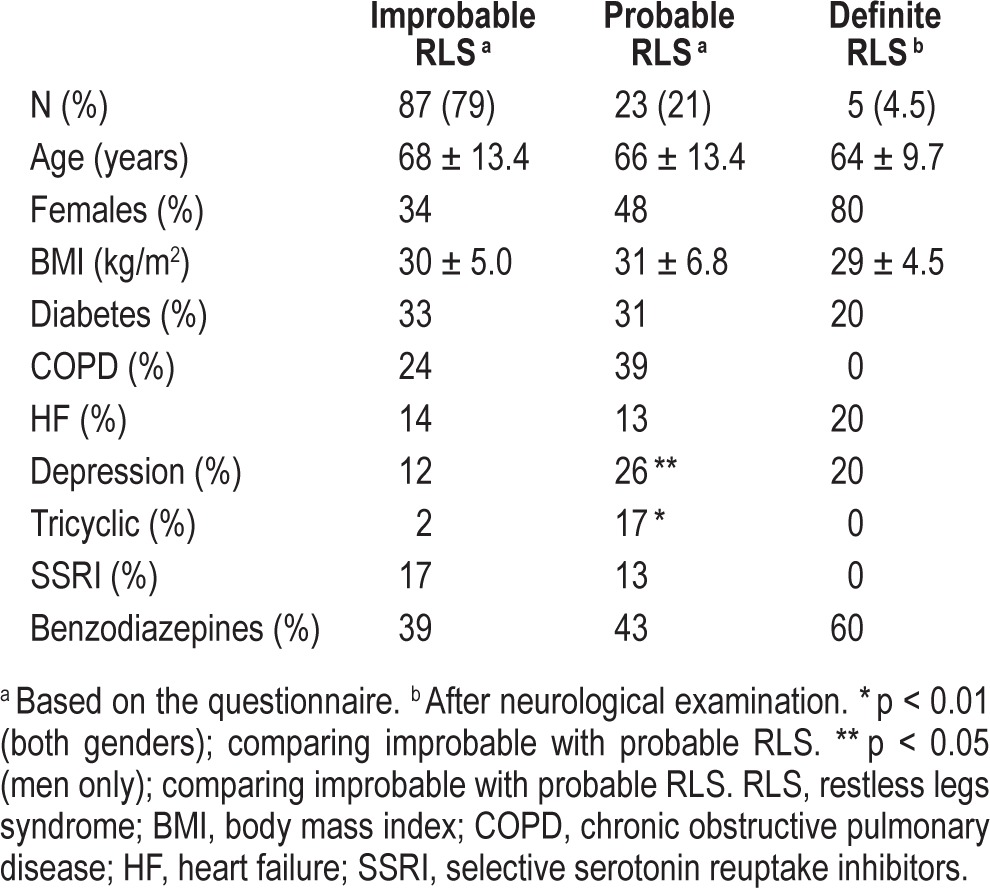
Table 2.
Patient laboratory data according to probability of RLS.
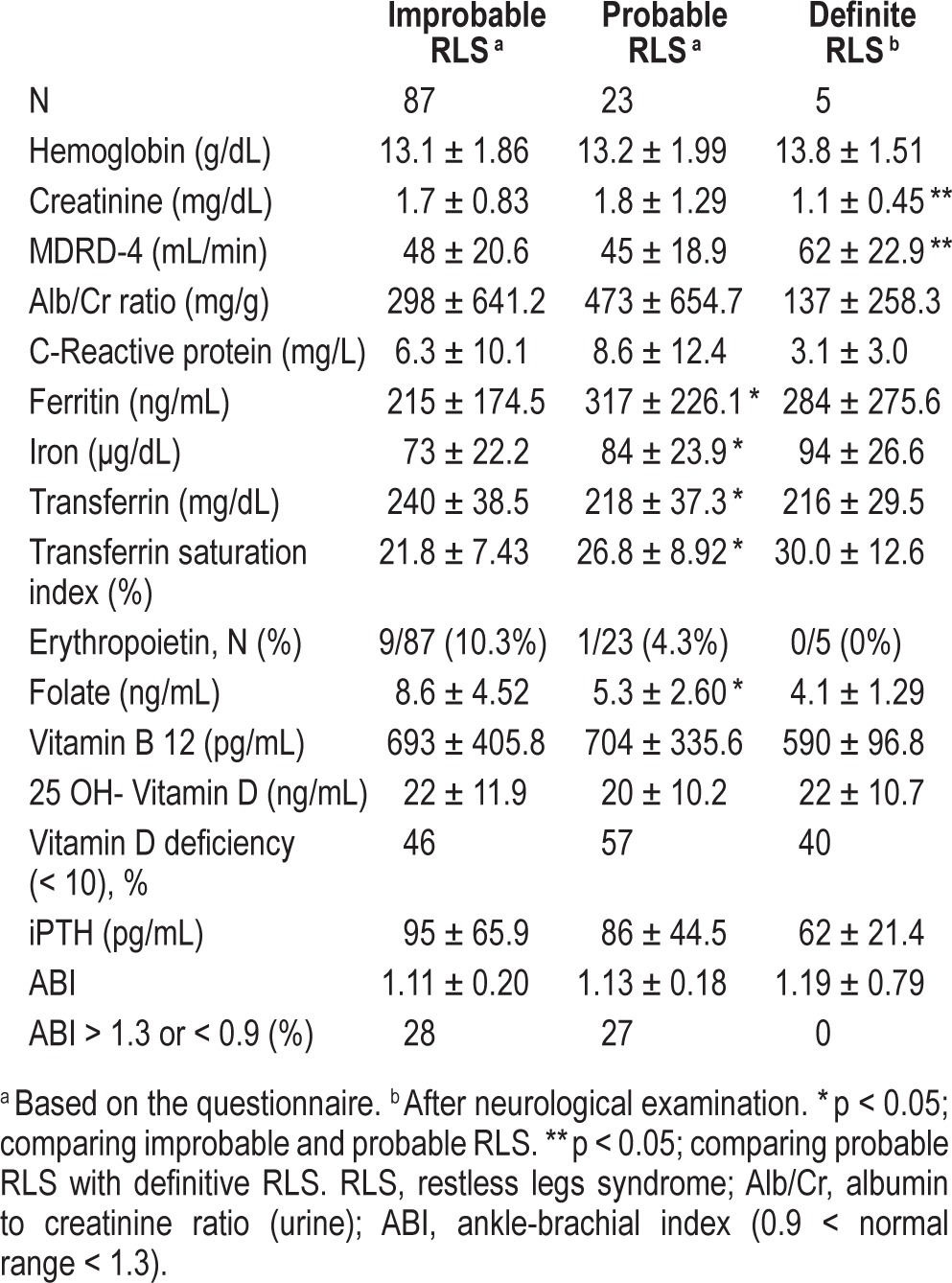
Of 23 patients classified as probable RLS based on the questionnaire, 21 were systematically examined by a neurologist (one patient died and another moved to another city). Interestingly, after careful neurological evaluation, the diagnosis of RLS was only confirmed in 4 women and 1 man (5 of 21 examined patients, 24%). This rendered a true global RLS frequency of 4.5%, more prevalent among women (9.7% vs 0.2%). Although the number of definite RLS patients did not allow powered statistical analysis, their clinical and biochemical characteristics were also included in Tables 1 and 2 for comparison. Among them, the IRLS score was mild in 2 patients, moderate in 2 patients, and very severe in one case. Surprisingly, definitive RLS patients had less impaired renal function than individuals for whom the diagnosis of RLS was not confirmed after neurological evaluation.
In the remaining 16 cases with probable RLS (76% of the subjects preselected by the questionnaire), oriented history taking and thorough neurological examination revealed that their symptoms did not meet RLS diagnostic criteria and were actually due to other causes: 3 ischemic pain of peripheral artery disease, 2 muscle cramps, 1 neurogenic claudication caused by lumbar spinal stenosis, 1 painful sensitive polyneuropathy, 1 nicturia, and 8 unspecific leg discomfort. Thus, a diagnosis of RLS was ruled out in these patients. Evaluation of the ABI did not show significant differences between subjects classified with probable or improbable RLS through the questionnaire (Table 2). Furthermore, none of the 5 patients with definite RLS had pathological ABI. The percentage of true RLS among patients preselected by the questionnaire was significantly higher for women (4/11; 36%) than for men (1/10; 10%). Of 8 patients with a positive questionnaire result and chronic obstructive pulmonary disease (COPD), none was finally classified with definite RLS. Moreover, none of the patients on tricyclic anti-depressants or SSRIs was diagnosed with definitive RLS.
DISCUSSION
Recent reports have highlighted that RLS is both under-recognized and misdiagnosed in several clinical settings, including renal disease.16,17 The association between RLS and ESRD has been widely described,1–4 and a “uremic” pathomechanism has been suggested given improvement after renal transplantation.18 A few studies have been carried out thus far in patients with CKD but not on dialysis, also suggesting a high RLS prevalence in this patient population (10% to 30%), regardless of CKD stage.14,19–21 In contrast, in the present study the prevalence of RLS among patients with non-dialysis CKD fell from 21%— probable RLS based on a self-administered questionnaire—to 4.5% after comprehensive neurological examination. Allen and collaborators also reported a drop in RLS prevalence when the patients were evaluated by a trained specialist.17,22 Our results are also in consonance with those of Mizuno and coworkers,23 who found that up to 77% of RLS in the elderly were false positives, and with the findings of another study that found that the positivity rate obtained in ESRD with questionnaires decreased significantly after expert examination.24 Similarly, in patients with early stage Parkinson disease—considered to have an etiopathogenic link with RLS—leg restlessness was more often due to akathisia and other confounders than to RLS.25
The diagnosis of RLS is based on the IRLSSG criteria.6 However, potential RLS mimics include cramps, arthritis, neuropathic pain, venous insufficiency, claudication, pruritus (e.g., in renal osteodystrophy), and other common conditions co-occurring with CKD.12,26 In our series, positional or nonspecific leg discomfort was the most frequent confounder. Peripheral artery disease was the second cause of misclassification. However, as ABI score was not different between patients with probable and improbable RLS (Table 2), this mimic did not appear to have been a significant contributor to bias questionnaire-based patient selection. In 2012 the IRLSSG included ruling out RLS mimics as a fifth diagnostic criterion (i.e., symptoms should not be solely accounted for by another disorder).22,26
It has been suggested that RLS in CKD might be related with anemia, iron status, GFR reduction, and metabolic bone disease.8,14,19–21 In order to clarify possible factors contributing both to RLS and to RLS mimics in our series, we compared several clinical and metabolic parameters between patient groups. Although we did not observe association between metabolic variables and RLS, these results must be interpreted with caution, since the small number of patients finally diagnosed with RLS in our series did not allow a powered statistical analysis stratifying for these factors. Unexpectedly, patients with definite RLS showed a better renal function compared with the probable RLS group, further supporting the hypothesis that RLS mimics may be frequently at play among CKD patients. The etiology of RLS has also been related to iron deficiency in the central nervous system.13 Interestingly, iron status parameters were better among patients with probable and definitive RLS than the improbable RLS group, suggesting that iron deficiency was not a significant factor underlying susceptibility to RLS/ RLS mimics in our series. Folic acid, necessary in the dopa-mine synthesis pathway, is usually scarce in renal diet.13,27 In our study folate levels were lowest in the definitive RLS group, perhaps contributing to this disorder. Low vitamin D levels have been previously reported in women with RLS.28 Although in our series, patients with probable RLS showed a trend to have lower levels of vitamin D, this difference was not statistically significant. It could be speculated whether routine vitamin D supplements might have contributed to ameliorate symptoms of RLS and thus to the low RLS prevalence in our study.
Another fact that might also have contributed to the low frequency of RLS in our series is predominance of men, since in large studies women had twice the likelihood to meet IRLSSG criteria.7,22 In fact, after neurological evaluation women showed a higher percentage of true RLS than men. Probable RLS was associated in our series with depression (statistically significant in men) and with tricyclic antidepressants. However, none of the definitive RLS patients was on antidepressants, perhaps pinpointing that somatizations and sleep disturbances frequently present in depression could be RLS mimics. COPD, another condition often associated with RLS, was common among patients classified as probable RLS by the questionnaire; however, none of the COPD patients was diagnosed with definitive RLS. The possibility that COPD-related symptoms might mimic RLS could also be inferred from the results of Calvacante and collaborators, who found that patients with RLS and COPD had better renal function.10
In summary, when RLS mimics were ruled out through careful examination, we observed that 4.5% of non-dialysis CKD patients had RLS, a prevalence strikingly similar to the 4.6% reported in the general Spanish population.22 Although our study protocol—based on a strict application of RLS diagnostic criteria—might have led to some false negatives, this is unlikely and would not invalidate the main outcomes. The subjective, difficult to describe symptoms of sleep disturbance and leg discomfort, as well as the relatively high overall frequency of RLS should be kept in mind to correctly interpret the prevalence and factors associated with RLS in CKD. Interestingly, the unadjusted prevalence found in our study through the written questionnaire (21%) was similar to that reported previously in CKD patients, suggesting that previous investigations may have overestimated RLS frequency in CKD and highlighting the need of expert examination, not routinely carried out in studies with renal endpoints. Our results also raise the question whether renal failure has an etiopathogenic role in RLS—as is generally believed—or, instead, could be a confounding factor when studying the etiology of RLS due to an increased occur-rence of disorders mimicking RLS among patients with CKD.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Pierratos A, Hanly PJ. Sleep disorders over the full range of chronic kidney disease. Blood Purif. 2011;31:146–50. doi: 10.1159/000321859. [DOI] [PubMed] [Google Scholar]
- 2.Mucsi I, Molnar MZ, Ambrus C, et al. Restless legs syndrome, Insomnia and quality of life in patients on maintenance dialysis. Nephrol Dial Transplant. 2005;20:571–7. doi: 10.1093/ndt/gfh654. [DOI] [PubMed] [Google Scholar]
- 3.La Manna G, Pizza F, Persici E, et al. Restless legs syndrome enhances cardiovascular risk and mortality in patients with end-stage kidney disease undergoing long-term haemodialysis treatment. Nephrol Dial Transplant. 2011;26:1976–83. doi: 10.1093/ndt/gfq681. [DOI] [PubMed] [Google Scholar]
- 4.Giannaki CD, Hadjigeorgiou GM, Karatzaferi C, Pantzaris MC, Stefanidis I, Sakkas GK. Epidemiology, impact, and treatment options of restless legs syndrome in endstage renal disease patients: an evidence-based review. Kidney Int. 2014;85:1275–82. doi: 10.1038/ki.2013.394. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Wang W, Winkelman JW, Malhotra A, Ma J, Gao X. Prospective study of restless legs syndrome and mortality among men. Neurology. 2013;81:52–9. doi: 10.1212/WNL.0b013e318297eee0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 7.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact - REST general population study. Arch Intern Med. 2005;165:1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 8.Quinn C, Uzbeck M, Saleem I, et al. Iron status and chronic kidney disease predict restless legs syndrome in an older hospital population. Sleep Med. 2011;12:295–301. doi: 10.1016/j.sleep.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Merlino G, Fratticci L, Valente M, et al. Association of restless legs syndrome in type 2 diabetes: a case-control study. Sleep. 2007;30:866–71. doi: 10.1093/sleep/30.7.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalcante AG, de Bruin PF, de Bruin VM, et al. Restless legs syndrome, sleep impairment, and fatigue in chronic obstructive pulmonary disease. Sleep Med. 2012;13:842–7. doi: 10.1016/j.sleep.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Borreguero D, Egatz R, Winkelmann J, Berger K. Epidemiology of restless legs syndrome: the current status. Sleep Med Rev. 2006;10:153–67. doi: 10.1016/j.smrv.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Ohayon MM, OHara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283–95. doi: 10.1016/j.smrv.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patrick LR. Restless legs syndrome: pathophysiology and the role of iron and folate. Altern Med Rev. 2007;12:101–12. [PubMed] [Google Scholar]
- 14.Lee J, Nicholl DD, Ahmed SB, et al. The prevalence of restless legs syndrome across the full spectrum of kidney disease. J Clin Sleep Med. 2013;9:455–9. doi: 10.5664/jcsm.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 16.Kutner NG, Zhang R, Bliwise DL. Restless legs syndrome is underdiagnosed in the US Renal Data System. QJM. 2013;106:487. doi: 10.1093/qjmed/hct014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budhiraja P, Budhiraja R, Goodwin JL, et al. Incidence of restless legs syndrome and its correlates. J Clin Sleep Med. 2012;8:119–24. doi: 10.5664/jcsm.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molnar MZ, Novak M, Ambrus C, et al. Restless legs syndrome in patients after renal transplantation. Am J Kidney Dis. 2005;45:388–96. doi: 10.1053/j.ajkd.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Merlino G, Lorenzut S, Gigli GL, et al. A case-control study on restless legs syndrome in nondialyzed patients with chronic renal failure. Mov Disord. 2010;25:1019–25. doi: 10.1002/mds.23010. [DOI] [PubMed] [Google Scholar]
- 20.Libório AB, Santos JP, Minete NF, de Diógenes CA, de Farias LA, de Bruin VM. Restless legs syndrome and quality of sleep in patients with glomerulopathy. BMC Nephrol. 2013;14:113–8. doi: 10.1186/1471-2369-14-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aritake-Okada S, Nakao T, Komada Y, et al. Prevalence and clinical characteristics of restless legs syndrome in chronic kidney disease patients. Sleep Med. 2011;12:1031–3. doi: 10.1016/j.sleep.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Allen RP, Stillman P, Myers AJ. Physician-diagnosed restless legs syndrome in a large sample of primary medical care patients in Western Europe: prevalence and characteristics. Sleep Med. 2010;11:31–7. doi: 10.1016/j.sleep.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno S, Miyaoka T, Inagaki T, Horiguchi J. Prevalence of restless legs syndrome in non-institutionalized Japanese elderly. Psychiatry Clin Neurosci. 2005;59:461–5. doi: 10.1111/j.1440-1819.2005.01399.x. [DOI] [PubMed] [Google Scholar]
- 24.Cirignotta F, Mondini S, Santoro A, Ferrari G, Gerardi R, Buzzi G. Reliability of a questionnaire screening restless legs syndrome in patients on chronic dialysis. Am J Kidney Dis. 2002;40:302–6. doi: 10.1053/ajkd.2002.34508. [DOI] [PubMed] [Google Scholar]
- 25.Gierstad MD, Tysnes OB, Larsen JP. Increased risk of leg motor restlessness but not RLS in early Parkinson disease. Neurology. 2011;77:1941–6. doi: 10.1212/WNL.0b013e31823a0cc8. [DOI] [PubMed] [Google Scholar]
- 26.Hening WA., Allen RP, Washburn M, Lesage SR, Earley CJ. The four diagnostic criteria for restless legs syndrome are unable to exclude confounding conditions (mimics) Sleep Med. 2009;10:976–81. doi: 10.1016/j.sleep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makoff R. Vitamin replacement therapy in renal failure patients. Miner Electrolyte Metab. 1999;25:349–51. doi: 10.1159/000057473. [DOI] [PubMed] [Google Scholar]
- 28.Balaban H, Yıldız ÖK, Çil G, et al. Serum 25-hydroxyvitamin D levels in restless legs syndrome patients. Sleep Med. 2012;13:953–7. doi: 10.1016/j.sleep.2012.04.009. [DOI] [PubMed] [Google Scholar]


