Abstract
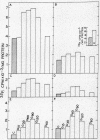
The pancreatic hormone, glucagon, stimulates the net uptake of inorganic 32P in vivo into rat liver and its incorporation into proteins of microsomes, mitochondrial membranes, and lysosomes. Incorporation into cytosolic proteins was enhanced only slightly by glucagon. More than 95% of the protein-bound phosphate is present as phosphoserine. Both the radioactivity and the total amount of protein-bound phosphate are increased after injection of glucagon. Glucagon treatment enhanced 32P incorporation into the alcohol-ether soluble lipids of mitochondria but did not alter the relative distribution of 32P in various phospholipid species.
Keywords: hormone, inorganic phosphate, phospholipids
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam P. A., Haynes R. C., Jr Control of hepatic mitochondrial CO2 fixation by glucagon, epinephrine, and cortisol. J Biol Chem. 1969 Dec 10;244(23):6444–6450. [PubMed] [Google Scholar]
- Allmann D. W., Bachmann E., Orme-Johnson N., Tan W. C., Green D. E. Membrane systems of mitochondria. VI. Membranes of liver mitochondria. Arch Biochem Biophys. 1968 Jun;125(3):981–1012. doi: 10.1016/0003-9861(68)90537-7. [DOI] [PubMed] [Google Scholar]
- Blat C., Loeb J. E. Effect of glucagon on phosphorylation of some rat liver ribosomal proteins in vivo. FEBS Lett. 1971 Oct 15;18(1):124–126. doi: 10.1016/0014-5793(71)80425-8. [DOI] [PubMed] [Google Scholar]
- DEVENANZI F., DIEZALTARES C., FORERO J. ORGAN DISTRIBUTION OF RADIOACTIVE ORTHOPHOSPHATE AND TOTAL PHOSPHORUS IN THE RAT; THE INFLUENCE OF GLUCAGON AND INSULIN. Diabetes. 1964 Nov-Dec;13:609–614. [PubMed] [Google Scholar]
- Exton J. H., Mallette L. E., Jefferson L. S., Wong E. H., Friedmann N., Miller T. B., Jr, Park C. R. The hormonal control of hepatic gluconeogenesis. Recent Prog Horm Res. 1970;26:411–461. doi: 10.1016/b978-0-12-571126-5.50014-5. [DOI] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. B., Rasmussen H., DiBella F., Guthrow C. E., Jr Cyclic adenosine 3':5'-monophosphate-stimulated phosphorylation of isolated neurotubule subunits. Proc Natl Acad Sci U S A. 1970 Oct;67(2):652–659. doi: 10.1073/pnas.67.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard O. The hormonal regulation of enzymes in penatal and postnatal rat liver. Effects of adenosine 3',5'-(cyclic)-monophosphate. Biochem J. 1969 Oct;115(1):19–24. doi: 10.1042/bj1150019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guder W., Hepp K. D., Wieland O. The catabolic action of glucagon in rat liver. The influence of age, nutritional state and adrenal function on the effect of glucagon on lysosomal N-acetyl-beta, D-glucosaminidase. Biochim Biophys Acta. 1970 Dec 29;222(3):593–605. [PubMed] [Google Scholar]
- Henning R., Kaulen H. D., Stoffel W. Biochemical analysis of the pinocytotic process. I. Isolation and chemical composition of the lysosomal and the plasma membrane of the rat liver cell. Hoppe Seylers Z Physiol Chem. 1970 Oct;351(10):1191–1199. doi: 10.1515/bchm2.1970.351.2.1191. [DOI] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. Characterization and regulatory aspects. Biochemistry. 1970 Oct 13;9(21):4160–4175. doi: 10.1021/bi00823a019. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. VII. Comparison of various histones as substrates for adenosine 3',5'-monophosphate-dependent and guanosine 3',5'-monophosphate-dependent protein kinases. Biochim Biophys Acta. 1970 Sep 16;212(3):434–440. doi: 10.1016/0005-2744(70)90249-4. [DOI] [PubMed] [Google Scholar]
- Langan T. A. Phosphorylation of liver histone following the administration of glucagon and insulin. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1276–1283. doi: 10.1073/pnas.64.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb J. E., Blat C. Phosphorylation of some rat liver ribosomal proteins and its activation by cyclic AMP. FEBS Lett. 1970 Sep 24;10(2):105–108. doi: 10.1016/0014-5793(70)80427-6. [DOI] [PubMed] [Google Scholar]
- MILLER L. L. Glucagon: a protein catabolic hormone in the isolated perfused rat liver. Nature. 1960 Jan 23;185:248–248. doi: 10.1038/185248a0. [DOI] [PubMed] [Google Scholar]
- Menahan L. A., Wieland O. Interactions of glucagon and insulin on the metabolism of perfused livers from fasted rats. Eur J Biochem. 1969 May 1;9(1):55–62. doi: 10.1111/j.1432-1033.1969.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Ragab H., Beck C., Dillard C., Tappel A. L. Preparation of rat liver lysosomes. Biochim Biophys Acta. 1967 Nov 28;148(2):501–505. doi: 10.1016/0304-4165(67)90148-1. [DOI] [PubMed] [Google Scholar]
- Rosa F. Ultrastructural changes produced by glucagon, cyclic 3'5'-AMP and epinephrine on perfused rat livers. J Ultrastruct Res. 1971 Feb;34(3):205–213. doi: 10.1016/s0022-5320(71)80068-0. [DOI] [PubMed] [Google Scholar]
- SAWANT P. L., DESAI I. D., TAPPEL A. L. DIGESTIVE CAPACITY OF PURIFIED LYSOSOMES. Biochim Biophys Acta. 1964 Apr 6;85:93–102. doi: 10.1016/0926-6569(64)90170-1. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Schnaitman C. A. Comparison of rat liver mitochondrial and microsomal membrane proteins. Proc Natl Acad Sci U S A. 1969 Jun;63(2):412–419. doi: 10.1073/pnas.63.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner J. B. A METHOD FOR THE COLORIMETRIC DETERMINATION OF PHOSPHORUS. Science. 1944 Nov 3;100(2601):413–414. doi: 10.1126/science.100.2601.413. [DOI] [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]