Abstract
The reaction of 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione (1) as a binucleophile with primary aromatic or heterocyclic amines and formaldehyde or aromatic (heterocyclic) aldehydes in a molar ratio (1:1:2) gave the pyrimido[4,5-d]pyrimidin-2,4-dione ring systems 2–5. Treatment of 1 with diamines and formalin in molar ratio (2:1:4) gave the bis-pyrimido[4,5-d]pyrimidin-2,4-diones 6–8. Furthermore, substituted pyrimido[4,5-d]pyrimidin-2,4-diones with uracil derivative 11 or spiro indole 16 were synthesized. Synthesis of pyrimido[4,5-d]pyrimidin-2,4-diones with different substitution at C-5 and C-7 was achieved to give 13 and 18, respectively.
Keywords: Uracil, Annulation, Double Mannich reaction, Aldimine, Ketimine
Introduction
A large number of pyrimidine derivatives were reported to exhibit interesting pharmacological activity [1], [2], [3], [4], [5], [6], [7], [8]. A class of compounds of biological relevance was used in the plant protection area as plant growth regulators [9]. In continuation of our studies, the development of expedient methods for the synthesis of pyrimido[4,5-d]pyrimidine-2,4-dione, were implemented [10]. Pyrimidopyrimidines are annulated uracils that have attracted considerable interest in recent years. Derivatives of pyrimidopyrimidine are known to display a wide range of pharmacological activities, and their potent inhibitory properties regarding the tyrosine kinase domain of epidermal growth factor receptor [11], 5-phosphoribosyl-1-pyrophosphate synthetase [12] and dihydrofolate-reductase [13], have been fully demonstrated. Numerous reports delineate the antitumour [14], antiviral [15], antioxidant [16], antifungal [17] and hepatoprotective [18] activities. The multicomponent reactions (MCR’s) [19], [20], are masterpieces of synthetic efficiency and reaction design.
Experimental
All melting points were measured on a Gallenkamp electric melting point apparatus. Infrared spectra measured using KBr discs on a Mattson 5000 FTIR spectrometer at the Microanalytical Center (Cairo University, Egypt). The 1H NMR and 13C NMR spectra were carried out on Varian Gemini 200 MHz spectrophotometer, Microanalytical center (Cairo University, Egypt), using TMS as an internal standard and chemical shifts were recorded in ppm on δ scale and coupling constants (J) are given in Hz. The mass spectra recorded on a GC–MS (Shimadzu QP-1000 EX). Reactions were monitored by thin layer chromatography (TLC) using silica gel (EM science) coated plates. The starting 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione (1) was purchased from Aldrich Company. Elemental analyses were measured with a ECS 4010 Elemental combustion system instrument at the Microanalytical Unit, Faculty of Science, Cairo University; their results were found to be in good agreement with the calculated values.
Synthesis of pyrimido[4,5-d]pyrimidone ring systems 2–5
General procedure: A solution of 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione (1) (1.01 g, 6.5 mmol) in ethanol (30 mL) was added to a mixture of amines [p-anisidine (0.8 g, 6.5 mmol), m-nitroaniline (0.898 g, 6.5 mmol), p-anisidine (0.8 g, 6.5 mmol), or 2-aminothiazole (0.65 g, 6.5 mmol)] and aldehydes [formaldehyde (0.39 g, 13 mmol), piperonal (1.95 g, 13 mmol), p-anisaldehde (1.77 g, 13 mmol), or furfural (1.25 g, 13 mmol)] in ethanol (20 mL). The reaction mixture was stirred at 35 °C for 2 h, then left to stand at room temperature for 3 days. The resulting precipitate was collected by filtration, dried and purified by crystallization from ethanol to give the corresponding products 2–5, respectively.
6-(4-Methoxyphenyl)-1,3-dimethyl-5,6,7,8-tetrahydropyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione (2)
Recrystallization from ethanol to give (25%) 2; white crystals. M.p.: 160 °C; Rf = 0.7 [pet. ether 40–60 °C/ethyl acetate, (1:2)]; IR (KBr): = 3257 (NH), 2971 (CH, str.), 1677, 1621 (CO), 1511 cm−1 (C C); 1H NMR (200 MHz, DMSO): δ = 3.32 (s, 3H, NCH3), 3.73 (s, 3H, OCH3), 4.59 (s, 2H, CH2), 5.04 (s, 1H, NH–CH2–N), 6.3 (br, 1H, NH), 6.65–7.12 (m, 12H, Ar–H); MS (m/z) (I%) = 302 (M+, 24), 303 (M++1, 5), 288 (13), 285 (3), 242 (1), 214 (1), 155 (5), 137 (2) 135 (100), 121 (10), 120 (54), 107 (3), 91 (2); Calc for (C15H18N4O3) C, 59.59; H, 6.00; N, 18.53; Found C 59.63, H, 5.97, N 18. 47.
5,7-Di(benzo[d][1,3]dioxol-5-yl)-1,3-dimethyl-6-(3-nitrophenyl)-5,6,7,8-tetrahydropyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione (3)
Recrystallization from ethanol to give to give (35%) 3; yellow crystals. M.p.: 212 °C; Rf = 0.57 [pet. ether 40–60 °C/ethyl acetate, (1:2)]; IR (KBr): = 3424 (NH), 1700, 1618 (CO), 1530 cm−1 (C C); 1H NMR (200 MHz, DMSO): δ = 3.32 (s, 3H, CH3), 3.37 (s, 3H, CH3), 4.6 (s, 4H, 2CH2), 5.4 (s, 1H, CH), 5.8 (s, 1H, CH), 6.9 (br., 1H, NH), 7.1–7.5 (m, 10 H, Ar–H); MS (m/z) (I%) = 555 (M+−2, 2), 380 (1), 313 (1), 288 (1), 196 (2), 138 (2), 137 (100), 65 (3); Calc for (C28H23N5O8) C, 60.32; H, 4.16; N, 12.56; Found C, 60.39; H, 4.23; N, 12.48.
5,6,7-Tris(4-methoxyphenyl)-1,3-dimethyl-5,6,7,8-tetrahydropyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione (4)
Recrystallization from ethanol to give (93%) 4; white crystal. M.p.: 130 °C; Rf = 0.56 [pet. ether 40–60 °C/ethyl acetate, (1:1)]; IR (KBr): = 3402 (NH), 3129, 2948 (CH, str.), 1625 cm−1 (CO); 1H NMR (200 MHz, DMSO): δ = 3.32 (s, 6H, 2NCH3), 3.73 (s, 9H, 3OCH3), 4.59 (s, 1H, CH), 5.04 (s, 1H, NH–CH), 6.3 (br., 1H, NH), 6.65-7.1 (m, 12H, Ar–H); MS (m/z) (I%) = 514 (M+, 14), 330 (30), 253 (20), 193 (44), 175 (43), 122 (100); Calc for (C29H30N4O5) C, 67.69; H, 5.88; N, 10.89; Found C, 67.74; H, 5.93; N, 10.78.
5,7-Di(furan-2-yl)-1,3-dimethyl-6-(thiazol-2-yl)-5,6,7,8-tetrahydro-pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione (5)
Recrystallization from ethanol to give (31%) 5; green crystals. M.p.: 325 °C; Rf = 0.46 [pet. ether 40–60 °C/ ethyl acetate (1:2)]; IR (KBr): = 3418, 3357 (NH), 3144, 2955 (CH, str.), 1675, 1615 (CO), 1554 cm−1 (C C); 1H NMR (200 MHz, DMSO): δ = 3.35 (s, 3H, NCH3), 3.77 (s, 3H, OCH3), 4.59 (s, 1H, CH), 5.04 (s, 1H, N–CH–N), 6.45 (br, 1H, NH), 6.65–7.16 (m, 12H, Ar–H); MS (m/z) (I%) = 411 (M+, 17), 383 (21), 354 (8), 271 (20), 225 (18), 178 (2), 55 (100); Calc for (C19H17N5O4S) C, 55.47; H, 4.16; N, 17.02; Found C, 55.38; H, 4.23; N, 17.00.
General procedure for the synthesis of bis-pyrimido[4,5-d]pyrimidones ring system 6, 7 and 8
A solution of 1 (1.01 g, 6.5 mmol) in ethanol (30 mL) was added to a solution of formaldehyde (13 mmol, 40% solution) with diamine namely; p-phenylenediamine (0.35 g, 3.25 mmol), ethane-1,2-diamine (0.195 g, 3.25 mmol) and hydrazine hydrate (0.16 g, 3.25 mmol) in ethanol (20 mL), the reaction mixture was stirred for 2 h then left to stand at room temperature for 3 days. The resulting precipitate was collected by filtration, then purified by recrystallization from ethanol to gave bis-pyrimido[4,5-d]pyrimidines 6-8, respectively.
6-(4-(6,8-Dimethyl-5,7-dioxooctahydropyrimido[4,5-d]pyrimidin-3(4H)-yl)phenyl)-1,3-dimethyl-5,6,7,8-tetrahydropyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione (6)
Recrystallization from ethanol to give (39%) 6; green crystals. M.p.: 224 °C; Rf = 0.5 [pet. ether 40–60 °C/ethyl acetate (1:2)]; IR (KBr): = 3399, 3358 (NH), 3225 (CH, str.) 1687, 1658 (CO), 1607 cm−1 (C C); 1H NMR (200 MHz, DMSO): δ = 3.12 (s, 3H, CH3), 3.16 (s, 3H, CH3), 3.17 (s, 3H, CH3), 3.2 (s, 3H, CH3), 3.94 (s, 4H, 2CH2), 4.51 (s, 4H, 2CH2), 6.5–6.9 (m, 4H, Ar–H), 7.3 (br., 2H, 2NH); MS (m/z) (I%) = 465 (M+−1, 2), 461 (2), 311 (10), 272 (2), 196 (4), 181 (3), 168 (6), 154 (10), 120 (100); Calc for (C22H28N8O4) C, 56.40; H, 6.02; N, 23.92; Found C, 56.57; H, 6.08; N, 24.01.
6-(2-(6,8-Dimethyl-5,7-dioxooctahydropyrimido[4,5-d]pyrimidin-3(4H)-yl)ethyl)-1,3-dimethyl-5,6,7,8-tetrahydropyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione (7)
Recrystallization from ethanol to give (40%) 7; white crystals. M.p.: 255 °C; Rf = 0.76 [pet. ether 40–60 °C/ethyl acetate, (1:1)]; IR (KBr): = 3402 (NH), 3129 (CH, str.), 1692, 1670, 1620 cm−1 (CO); 1H NMR (200 MHz, DMSO): δ = 3.12 (s, 6H, 2CH3), 3.21 (s, 6H, 2CH3), 3.29 (t, J = 6.4 Hz, 4H, 2CH2), 3.42 (s, 4H, 2CH2), 4.02 (s, 4H, 2CH2), 7.1 (br., 2H, 2NH); MS (m/z) (I%) = 209 (M+−2, 5), 222 (4), 196 (2), 140 (7), 56 (35), 55 (100); Calc for (C18H28N8O4) C, 51.42; H, 6.71; N, 26.65; Found C, 51.47; H, 6.74; N, 26.72.
6,6′,8,8′-Tetramethyl-4′,4′a-dihydro-1H,1′H-3,3′-bipyrimido[4,5-d]pyrimidine-5,5′,7,7′(2H,2′H,4H,6H,6′H,8H,8′H,8′aH)-tetraone (8)
Recrystallization from ethanol to give (40%) 8; white crystals. M.p.: 230–233 °C; Rf = 0.58 [pet. ether 40–60 °C/ethyl acetate (1:2)]; IR (KBr): = 3402 (NH), 3135, 2948 (CH, str.), 1693, 1680 (CO), 1584 cm−1 (C C); MS (m/z) (I%) = 390 (M+−2, 2), 371 (2), 357 (3), 322 (35), 304 (5), 233 (6), 190 (4), 168 (100), 111 (2), 57 (76); Calc for (C16H24N8O4) C, 48.97; H, 6.16; N, 28.56; Found C, 48.93; H, 6.19; N, 28.62.
Synthesis of 6-((6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-pyrimidin-5-yl)methyl)-1,3-dimethyl-5,6,7,8-tetrahydropyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione (11)
A mixture of 10 (1.05 g, 3 mmol) and formaldehyde (2 mL, 40% solution) was heated at 60 °C in ethanol (10 mL). The reaction mixture was cooled, product filtered, dried and crystallized from ethanol to give (55%) 11; white crystals. M.p.: 150 °C; IR (KBr): = 3350, 3283 (NH2), 3209 (NH), 1695, 1677 cm−1 (CO); 1H NMR (200 MHz, DMSO): δ = 3.32 (s, 6H, 2CH3), 3.37 (s, 6H, 2CH3), 3.6 (s, 2H, NH2), 4.2 (s, 4H, 2CH2, pyrimidine), 4.3 (s, 2H, CH2), 6.3 (br., 1H, NH); 13C NMR (200 MHz, DMSO): δ = 162.8 (CO–N), 162.5 (CO–N), 154.8, 152.6, 151.5, 83.3, 62.3, 45.9, 43.5, 31.0, 30.7, 29.7, 29.5; MS (m/z) (I%) = 363 (M+, 8), 303 (41), 304 (100), 209 (3), 208 (14), 192 (11), 154 (5); Calc for (C15H21N7O4) C, 49.58; H, 5.83; N, 26.98; Found C, 49.62; H, 5.72; N, 26.86.
Synthesis of secondary Mannich bases 12 and 15
General procedure: A mixture of 1 (1.01 g, 6.5 mmol), aldimines namely; N-(4-chlorobenzylidene)-3-nitroaniline (1.69 g, 6.5 mmol) or 14 (1.44 g, 6.5 mmol) in ethanol (30 mL) containing three drops of acetic acid was heated under reflux for 9 h. The reaction mixture was kept in refrigerator overnight whereby the formed precipitate was filtered off, dried and recrystallized from appropriate to furnish compounds 12 and 15, respectively.
6-Amino-5-((4-chlorophenyl)(3-nitrophenylamino)methyl)-1,3-dimethyl-pyrimidine-2,4(1H,3H)-dione (12)
Recrystallized from acetic acid to give (45%) 12; yellow crystals. M.p.: 260 °C; Rf = 0.64 [pet. ether 40–60 °C/ethyl acetate (1:2)]; IR (KBr): = 3350, 3225 (NH2), 3209 (NH), 3138 (CH, str.) 1693, 1668 (CO), 1592 cm−1 (C C); 1H NMR (200 MHz, DMSO): δ = 3.1 (s, 3H, CH3) 3.3 (s, 3H, CH3), 5.57 (s, 1H, CH), 7.6 (br., 2H, NH2), 7.7–8.0 (m, 8H, Ar–H), 8.78 (s, 1H, NH); MS (m/z) (I%) = 415 (M+, 27), 413 (79), 278 (72), 276 (100), 242 (8), 166 (10), 155 (27), 137 (8); Calc for (C19H18ClN5O4) C, 54.88; H, 4.36; N, 16.84; Found C, 54.69; H, 4.43; N, 16.79.
6-Amino-1,3-dimethyl-5-(2-oxo-3-(phenylamino)indolin-3-yl)pyrimidine-2,4(1H,3H)-dione (15)
Recrystallized from acetic acid to give (54%) 15; yellow crystals. M.p.: 330–332 °C; Rf = 0.67 [chloroform/methanol (1:4)]; IR (KBr): = 3465, 3409 (NH2), 3282, 3198 (NH), 1754 (CO, isatin), 1696, 1689 (CO, amidic), 1600 cm−1 (C C); 1H NMR (200 MHz, DMSO): δ = 3.02 (s, 3H, CH3), 3.18 (s, 3H, CH3), 6.95 (br., 2H, NH2), 6.98–7.32 (m, 9H, Ar–H), 9.36 (s, 1H, NH), 11.55 (br., 1H, NH); MS (m/z) (I%) = 378 (M++1, 17), 313 (25), 285 (11), 153 (7), 152 (23), 133 (13), 91 (27), 57 (100), 56 (43); Calc for (C20H19N5O3) C, 63.65; H, 5.07; N, 18.56; Found C, 63.59; H, 5.16; N, 18.46.
Annulations of secondary Mannich bases: Synthesis of 13 and 16
A mixture of 12 (1.25 g, 3 mmol) or 15 (1.13 g, 3 mmol) and formaldehyde (2 mL, 40% solution) was heated at 60 °C in ethanol (10 mL) for 3 h. The reaction mixture was cooled, product filtered, dried and recrystallized from ethanol to give 13 and 16, respectively.
5-(4-Chlorophenyl)-1,3-dimethyl-6-(3-nitrophenyl)-5,6,7,8-tetrahydropyrimido[4,5-d] pyrimidine-2,4(1H,3H)-dione (13)
Recrystallized from ethanol to give (75%) 13; white crystals. M.p.: 120 °C; IR (KBr): = 3314, 3181 (NH), 2922 (CH, str.), 1651 (CO), 1600 cm−1 (C C); MS (m/z) (I%) = 429 (M++1, 15), 372 (41), 310 (16), 256 (29), 222 (36), 165 (56), 52 (100); Calc for (C20H18ClN5O4) C, 56.15; H, 4.24; N, 16.37; Found C, 56.21; H, 4.32; N, 16.35.
6′,8′-Dimethyl-3′-phenyl-2′,3′-dihydro-1′H-spiro[indoline-3,4′-pyrimido[4,5-d]pyrimidine]-2,5′,7′(6′H,8′H)-trione (16)
Recrystallized from ethanol to give (45%) 16; white crystals. M.p.: 190 °C; IR (KBr): = 3308, 3185 (2NH), 2983, 2921 (CH, str.), 1752, 1697, 1648 cm−1 (CO); 1H NMR (200 MHz, DMSO): δ = 3.32 (s, 3H, CH3), 3.37 (s, 3H, CH3), 4.6 (s, 2H, CH2), 6.2 (br., s, 1H, CH2–NH), 7.1–7.52 (m, 9H, Ar–H), 8.6 (s, 1H, CO–NH); 13C NMR (200 MHz, DMSO): δ = 168.7, 163.4, 155.1, 154.1, 149.6, 141.2, 129.9, 129.7, 127.8, 126.9, 122.0, 118.3, 114.3, 95.6, 63.6, 47.2, 30.9, 29.7; MS (m/z) (I%) = 390 (M++1, 15), 368 (29), 358 (24), 264 (100), 227 (22); Calc for (C21H19N5O3) C, 64.77; H, 4.92; N, 17.98; Found C, 64.73; H, 4.83; N, 17.82.
Synthesis of 6-amino-1,3-dimethyl-2,4-dioxo-N-phenyl-1,2,3,4-tetrahydro-pyrimidine-5-carbo-thioamide (17)
A mixture of 1 (1.01 g, 6.5 mmol) and phenyl isothiocyanate (0.88 g, 6.5 mmol) in toluene (20 mL), was refluxed for 9 h until the disappearance of the starting materials as evidenced by the TLC. The formed precipitate was collected by filtration, dried and crystallized from ethanol to give (55%) 17, yellow crystals. M.p.: 300 °C; Rf = 0.67 [chloroform: methanol (5:1)]; IR (KBr): = 3400, 3356 (NH2) 3227 (NH), 2948 (CH, str.), 1660, 1613 (CO), 1236, 1213 (C S); MS (m/z) (I%) = 290 (M+, 0.13), 257 (0.31), 222 (0.09), 154 (100), 136 (6.77), 127 (13.67), 82 (76.88), 77 (0.35); Calc for (C13H14N4O2S) C, 53.78; H, 4.86; N, 19.30; Found C, 53.65; H, 4.93; N, 19.26.
Synthesis of 1,3-dimethyl-6,7-diphenyl-5-thioxo-5,6,7,8-tetrahydropyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione (18)
A mixture of 17 (0.87 g, 3 mmol) and benzyldehyde (0.32 g, 3 mmol) was refluxed in ethanol (10 mL) for 3 h. The reaction mixture was cooled, product filtered, dried and crystallized from ethanol to give (61%) 18, yellow crystal. M.p.: 125 °C; IR (KBr): = 3068, 3005 (NH), 2880, 2834 (CH, str.), 1789, 1686 (CO), 1326, 1292 cm−1 (C S); 1H NMR (200 MHz, DMSO): δ = 3.32 (s, 3H, CH3), 3.37 (s, 3H, CH3), 5.06 (s, 1H, CH), 6.3 (s, 1H, NH), 6.5–7.3 (m, 10H, Ar–H); MS (m/z) (I%) = 379 (M++1, 2), 378 (M+, 6), 331 (3), 269 (2), 256 (5), 213 (4), 105 (36), 55 (100); Calc for (C20H18N4O2S) C, 63.47; H, 4.79; Found C, 63.53; H, 4.83.
Results and discussion
The objective of the present work was to investigate the behavior of Mannich reaction towards 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione (1) as a bifunctional nucleophile with primary amines. Mannich reaction of 1 with primary aromatic or heterocyclic amines, namely, p-anisidine and m-nitroaniline, 2-aminothiazole, aromatic and heterocyclic aldehydes, namely; formaldehyde, piperonal, p-anisaldehyde as well as furfural in a molar ratio (1:1:2) gave pyrimido[4,5-d]pyrimidine ring systems 2–5 via a double Mannich reaction (Scheme 1), similar methods to obtain pyrimido[4,5-d]pyrimidines have been reported [21], [22], [23], [24], [25]. Furthermore, involvement of both C-5 and 6-amino group in this reaction is in line with our reported work [10], [26], [27] and to Roth & Hagen work [28]. The 1H NMR spectrum of 3 showed singlet signals at δ 3.32 and 3.37 ppm for two N–CH3 groups, singlet signals at δ 4.6, 5.4 and 5.8 ppm due to two (–O–CH2–O–), (CH–N) and (N–CH–N) groups, respectively, broad signal at δ 6.9 ppm for NH and multiplet signals at δ 7.1–7.5 ppm for ten aromatic protons. The mass spectra of compounds 2–5 showed the molecular ion peaks at m/z 302 (M+, 24%), 555 (M+−2, 2%), 514 (M+, 14%) and 411 (M+, 17%), respectively.
Scheme 1.
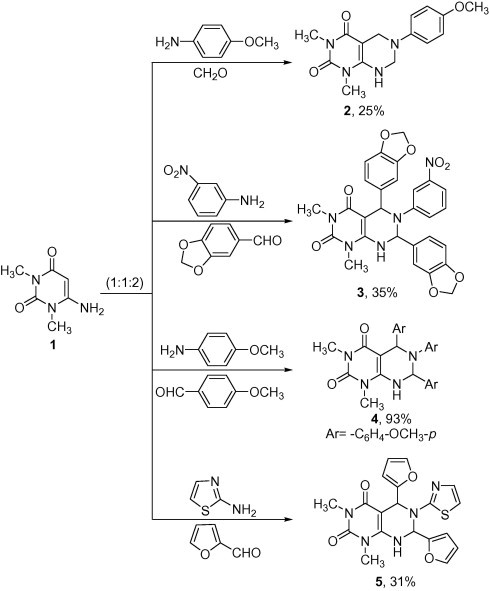
Reaction of 1 with primary amines and different aldehyde in a molar ratio (1:1:2).
On the other hand, treatment of 1 with primary aliphatic or aromatic diamines and formalin in a molar ratio (2:1:4) gave bis-pyrimido[4,5-d]pyrimidine ring systems 6–8 (Scheme 2). The 1H NMR of 6 showed singlet signals at δ 3.12, 3.16, 3.17 and 3.2 ppm for four (N–CH3) groups, singlet signals at 3.94 and 4.51 ppm for two (N–CH2) and two (N–CH2–N) groups, respectively, multiplet signals at δ 6.5–6.9 for four aromatic protons and broad signal at δ 7.3 ppm for two NH protons. Additionally, the mass spectra of compounds 6, 7 and 8 showed the molecular ion peaks at m/z 463 (M+−2), 209 (M+−2) and 390 (M+), respectively.
Scheme 2.
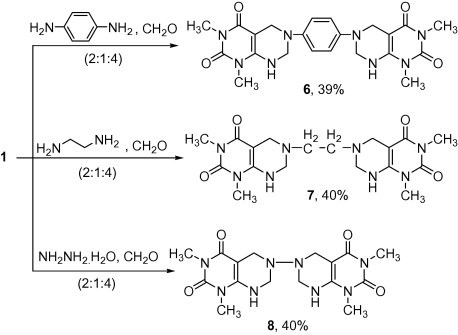
Synthesis of bis-pyrimido[4,5-d]pyrimidine ring systems 6-8.
Therefore, 6-amino-1,3-dimethyl-5-(morpholinomethyl)pyrimidine-2,4(1H,3H)-dione (9) [10], was trans-aminated with ammonium carbonate to the symmetrical bis-Mannich base 10, which annulated by formalin to give 6-((6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)methyl)-1,3-dimethyl-5,6,7,8-tetrahydropyrimido[4,5-d]pyrimidine-2,4-(1H,3H)-dione (11) (Scheme 3). The mass spectrum of 11 showed the molecular ion peak at m/z 363 (M+, 7.5%).
Scheme 3.
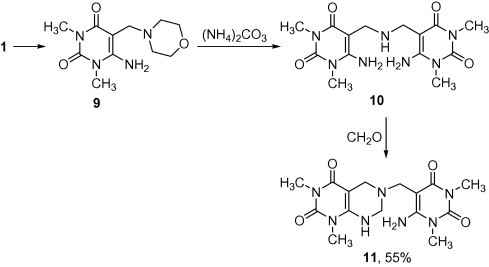
Synthesis of pyrimido[4,5-d]pyrimidine 11.
Another route was also accomplished to obtain the pyrimido[4,5-d]pyrimidine derivative with different substitution at C-5 and C-7 [since in Mannich reaction the new constructed pyrimidine moieties have symmetrical substitutions at C-5 and C-7 according to the aldehyde used]. Therefore, the present work has focused on the reaction of 1 with aldimine or ketimine as a route to the corresponding secondary Mannich bases [27], [28]. Treatment of 1 with N-(4-chlorobenzylidene)-3-nitroaniline afforded 12. Additionally, compound 12 was annulated with formalin to afford the target compounds 13. The assigned structure 12 was established on the basis of analytical and spectral data. Its mass spectrum showed the molecular ion peak at m/z 415 (M+, 26.5) and 413 (M+−2, 78.7) by relative intensity (1:3). In addition, treatment of 1 with 3-(phenylimino)indolin-2-one (14) afforded 15 which annulated with formalin to give the corresponding spiro[indoline-3,4′-pyrimido[4,5-d]pyrimidine]-2,5′,7′(6′H,8′H)-trione (16). The mass spectrum of compound 15 showed the molecular ion peak at m/z 378 (M++1, 17). Once more, the synthesis of pyrimido[4,5-d]pyrimidine-5-thione derivative 18 was conducted. Hence, reaction of 1 with phenyl isothiocyanate afforded compound 17. Annulation of 17 with benzaldehyde gave the target product 18 (Scheme 4) which was established on the basis of its analytical and spectral data. The mass spectrum of 17 showed the molecular ion peak at m/z 290 (M+, 0.13%).
Scheme 4.
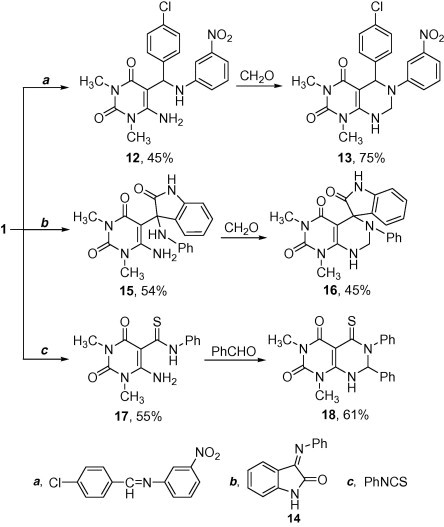
Synthesis of pyrimido[4,5-d]pyrimidine derivatives 13, 16 and 18.
In conclusion, 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione (1) contain enamino system including bis-nucleophilic centers as amino group and beta position of amino group which reacted with two moles of different aldehydes to give dimethylol followed by its reaction with one mole of primary amine via double Mannich reaction. Eleven pyrimido[4,5-d]pyrimidine derivatives and their related analogs were synthesized and elucidated on the basis of elemental analyses and spectral data (C.f. Experimental section). Newly prepared compounds were obtained through reaction of 1 with primary amines and formaldehyde or aldehydes in a molar ratio (1:1:2). Bis-pyrimido[4,5-d]pyrimidine ring systems 6–8 were obtained through reaction of 1 with primary amines and formaldehyde in molar ration (2:1:4). Furthermore, transamination of 9 with ammonium carbonate gave symmetrical bis-Mannich base 10 which upon reaction with formalin gave the target pyrimido[4,5-d]pyrimidine 11. In other routes, compound 1 reacted with Schiff bases followed by reaction with formaldehyde or reacted with phenyl isothiocyanate followed by reaction with benzaldehyde. Yields are low (around 30%) for most of the substrates except for compound 4 (93%). because aldehyde and amine contains electron donating group “OCH3” which activate the two substrates then activate the reaction and therefore gave high yield.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Aly A.A. Synthesis and pharmacological activity of annelated pyrimidine derivatives. Phosphorus Sulfur Silicon Relat Elem. 2006;181(6):1285–1298. [Google Scholar]
- 2.Pinna C., Glass R., Knight G.E., Bolego C., Puglisi L., Burnstock G. Purine- and pyrimidine-induced responses and P2Y receptor characterization in the hamster proximal urethra. Br J Pharmacol. 2005;144(4):510–518. doi: 10.1038/sj.bjp.0706047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reznik V.S., Pashkurov N.G., Mikhailov A.S., Akamsin V.D., Galyametdinova I.V., Zobov V.V., et al. Synthesis and pharmacological activity of ω-(4-phenylpiperazin-1-yl)alkylthiopyrimidines. Pharm Chem J. 2004;38(12):654–658. [Google Scholar]
- 4.Sondhi S.M., Singh N., Johar M., Kumar A. Synthesis, anti-inflammatory and analgesic activities evaluation of some mono, bi and tricyclic pyrimidine derivatives. Bioorg Med Chem. 2005;13(22):6158–6166. doi: 10.1016/j.bmc.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 5.Bruno O., Brullo C., Ranise A., Schenone S., Bondavalli F., Barocelli E., et al. Synthesis and pharmacological evaluation of 2,5-cycloamino-5H-[1]benzopyrano[4,3-d]pyrimidines endowed within vitro antiplatelet activity. Bioorg Med Chem Lett. 2001;11(11):1397–1400. doi: 10.1016/s0960-894x(01)00221-9. [DOI] [PubMed] [Google Scholar]
- 6.Gangjee A., Vidwans A., Elzein E., Mc Cuire J.J., Queener S.F., Kisliuk R.L. Synthesis, antifolate, and antitumor activities of classical and nonclassical 2-amino-4-oxo-5-substituted-pyrrolo[2,3-d]pyrimidines. J Med Chem. 2001;44(12):1993–2003. doi: 10.1021/jm0100382. [DOI] [PubMed] [Google Scholar]
- 7.Banjanac M., Tatic I., Ivezic Z., Tomic S., Dumic J. Pyrimidopyrimidines: a novel class of dihydrofolate reductase inhibitors. Food Technol Biotechnol. 2009;47(3):236–245. [Google Scholar]
- 8.Mangalagiu G., Ungureanu M., Grosu G., Mangalagiu I., Petrovanu M. New pyrrolopyrimidine derivatives with antifungal or antibacterial properties. Ann Pharm Fr. 2001;59(2):139–140. [PubMed] [Google Scholar]
- 9.Wamhoff H., Dzenis J., Hirota K. In: 2nd ed. Katritzky A.R., editor. vol. 55. Academic Press, Inc.; New York: 1992. pp. 163–165. (Advances in Heterocyclic Chemistry). [Google Scholar]
- 10.Hamama W.S. Enaminouracils as precursors for the synthesis of pyrimido[4,5-d]pyrimidine-2,4-diones. Z Naturforsch. 2000;55b:443–447. [Google Scholar]
- 11.Rewcastle G.W., Bridges A.J., Fry D.W., Rubin J.R., Denny W.A. Tyrosine kinase inhibitors. 12. Synthesis and structure−activity relationships for 6-substituted 4-(phenylamino)pyrimido[5,4-d]pyrimidines designed as inhibitors of the epidermal growth factor receptor. J Med Chem. 1997;40(12):1820–1826. doi: 10.1021/jm960879m. [DOI] [PubMed] [Google Scholar]
- 12.Fry D.W., Becker M.A., Switzer R.L. Inhibition of human 5-phosphoribosyl-1-pyrophosphate synthetase by 4-amino-8-[β-d-ribofuranosylamino]pyrimido[5,4-d]pyrimidine-5′-monophosphate: evidence for interaction at the adenosine-5′-diphosphate allosteric site. Mol Pharmacol. 1995;47(4):810–815. [PubMed] [Google Scholar]
- 13.Gready J.E., McKinlay C., Gebauer M.G. Synthesis of quaternised 2-aminopyrimido[4,5-d]pyrimidin-4(3H)-ones and their biological activity with dihydrofolate reductase. Eur J Med Chem. 2003;38(7):719–728. doi: 10.1016/s0223-5234(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 14.Sanghhvi Y.S., Larson S.B., Matsumoto S.S., Nord L.D., Smee D.F., Willis R.C., et al. Antitumor and antiviral activity of synthetic alpha- and beta-ribonucleosides of certain substituted pyrimido[5,4-d]pyrimidines: a new synthetic strategy for exocyclic aminonucleosides. J Med Chem. 1989;32(3):629–637. doi: 10.1021/jm00123a022. [DOI] [PubMed] [Google Scholar]
- 15.Tenser R.B., Gaydos A., Hay K.A. Inhibition of herpes simplex virus reactivation by dipyridamole. Antimicrob Agent Chemother. 2001;45(12):3657–3659. doi: 10.1128/AAC.45.12.3657-3659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De la Cruz J.P., Carrasco T., Ortega G., Sanchez De la Cuesta F. Inhibition of ferrous-induced lipid peroxidation by pyrimidopyrimidine derivatives in human liver membranes. Lipids. 1992;27(3):192–194. doi: 10.1007/BF02536177. [DOI] [PubMed] [Google Scholar]
- 17.Furuya S, Ohtaki T. Pyrido[2,3-d]pyrimidines and their uses as anatagonists. Eur Pat Appl Ep. 608565, 1994; Chem Abstr.; 1994, 121:205395w.
- 18.Singh G., Singh G., Yadav A.K., Mishraa K. Synthesis and antimicrobial evaluation of some new pyrido[2,3-d]pyrimidines and their riboufuranosides. Ind J Chem. 2002;41(2):430–432. [Google Scholar]
- 19.Domling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev. 2006;106(1):17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 20.Ram V.J., Goel A., Sarkhel S., Maulik P.R. A convenient synthesis and hepatoprotective activity of imidazo[1,2-c]pyrimido[5,4-e]pyrimidine, tetraazaacenaphthene and tetraazaphenalene from cyclic ketene aminals through tandem addition-cyclization reactions. Bioorg Med Chem. 2002;10(5):1275–1280. doi: 10.1016/s0968-0896(01)00423-0. [DOI] [PubMed] [Google Scholar]
- 21.Bernier J.L., Henichart J.P., Warin V., Baert F. Synthesis and structure–activity relationship of a pyrimido[4,5-d]pyrimidine derivative with antidepressant activity. J Pharm Sci. 1980;69(11):1343–1345. doi: 10.1002/jps.2600691128. [DOI] [PubMed] [Google Scholar]
- 22.Abonia R., Albornoz A., Larrahondo H., Quiroga J., Insuasty B., Insuasty H., et al. Synthesis of pyrazole and pyrimidine Tröger’s-base analogues. J Chem Soc Perkin Trans. 2002;1:1588–1591. [Google Scholar]
- 23.Youssif S. 6-Aminouracil as precursors for the synthesis of fused di- and tricyclic pyrimidines. J Chem Res. 2004;2004(5):341–343. [Google Scholar]
- 24.Aksinenko AYu, Sokolov V.B., Goreva T.V., Epishina T.A., Pushin A.N. Synthesis of 6-substituted 5,6,7,8-tetrahydropyrimido[4,5-d]pyrimidine-2,4-diones and 2-thioxo-5,6,7,8-tetrahydropyrimido[4,5-d]pyrimidin-4-ones. Russ Chem Bull. 2008;57(7):1543–1546. [Google Scholar]
- 25.Youssif S., Agili F. One-Pot Synthesis of fused 2-thiouracils: pyrimido-pyrimidines, pyridopyrimidines and imidazolopyrimidines. Z Naturforsch B. 2008;63b:860–864. [Google Scholar]
- 26.Hammouda M., Hamama W.S., Afsah E.M. A study on the Mannich reaction with 1-phenylam-ino-3-indenone. Z Naturforsch. 1987;42b:94–96. [Google Scholar]
- 27.Hamama W.S., Hammouda M., Afsah E.M. Mannich reaction with 5,5-dimethyl-3-phenylamino-2-cyclohexen-1-one. Z Naturforsch. 1988;43b:483–486. [Google Scholar]
- 28.Roth H.J., Hagen H.-E. Aminomethylation of enamines of cyclic β-dicarbonyl compounds. Arch Pharm. 1971;304(5):331–341. doi: 10.1002/ardp.19713040504. [DOI] [PubMed] [Google Scholar]



