Abstract
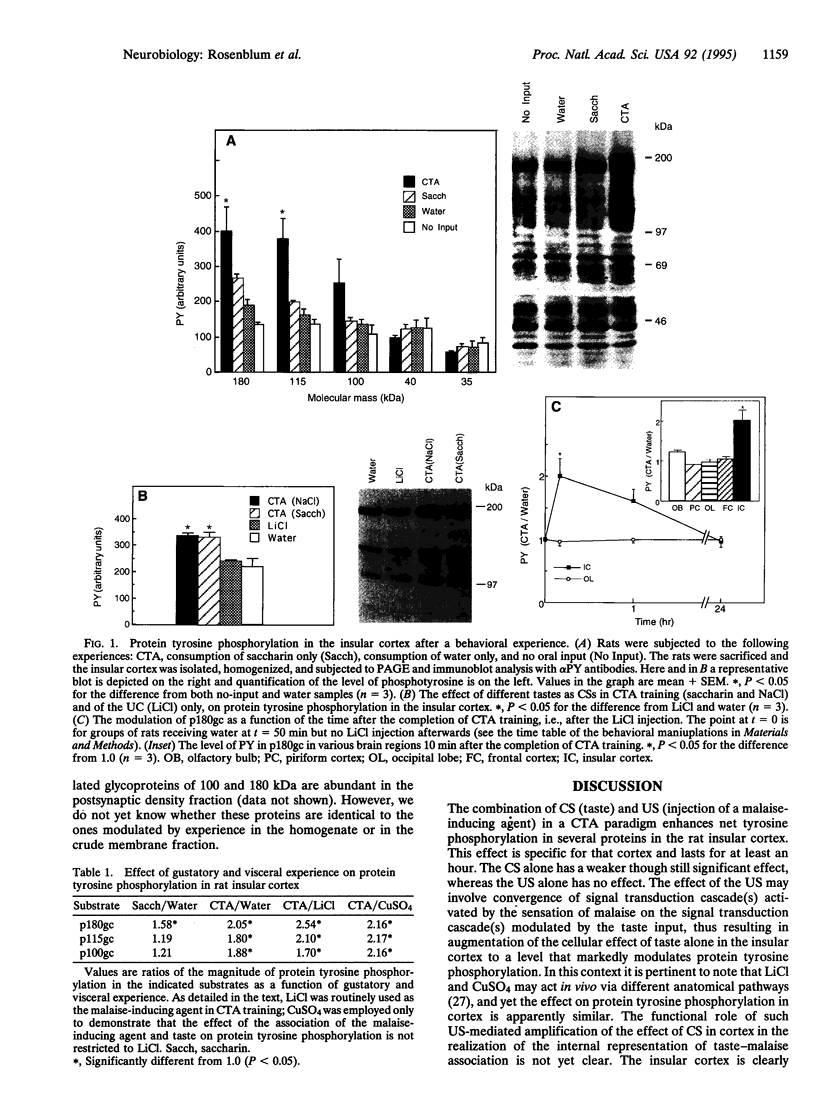
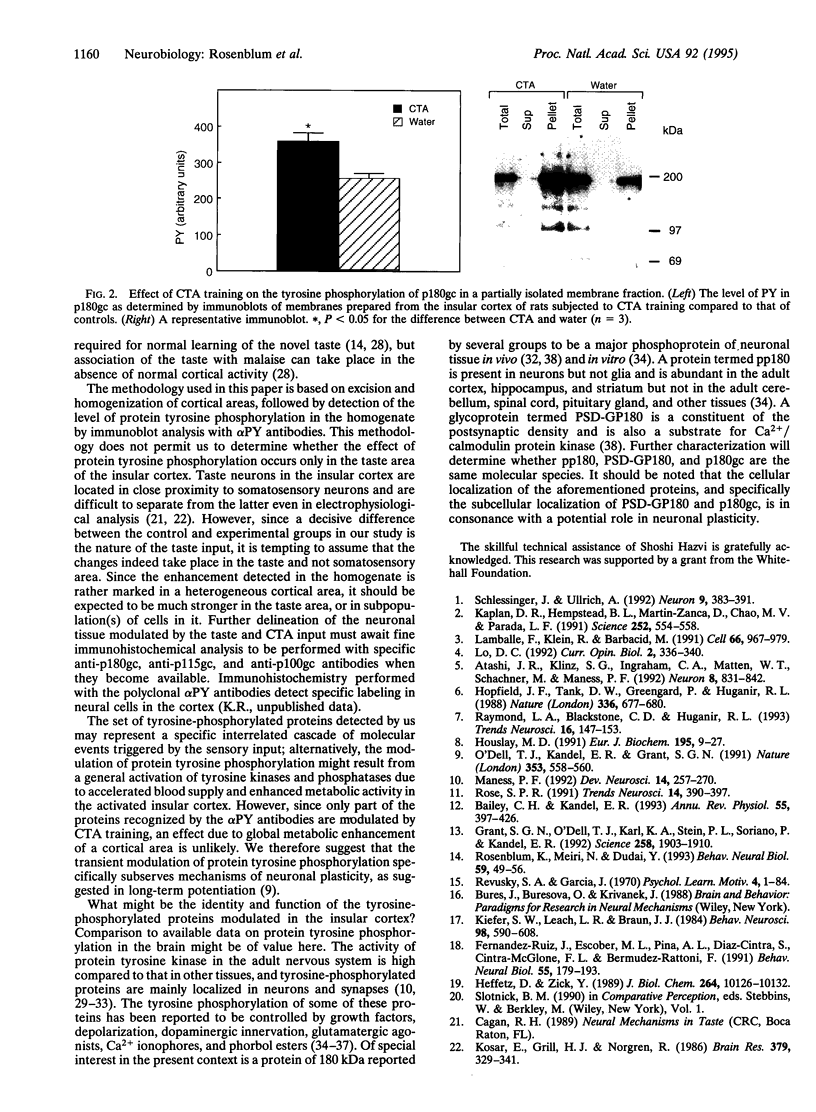
Protein tyrosine phosphorylation is a major signal transduction pathway involved in cellular metabolism, growth, and differentiation. Recent data indicate that tyrosine phosphorylation also plays a role in neuronal plasticity. We are using conditioned taste aversion, a fast and robust associative learning paradigm subserved among other brain areas by the insular cortex, to investigate molecular correlates of learning and memory in the rat cortex. In conditioned taste aversion, rats learn to associate a novel taste (e.g., saccharin) with delayed poisoning (e.g., by LiCl injection). Here we report that after conditioned taste aversion training, there is a rapid and marked increase in tyrosine phosphorylation of a set of proteins in the insular cortex but not in other brain areas. A major protein so modulated, of 180 kDa, is abundant in a membrane fraction and remains modulated for more than an hour after training. Exposure of the rats to the novel taste alone results in only a small modulation of the aforementioned proteins whereas administration of the malaise-inducing agent per se has no effect. To the best of our knowledge, this is the first demonstration of modulation of protein tyrosine phosphorylation in the brain after a behavioral experience.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atashi J. R., Klinz S. G., Ingraham C. A., Matten W. T., Schachner M., Maness P. F. Neural cell adhesion molecules modulate tyrosine phosphorylation of tubulin in nerve growth cone membranes. Neuron. 1992 May;8(5):831–842. doi: 10.1016/0896-6273(92)90197-l. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Kandel E. R. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Cudmore S. B., Gurd J. W. Postnatal age and protein tyrosine phosphorylation at synapses in the developing rat brain. J Neurochem. 1991 Oct;57(4):1240–1248. doi: 10.1111/j.1471-4159.1991.tb08285.x. [DOI] [PubMed] [Google Scholar]
- Ellis P. D., Bissoon N., Gurd J. W. Synaptic protein tyrosine kinase: partial characterization and identification of endogenous substrates. J Neurochem. 1988 Aug;51(2):611–620. doi: 10.1111/j.1471-4159.1988.tb01082.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J., Escobar M. L., Piña A. L., Diaz-Cintra S., Cintra-McGlone F. L., Bermúdez-Rattoni F. Time-dependent recovery of taste aversion learning by fetal brain transplants in gustatory neocortex-lesioned rats. Behav Neural Biol. 1991 Mar;55(2):179–193. doi: 10.1016/0163-1047(91)80138-5. [DOI] [PubMed] [Google Scholar]
- Gallo M., Roldan G., Bures J. Differential involvement of gustatory insular cortex and amygdala in the acquisition and retrieval of conditioned taste aversion in rats. Behav Brain Res. 1992 Nov 30;52(1):91–97. doi: 10.1016/s0166-4328(05)80328-6. [DOI] [PubMed] [Google Scholar]
- Gamzu E., Vincent G., Boff E. A pharmacological perspective of drugs used in establishing conditioned food aversions. Ann N Y Acad Sci. 1985;443:231–249. doi: 10.1111/j.1749-6632.1985.tb27077.x. [DOI] [PubMed] [Google Scholar]
- Girault J. A., Chamak B., Bertuzzi G., Tixier H., Wang J. K., Pang D. T., Greengard P. Protein phosphotyrosine in mouse brain: developmental changes and regulation by epidermal growth factor, type I insulin-like growth factor, and insulin. J Neurochem. 1992 Feb;58(2):518–528. doi: 10.1111/j.1471-4159.1992.tb09751.x. [DOI] [PubMed] [Google Scholar]
- Girault J. A., Siciliano J. C., Robel L., Hervé D. Stimulation of protein-tyrosine phosphorylation in rat striatum after lesion of dopamine neurons or chronic neuroleptic treatment. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2769–2773. doi: 10.1073/pnas.89.7.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S. G., O'Dell T. J., Karl K. A., Stein P. L., Soriano P., Kandel E. R. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992 Dec 18;258(5090):1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Gurd J. W., Bissoon N. Phosphorylation of proteins of the postsynaptic density: effect of development on protein tyrosine kinase and phosphorylation of the postsynaptic density glycoprotein, PSD-GP180. J Neurosci Res. 1990 Mar;25(3):336–344. doi: 10.1002/jnr.490250310. [DOI] [PubMed] [Google Scholar]
- Heffetz D., Zick Y. H2O2 potentiates phosphorylation of novel putative substrates for the insulin receptor kinase in intact Fao cells. J Biol Chem. 1989 Jun 15;264(17):10126–10132. [PubMed] [Google Scholar]
- Hirano A. A., Greengard P., Huganir R. L. Protein tyrosine kinase activity and its endogenous substrates in rat brain: a subcellular and regional survey. J Neurochem. 1988 May;50(5):1447–1455. doi: 10.1111/j.1471-4159.1988.tb03029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. F., Tank D. W., Greengard P., Huganir R. L. Functional modulation of the nicotinic acetylcholine receptor by tyrosine phosphorylation. Nature. 1988 Dec 15;336(6200):677–680. doi: 10.1038/336677a0. [DOI] [PubMed] [Google Scholar]
- Houslay M. D. 'Crosstalk': a pivotal role for protein kinase C in modulating relationships between signal transduction pathways. Eur J Biochem. 1991 Jan 1;195(1):9–27. doi: 10.1111/j.1432-1033.1991.tb15671.x. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Hempstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991 Apr 26;252(5005):554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Kiefer S. W., Leach L. R., Braun J. J. Taste agnosia following gustatory neocortex ablation: dissociation from odor and generality across taste qualities. Behav Neurosci. 1984 Aug;98(4):590–608. doi: 10.1037//0735-7044.98.4.590. [DOI] [PubMed] [Google Scholar]
- Kosar E., Grill H. J., Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1986 Aug 6;379(2):329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- Lamballe F., Klein R., Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991 Sep 6;66(5):967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- Li P. P., Sibony D., Green M. A., Warsh J. J. Lithium modulation of phosphoinositide signaling system in rat cortex: selective effect on phorbol ester binding. J Neurochem. 1993 Nov;61(5):1722–1730. doi: 10.1111/j.1471-4159.1993.tb09809.x. [DOI] [PubMed] [Google Scholar]
- Lo D. C. Signal transduction and regulation of neurotrophins. Curr Opin Neurobiol. 1992 Jun;2(3):336–340. doi: 10.1016/0959-4388(92)90125-5. [DOI] [PubMed] [Google Scholar]
- Maness P. F. Nonreceptor protein tyrosine kinases associated with neuronal development. Dev Neurosci. 1992;14(4):257–270. doi: 10.1159/000111670. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R., Grant S. G. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991 Oct 10;353(6344):558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Pang D. T., Wang J. K., Valtorta F., Benfenati F., Greengard P. Protein tyrosine phosphorylation in synaptic vesicles. Proc Natl Acad Sci U S A. 1988 Feb;85(3):762–766. doi: 10.1073/pnas.85.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A., Steward O. Evidence that protein constituents of postsynaptic membrane specializations are locally synthesized: analysis of proteins synthesized within synaptosomes. J Neurosci. 1991 Sep;11(9):2881–2895. doi: 10.1523/JNEUROSCI.11-09-02881.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond L. A., Blackstone C. D., Huganir R. L. Phosphorylation of amino acid neurotransmitter receptors in synaptic plasticity. Trends Neurosci. 1993 Apr;16(4):147–153. doi: 10.1016/0166-2236(93)90123-4. [DOI] [PubMed] [Google Scholar]
- Rose S. P. How chicks make memories: the cellular cascade from c-fos to dendritic remodelling. Trends Neurosci. 1991 Sep;14(9):390–397. doi: 10.1016/0166-2236(91)90027-r. [DOI] [PubMed] [Google Scholar]
- Rosenblum K., Meiri N., Dudai Y. Taste memory: the role of protein synthesis in gustatory cortex. Behav Neural Biol. 1993 Jan;59(1):49–56. doi: 10.1016/0163-1047(93)91145-d. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992 Sep;9(3):383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Siciliano J. C., Gelman M., Girault J. A. Depolarization and neurotransmitters increase neuronal protein tyrosine phosphorylation. J Neurochem. 1994 Mar;62(3):950–959. doi: 10.1046/j.1471-4159.1994.62030950.x. [DOI] [PubMed] [Google Scholar]
- Wagner K. R., Mei L., Huganir R. L. Protein tyrosine kinases and phosphatases in the nervous system. Curr Opin Neurobiol. 1991 Jun;1(1):65–73. doi: 10.1016/0959-4388(91)90011-u. [DOI] [PubMed] [Google Scholar]
- Woodrow S., Bissoon N., Gurd J. W. Depolarization-dependent tyrosine phosphorylation in rat brain synaptosomes. J Neurochem. 1992 Sep;59(3):857–862. doi: 10.1111/j.1471-4159.1992.tb08323.x. [DOI] [PubMed] [Google Scholar]