Abstract
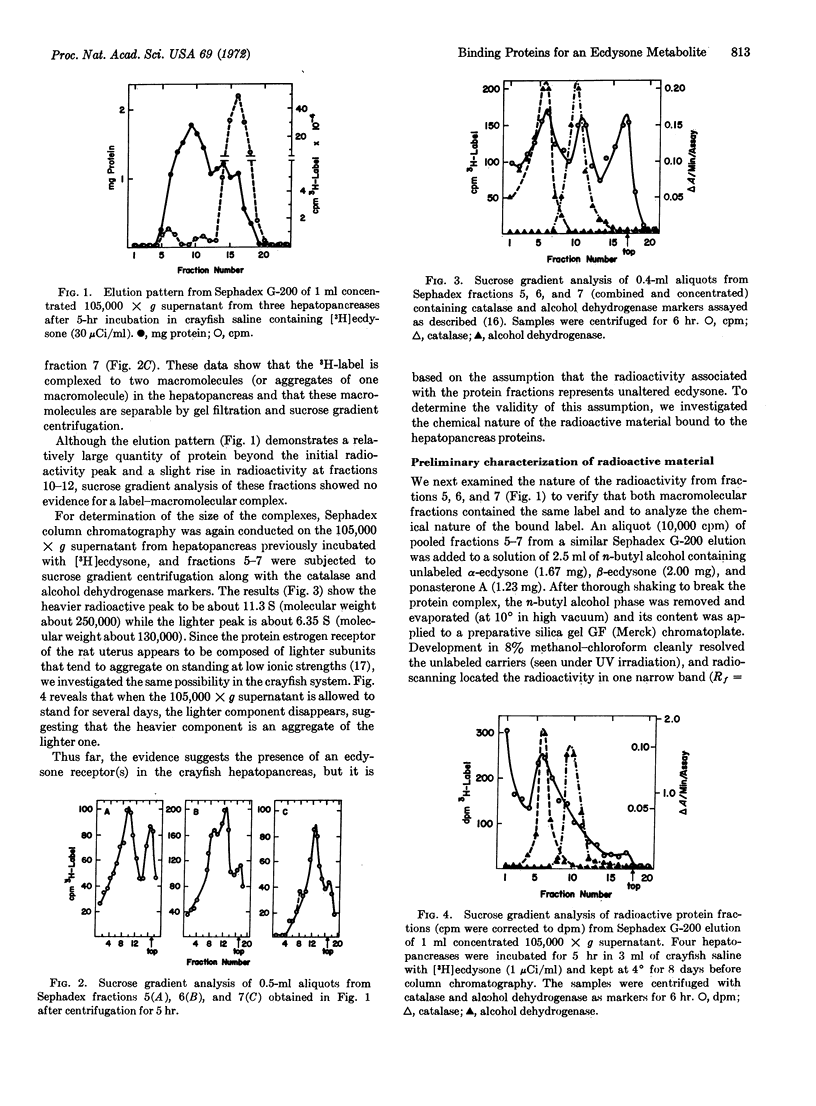
When crustacean hepatopancreas is incubated in the presence of α-3H]ecdysone of high specific activity and is then homogenized and centrifuged, a peak of protein-radioactivity is recovered after gel filtration of the 105,000 × g supernatant. Analysis of this peak by sucrose gradient centrifugation revealed the presence of two complexes of protein and labeled material (∼11.5 S and 6.35 S). The same results were obtained in vivo. On standing at low ionic strength, the lighter component disappeared, suggesting that the heavier component is an aggregate of the lighter one. Chemical analysis of radioactive material in the complex revealed that it is not α- or β-ecdysone nor any previously described metabolite of the ecdysones. This new metabolite of α-ecdysone is found mainly in the incubated hepatopancreas. Partial structures consistent with the analytical data are inferred for this metabolite. It is suggested that the metabolite may be active in the action of molting hormone.
Keywords: molting hormone, steroid, sucrose gradient centrifugation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulieu E. E., Lasnitzki I., Robel P. Testosterone, prostate gland and hormone action. Biochem Biophys Res Commun. 1968 Aug 13;32(3):575–577. doi: 10.1016/0006-291x(68)90702-x. [DOI] [PubMed] [Google Scholar]
- Chino H., Gilbert L. I., Siddall J. B., Hafferl W. Studies on ecdysone transport in insect haemolymph. J Insect Physiol. 1970 Nov;16(11):2033–2040. doi: 10.1016/0022-1910(70)90076-4. [DOI] [PubMed] [Google Scholar]
- Gorell T. A., Gilbert L. I. Stimulation of protein and RNA synthesis in the crayfish hepatopancreas by crustecdysone. Gen Comp Endocrinol. 1969 Oct;13(2):308–310. doi: 10.1016/0016-6480(69)90253-6. [DOI] [PubMed] [Google Scholar]
- Gorski J., Toft D., Shyamala G., Smith D., Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res. 1968;24:45–80. doi: 10.1016/b978-1-4831-9827-9.50008-3. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Numata M., Smith S., DeSombre E. R. Estrogen-binding substances of target tissues. Steroids. 1969 Apr;13(4):417–427. doi: 10.1016/0039-128x(69)90053-1. [DOI] [PubMed] [Google Scholar]
- King D. S., Siddall J. B. Conversion of alpha ecdysone to beta ecdysone by crustaceans and insects. Nature. 1969 Mar 8;221(5184):955–956. doi: 10.1038/221955a0. [DOI] [PubMed] [Google Scholar]
- Krishnakumaran A., Schneiderman H. A. Chemical control of moulting in arthropods. Nature. 1968 Nov 9;220(5167):601–603. doi: 10.1038/220601a0. [DOI] [PubMed] [Google Scholar]
- Krishnakumaran A., Schneiderman H. A. Induction of molting in Crustacea by an insect molting hormone. Gen Comp Endocrinol. 1969 Jun;12(3):515–518. doi: 10.1016/0016-6480(69)90168-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowe M. E., Horn D. H., Galbraith M. N. The role of crustecdysone in the moulting crayfish. Experientia. 1968 May 15;24(5):518–519. doi: 10.1007/BF02144428. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Moriyama H., Nakanishi K., King D. S., Okauchi T., Siddall J. B., Hafferl W. On the origin and metabolic fate of alpha-ecdysone in insects. Gen Comp Endocrinol. 1970 Aug;15(1):80–87. doi: 10.1016/0016-6480(70)90099-7. [DOI] [PubMed] [Google Scholar]
- O'Connor J. D., Gilbert L. I. Aspects of lipid metabolism in crustaceans. Am Zool. 1968 Aug;8(3):529–539. doi: 10.1093/icb/8.3.529. [DOI] [PubMed] [Google Scholar]


