Abstract
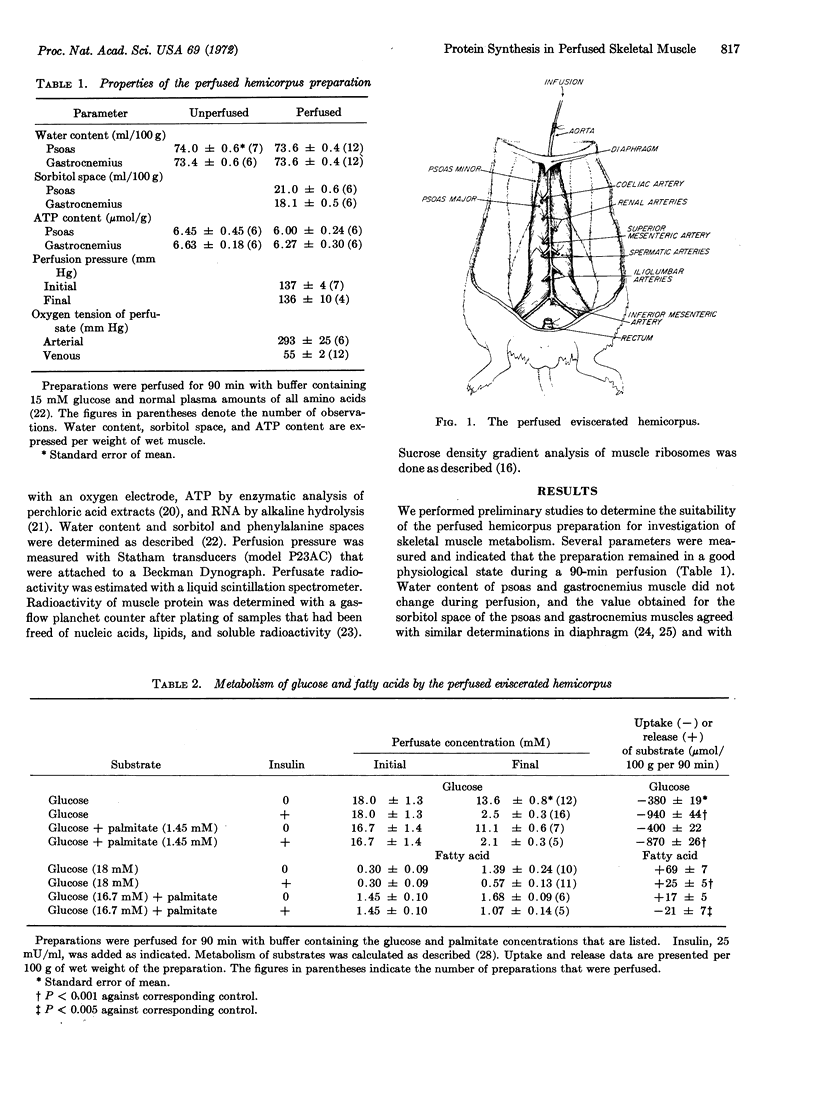
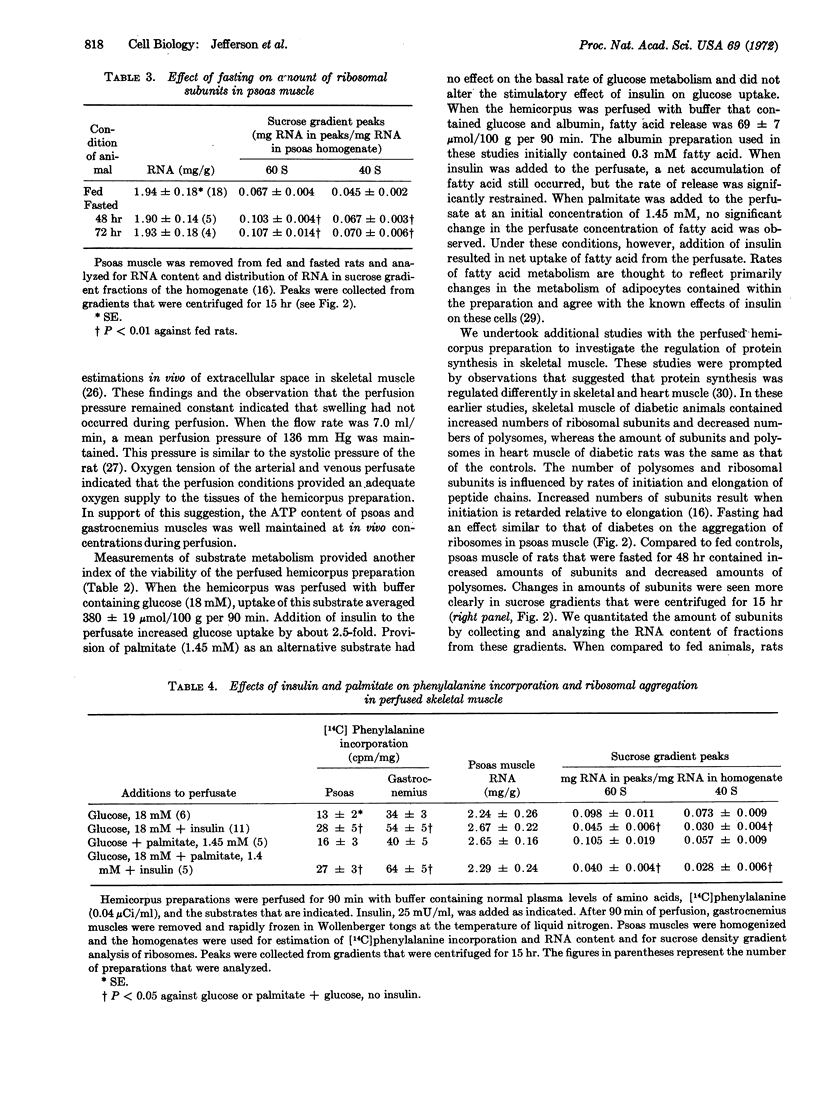
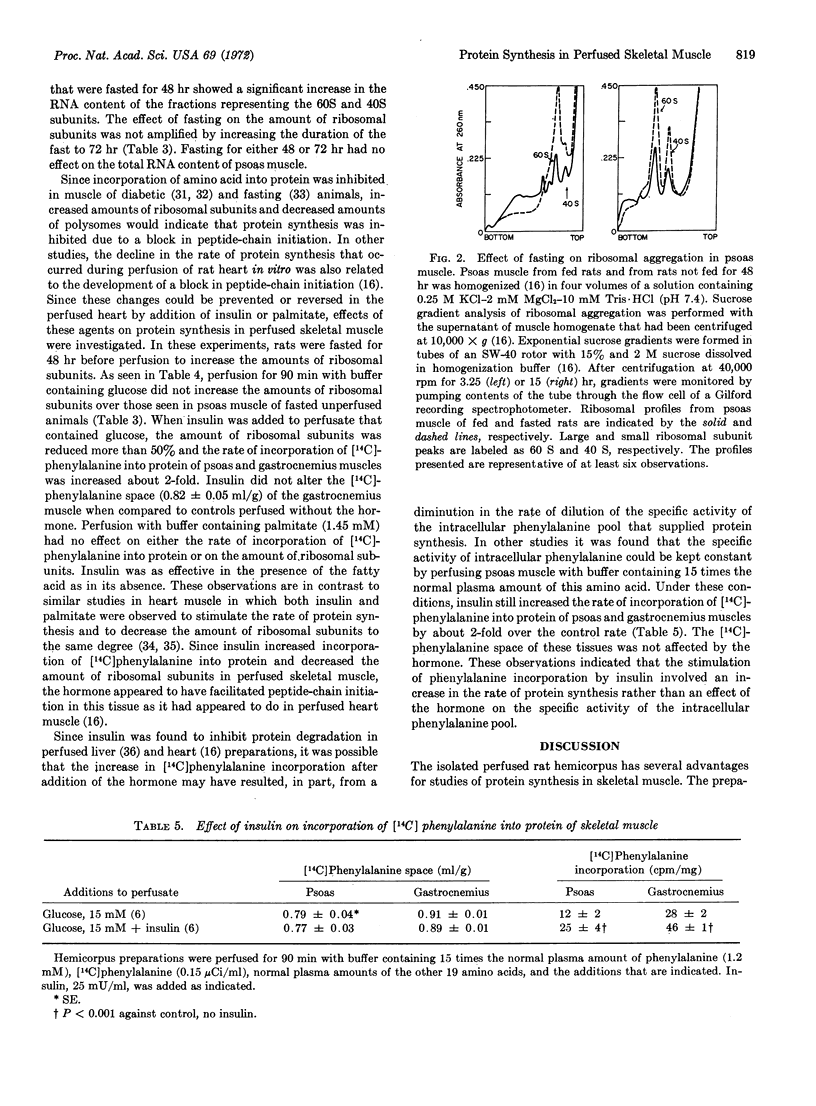
A method for perfusion in vitro of a preparation of rat hemicorpus was developed for study of the metabolism of skeletal muscle. The preparation was stable during perfusion, as indicated by maintenance of ATP concentration, perfusion pressure, and oxygen consumption for up to 90 min. The perfused hemicorpus provided the following advantages for study of protein synthesis in skeletal muscle: (a) hormones and substrates reached the muscle cells through an intact capillary bed, and (b) the preparation included the psoas muscle, which was sufficiently large to allow measurements of intermediates in the pathway of protein synthesis and was readily homogenized for preparation of ribosomes and ribosomal subunits. Perfusion of psoas muscle from fasted rats with buffer containing glucose and insulin reduced the concentration of ribosomal subunits and increased phenylalanine incorporation as compared to perfusion with buffer containing glucose alone. In addition, the hormone increased glucose uptake from the perfusate and inhibited release of free fatty acids from the preparation. When the muscle was perfused with buffer that contained glucose and palmitate, the concentration of ribosomal subunits and phenylalanine incorporation were unchanged. Since fatty acid is known to stimulate protein synthesis in heart muscle, these results indicated that rates of protein synthesis in heart, but not in skeletal, muscle would be maintained during fasting or in diabetic animals by increased plasma concentration of fatty acid.
Keywords: psoas muscle, gastrocnemius muscle, ribosomal subunits, fatty acid, fasting
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALL E. G., JUNGAS R. L. SOME EFFECTS OF HORMONES ON THE METABOLISM OF ADIPOSE TISSUE. Recent Prog Horm Res. 1964;20:183–214. [PubMed] [Google Scholar]
- BATTAGLIA F. C., RANDLE P. J. Regulation of glucose uptake by muscle. 4. The specificity of monosaccharide-transport systems in rat-diaphragm muscle. Biochem J. 1960 May;75:408–416. doi: 10.1042/bj0750408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORSOOK H., DEASY C. L., HAAGENSMIT A. J., KEIGHLEY G., LOWY P. H. Incorporation in vitro of labeled amino acids into rat diaphragm proteins. J Biol Chem. 1950 Sep;186(1):309–315. [PubMed] [Google Scholar]
- Beatty C. H., Bocek R. M. Interrelation of carbohydrate and palmitate metabolism in skeletal muscle. Am J Physiol. 1971 Jun;220(6):1928–1934. doi: 10.1152/ajplegacy.1971.220.6.1928. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Henshaw E. C., Hirsch C. A., Morton B. E., Hiatt H. H. Control of protein synthesis in mammalian tissues through changes in ribosome activity. J Biol Chem. 1971 Jan 25;246(2):436–446. [PubMed] [Google Scholar]
- Hider R. C., Fern E. B., London D. R. Identification in skeletal muscle of a distinct extracellular pool of amino acids, and its role in protein synthesis. Biochem J. 1971 Mar;121(5):817–827. doi: 10.1042/bj1210817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hider R. C., Fern E. B., London D. R. Relationship between intracellular amino acids and protein synthesis in the extensor digitorum longus muscle of rats. Biochem J. 1969 Sep;114(2):171–178. doi: 10.1042/bj1140171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton C. R., Ruderman N. B. Acetoacetate as a fuel for perfused rat skeletal muscle. Biochem J. 1971 Jan;121(1):15P–16P. doi: 10.1042/bj1210015pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. III. The effect of insulin on pentose uptake by the diaphragm. J Biol Chem. 1957 Feb;224(2):681–693. [PubMed] [Google Scholar]
- KIPNIS D. M., REISS E., HELMREICH E. Functional heterogeneity of the intracellular amino acid pool in mammalian cells. Biochim Biophys Acta. 1961 Aug 19;51:519–524. doi: 10.1016/0006-3002(61)90608-4. [DOI] [PubMed] [Google Scholar]
- KOSTYO J. L., HOTCHKISS J., KNOBIL E. Stimulation of amino acid transport in isolated diaphragm by growth hormone added in vitro. Science. 1959 Dec 11;130(3389):1653–1654. doi: 10.1126/science.130.3389.1653. [DOI] [PubMed] [Google Scholar]
- KOSTYO J. L. SEPARATION OF THE EFFECTS OF GROWTH HORMONE ON MUSCLE AMINO ACID TRANSPORT AND PROTEIN SYNTHESIS. Endocrinology. 1964 Jul;75:113–119. doi: 10.1210/endo-75-1-113. [DOI] [PubMed] [Google Scholar]
- KRAHL M. E. Incorporation of C14-amino acids into glutathione and protein fractions of normal and diabetic rat tissues. J Biol Chem. 1953 Jan;200(1):99–109. [PubMed] [Google Scholar]
- KRAHL M. E. Incorporation of C14-amino acids into glutathione and protein fractions of normal and diabetic rat tissues. J Biol Chem. 1953 Jan;200(1):99–109. [PubMed] [Google Scholar]
- Kurihara K., Wool I. G. Effect of insulin on the synthesis of sarcoplasmic and ribosomal proteins of muscle. Nature. 1968 Aug 17;219(5155):721–724. doi: 10.1038/219721a0. [DOI] [PubMed] [Google Scholar]
- Little J. R., Goto M., Spitzer J. J. Effect of ketones on metabolism of FFA by dog myocardium and skeletal muscle in vivo. Am J Physiol. 1970 Nov;219(5):1458–1463. doi: 10.1152/ajplegacy.1970.219.5.1458. [DOI] [PubMed] [Google Scholar]
- MANCHESTER K. L., YOUNG F. G. The effect of insulin on incorporation of amino acids into protein of normal rat diaphragm in vitro. Biochem J. 1958 Nov;70(3):353–358. doi: 10.1042/bj0700353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem. 1961 Feb;236:253–261. [PubMed] [Google Scholar]
- MORTIMORE G. E., TIETZE F., STETTEN D., Jr Metabolism of insulin-I 131; studies in isolated, perfused rat liver and hindlimb preparations. Diabetes. 1959 Jul-Aug;8(4):307–314. doi: 10.2337/diab.8.4.307. [DOI] [PubMed] [Google Scholar]
- Mahler R. J., Szabo O., Penhos J. C. Antagonism to insulin action on the perfused hind limb of the rat by a reduced insulin B chain-albumin complex. Diabetes. 1968 Jan;17(1):1–7. doi: 10.2337/diab.17.1.1. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Earl D. C., Broadus A., Wolpert E. B., Giger K. E., Jefferson L. S. Regulation of protein synthesis in heart muscle. I. Effect of amino acid levels on protein synthesis. J Biol Chem. 1971 Apr 10;246(7):2152–2162. [PubMed] [Google Scholar]
- Morgan H. E., Jefferson L. S., Wolpert E. B., Rannels D. E. Regulation of protein synthesis in heart muscle. II. Effect of amino acid levels and insulin on ribosomal aggregation. J Biol Chem. 1971 Apr 10;246(7):2163–2170. [PubMed] [Google Scholar]
- Mortimore G. E., Mondon C. E. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970 May 10;245(9):2375–2383. [PubMed] [Google Scholar]
- Munro A. J., Jackson R. J., Korner A. Studies on the nature of polysomes. Biochem J. 1964 Aug;92(2):289–299. doi: 10.1042/bj0920289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLS G., Jr, NICHOLS N., WEIL W. B., WALLACE W. M. The direct measurement of the extracellular phase of tissues. J Clin Invest. 1953 Dec;32(12):1299–1308. doi: 10.1172/JCI102858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely J. R., Bowman R. H., Morgan H. E. Effects of ventricular pressure development and palmitate on glucose transport. Am J Physiol. 1969 Apr;216(4):804–811. doi: 10.1152/ajplegacy.1969.216.4.804. [DOI] [PubMed] [Google Scholar]
- Noble R. P. Automatic titration of plasma fatty acids by photocolorimetry. J Lipid Res. 1966 Nov;7(6):745–749. [PubMed] [Google Scholar]
- ROBINSON D. S., HARRIS P. M. The production of lipolytic activity in the circulation of the hind limb in response to heparin. Q J Exp Physiol Cogn Med Sci. 1959 Jan;44(1):80–90. doi: 10.1113/expphysiol.1959.sp001378. [DOI] [PubMed] [Google Scholar]
- Rannels D. E., Jefferson L. S., Hjalmarson A. C., Wolpert E. B., Morgan H. E. Maintenance of protein synthesis in hearts of diabetic animals. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1110–1116. doi: 10.1016/0006-291x(70)90909-5. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINEX F. M., MACMULLEN J., HASTINGS A. B. The effect of insulin on the incorporation of C14 into the protein of rat diaphragm. J Biol Chem. 1952 Oct;198(2):615–619. [PubMed] [Google Scholar]
- Schonfeld G., Kipnis D. M. Effects of fatty acids on carbohydrate and fatty acid metabolism of rat diaphragm. Am J Physiol. 1968 Aug;215(2):513–522. doi: 10.1152/ajplegacy.1968.215.2.513. [DOI] [PubMed] [Google Scholar]
- WOOL I. G., KRAHL M. E. Incorporation of C14-amino acids into protein of isolated diaphragms: an effect of insulin independent of glucose entry. Am J Physiol. 1959 May;196(5):961–964. doi: 10.1152/ajplegacy.1959.196.5.961. [DOI] [PubMed] [Google Scholar]