Abstract
Purpose
To compare the perioperative outcomes of laparoscopic partial nephrectomy (LPN) and robotic partial nephrectomy (RPN) for moderately or highly complex tumors (RENAL nephrometry score≥7).
Materials and Methods
A retrospective analysis was performed for 127 consecutive patients who underwent either LPN (n=38) or RPN (n=89) between 2007 and 2013. Perioperative outcomes were compared.
Results
There were no significant differences between the two groups with respect to patient gender, laterality, RENAL nephrometry score, or body mass index. The RPN group had a slightly higher RENAL nephrometry score (7.8 vs. 7.5, p=0.061) and larger tumor size (3.0 cm vs. 2.5 cm, p=0.044) but had a lower Charlson comorbidity index (3.7 vs. 4.4, p=0.017) than did the LPN group. There were no significant differences with respect to warm ischemia time, estimated blood loss, intraoperative complications, or operative time. Only one patient who underwent LPN had a positive surgical margin. There were statistically significant differences in surgical marginal width between the LPN and RPN groups (0.6 cm vs. 0.4 cm, p=0.001). No significant differences in postoperative complications were found between the two groups. Owing to potential baseline differences between the two groups, we performed a propensity-based matching analysis, in which differences in surgical margin width between the LPN and RPN groups remained statistically significant (0.6 cm vs. 0.4 cm, p=0.029).
Conclusions
RPN provides perioperative outcomes comparable to those of LPN and has the advantage of healthy parenchymal preservation for complex renal tumors (RENAL score≥7).
Keywords: Laparoscopy, Nephrectomy, Renal cell carcinoma, Robotics
INTRODUCTION
Partial nephrectomy currently represents the gold standard of care for clinical T1 renal masses, because it can provide oncologic outcomes equivalent to those of radical nephrectomy [1,2]. Accumulating data suggest that partial nephrectomy can reduce the morbidity and mortality resulting from renal insufficiency [1,2,3,4,5]. The expansion of minimally invasive techniques has allowed laparoscopic partial nephrectomy (LPN) to be performed for small renal masses with oncologic and functional outcomes comparable to those of open partial nephrectomy [6,7,8]. However, LPN involves certain technical challenges because it requires intracorporeal suturing skills and shortened warm ischemia time (WIT) [9]. Robot-assisted partial nephrectomy (RPN) has emerged as a viable alternative to help mitigate the technical challenges of LPN, demonstrating perioperative outcomes at least comparable to LPN, with the benefit of reduced WIT [9,10,11]. The unique technology of the robotic platform (wristed instrumentation and three-dimensional, high-definition magnified vision) may make nephron-sparing surgeries more readily available to urological surgeons and expand minimally invasive management to more difficult tumors [12,13,14]. Recent studies have shown that increasing tumor complexity is less detrimental in terms of operative parameters for RPN than it is for LPN. The indications for RPN have been expanded to treat patients with both moderately and highly complex renal masses. For characterization of renal tumor anatomy in a quantifiable manner, we chose to use the RENAL nephrometry score to designate the complexity of renal masses [8]. There are few comparative studies on perioperative outcomes with RPN and LPN among moderate- to high-complexity tumors. We report our single-surgeon experience with RPN and LPN in patients with moderately and highly complex renal masses. The aim of this study was to compare the perioperative outcomes of patients undergoing LPN and RPN for a single renal mass of moderate or high complexity (RENAL nephrometry score≥7).
MATERIALS AND METHODS
After obtaining Institutiotional Review Board approval at Samsung Medical Center (No. 2014-05-118), we reviewed data of 322 patients who underwent minimally invasive partial nephrectomy by a single surgeon between September 2007 and May 2013 at Samsung Medical Center. A total of 150 patients who underwent LPN or RPN for a complex renal mass (RENAL nephrometry score≥7) were identified. Of the 150 patients, 23 cases were excluded from the study because of preoperative chronic kidney disease stage 5, a solitary kidney, multiple or bilateral tumors, metastatic disease, or loss to follow-up. Retrospective analysis was performed for 127 consecutive patients, 89 of whom underwent RPN. RPN was performed by using the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA).
RENAL nephrometry scores were determined by a retrospective review of imaging. A total of 89 patients underwent RPN, with 72 patients (80.9%) having a RENAL score of 7 to 8 and 17 patients (19.1%) having a RENAL score of 9 to 10. A total of 38 patients underwent LPN, with 34 patients (89.5%) having a RENAL score of 7 to 8 and 4 patients (10.5%) having a RENAL score of 9 to 10. No patients had a RENAL score≥11 in either group.
Methods of hilar control were consistent between the LPN and RPN groups. For most lesions, the renal artery was controlled with bulldog clamps. The outcomes of these patients were compared with those of patients who underwent LPN (n=38).
Perioperative data and clinical and pathologic outcomes were retrospectively reviewed. The operative time was considered to be from the initial intraperitoneal gas insufflation to intraperitoneal gas discharge to avoid biases due to setup time or anesthesia time. Marginal width was defined as the shortest distance between the tumor capsule and the resection margin. Postoperative complications were identified within 30 days of the operation. Complications were stratified by severity of the Clavien-Dino classification. Ileus was defined as prolonged return of bowel function as identified radiographically. Respiratory complications (atelectasis, pleural effusion, pneumothorax, subcutaneous emphysema) were identified radiographically. Lymphatic leak was identified by Jackson-Pratt drain color and a drain fluid lab test. The Modification of Diet in Renal Disease (MDRD) calculator was used to calculate preoperative and postoperative estimated glomerular filtration rates (eGFRs).
Descriptive statistics focused on frequencies for categorical variables. Means were reported for continuous variables. Chi-square and independent-sample t-tests were used to compare the statistical significance of differences in frequencies and means, respectively. Propensity-based matching was performed to adjust for potential baseline differences between the two groups. Cohorts were matched by patient characteristics: age, gender, Charlson comorbidity index (CCI), and RENAL nephrometry score.
RESULTS
The patient demography and clinical and pathologic data are listed in Table 1. There were no significant differences between the two groups with respect to gender, laterality, RENAL nephrometry score, or body mass index. Patients undergoing RPN had a slightly higher RENAL nephrometry score, but this difference was not statistically significant (7.8 vs. 7.5, respectively, p=0.061). Patients undergoing RPN had a larger tumor size (3.0 cm vs. 2.5 cm, respectively, p=0.044), but had a lower CCI (3.7 vs. 4.4, respectively, p=0.017).
TABLE 1.
Demography and tumor characteristics
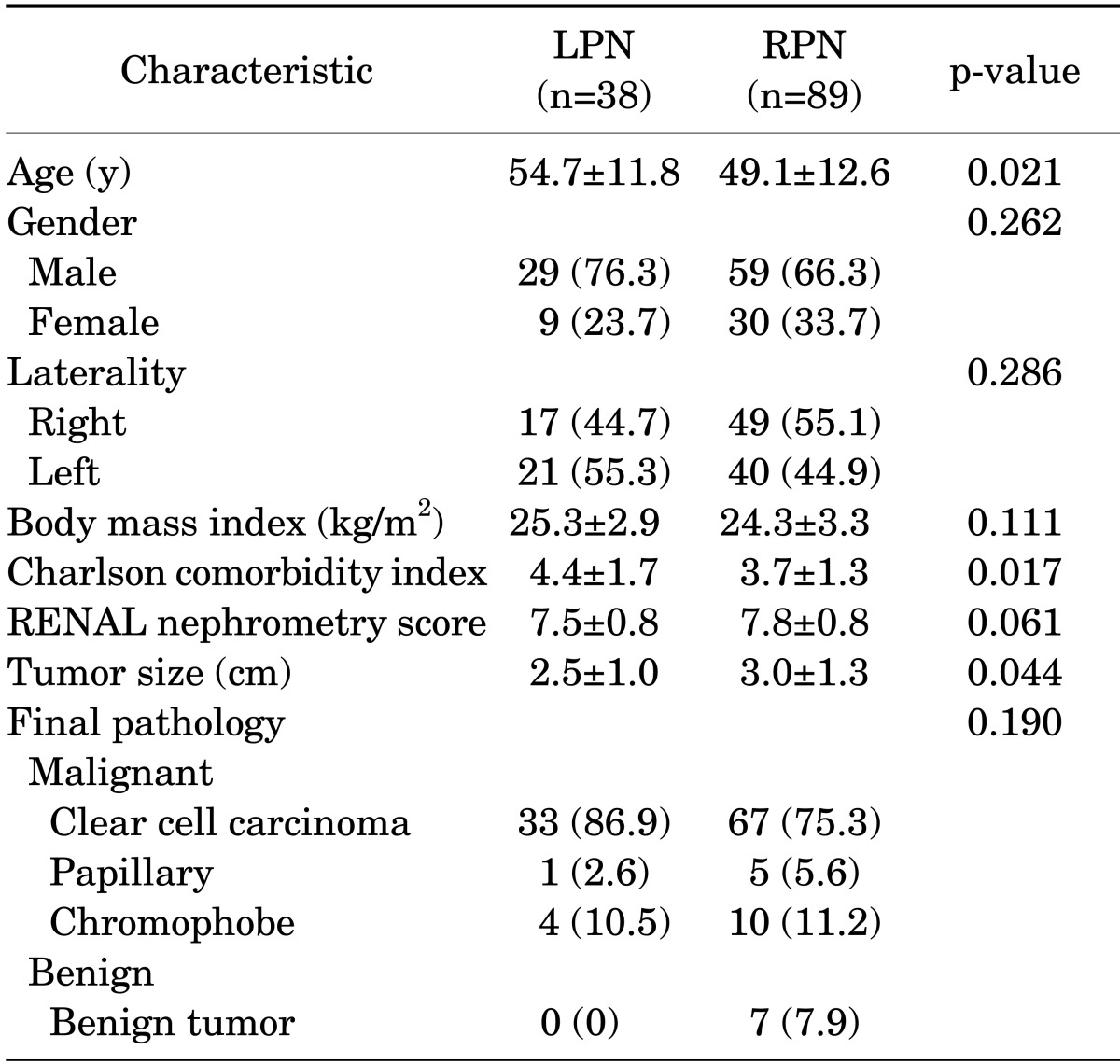
Values are presented as mean±standard deviation or number (%).
LPN, laparoscopic partial nephrectomy; RPN, robot-assisted laparoscopic partial nephrectomy.
Table 2 shows the operative and postoperative outcomes stratified according to LPN and RPN. Estimated blood loss, WIT, intraoperative complications, and hospital stay were not significantly different between the two groups. The operative time for LPN patients was longer than that for RPN patients, but the differences did not reach statistical significance (168.5 minutes vs. 143.9 minutes, p=0.061). There was no conversion to radical nephrectomy in either group. One of the patients who underwent only LPN had a positive surgical margin, but this was not significantly different from the RPN group (2.6% vs. 0%, respectively, p=0.299). There were statistically significant differences in surgical marginal width between the LPN and RPN groups (0.6 cm vs. 0.4 cm, respectively, p=0.001). Although the differences in transfusion rate between the two groups were not statistically significant, the rate of transfusion in the LPN group was higher than that in the RPN group (10.5% vs. 4.5%, respectively, p=0.239).
TABLE 2.
Operative and postoperative outcomes
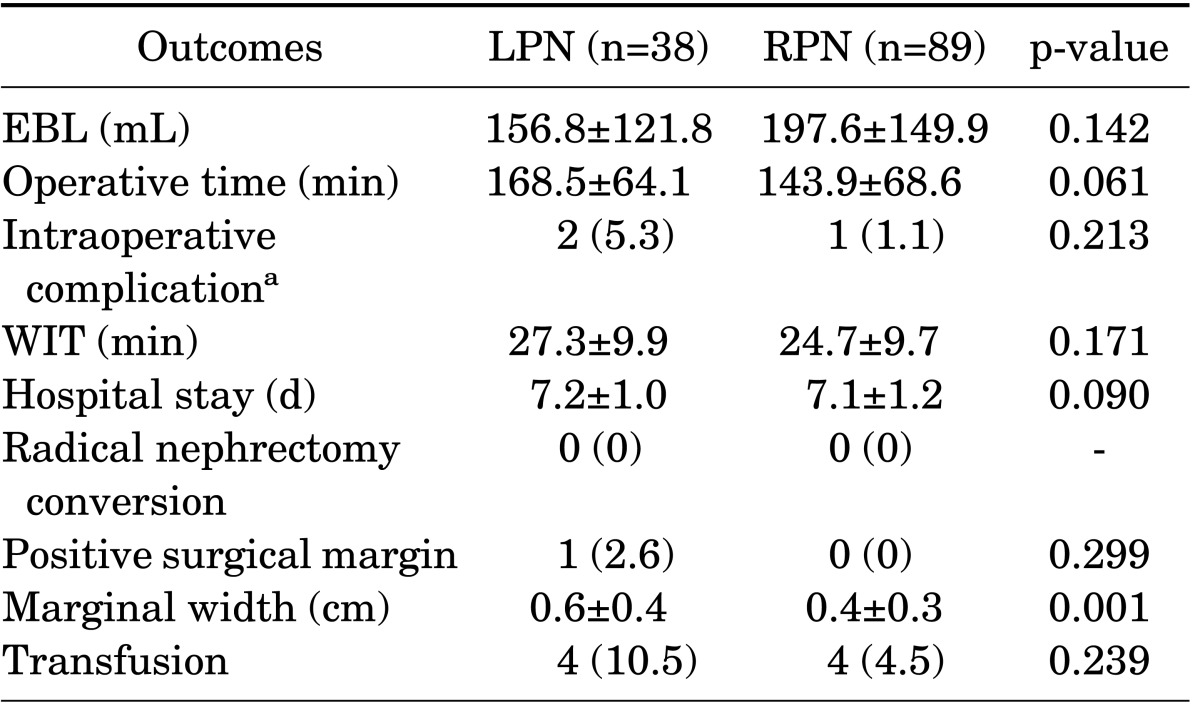
Values are presented as mean±standard deviation or number (%).
LPN, laparoscopic partial nephrectomy; RPN, robot-assisted laparoscopic partial nephrectomy; EBL, estimated blood loss; WIT, warm ischemic time.
a: Intraoperative complications: one transfusion due to bleeding and one pleural tearing in two LPN patients, one transfusion due to bleeding in RPN patients.
The postoperative complication data for both groups are listed in Table 3. No significant differences in postoperative complications were found between the two groups. Most of the postoperative complications occurred within the first month and were minor (Clavien-Dino I and II). There were only 2 (1.6%) major complications (Clavien-Dino grade III or greater). None of the 127 patients died.
TABLE 3.
Postoperative complications
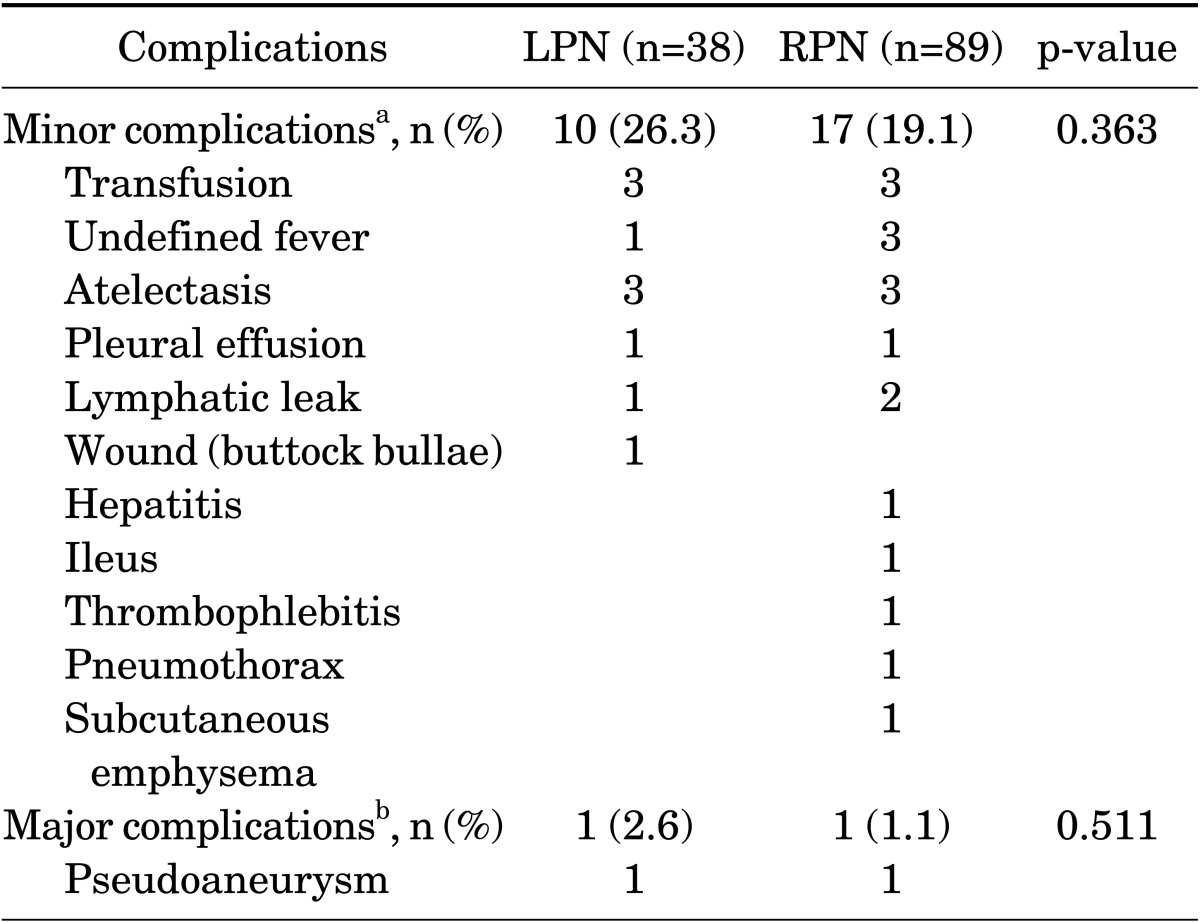
LPN, laparoscopic partial nephrectomy; RPN, robot-assisted laparoscopic partial nephrectomy.
a: Clavien-Dindo I and II. b: Clavien-Dindo III and IV.
Both preoperative eGFR and postoperative 1 month eGFR were significantly higher in the RPN group (88.6 mL/min/1.73 m2 vs. 81.4 mL/min/1.73 m2 preoperatively, p=0.041, and 77.3 mL/min/1.73 m2 vs. 69.8 mL/min/1.73 m2 postoperatively, p=0.035). However, there were no significant differences in eGFR change value and eGFR change percentage between the LPN and RPN groups (- 11.3 mL/min/1.73 m2 vs. -11.5 mL/min/1.73 m2, p=0.936, and -11.8% vs. -10.8%, p=0.750, respectively) (Table 4).
TABLE 4.
Renal function outcomes in the overall population
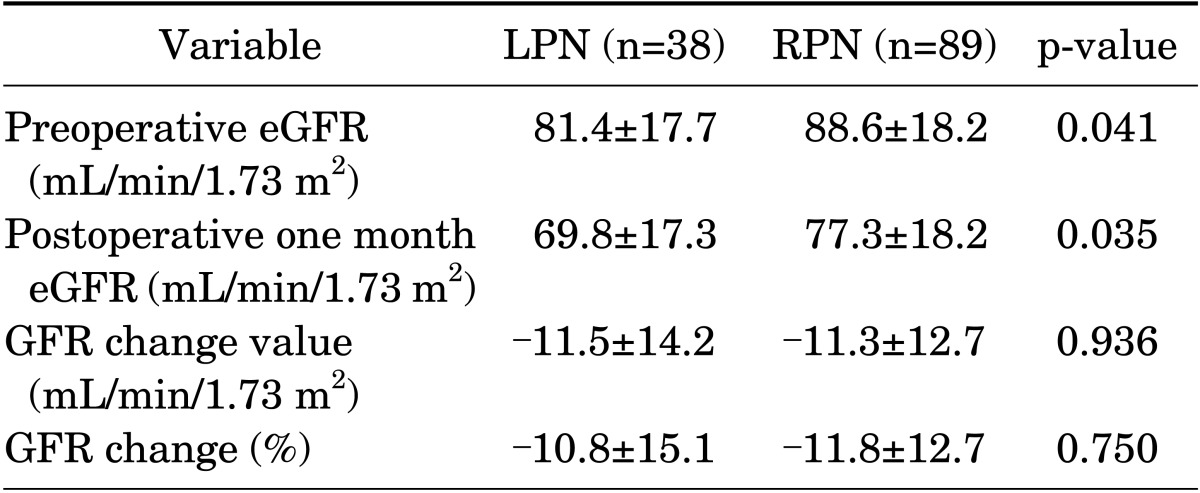
Values are presented as mean±standard deviation.
LPN, laparoscopic partial nephrectomy; RPN, robot-assisted laparoscopic partial nephrectomy; eGFR, estimated glomerular filtration rate.
There were significant differences between the two groups with respect to age, CCI, and tumor size. Patients undergoing RPN had a slightly higher RENAL nephrometry score. Owing to potential baseline differences between the two groups, we used a propensity-based matching analysis. Propensity-score matching resulted in a cohort of 30 LPN and 30 RPN patients. There were no significant differences between the two groups in demography or tumor characteristics.
Table 5 shows the operative and postoperative outcomes stratified according to group. There were statistically significant differences in surgical margin width between the LPN and RPN groups (0.6 cm vs. 0.4 cm, p=0.029). WIT and operative time of the RPN group were shorter than those of the LPN group, but there was no statistical significance (23.4 minutes vs. 26.6 minutes, p=0.232, and 148.5 minutes vs. 159.7 minutes, p=0.385, respectively).
TABLE 5.
Operative and postoperative outcomes (matching data)
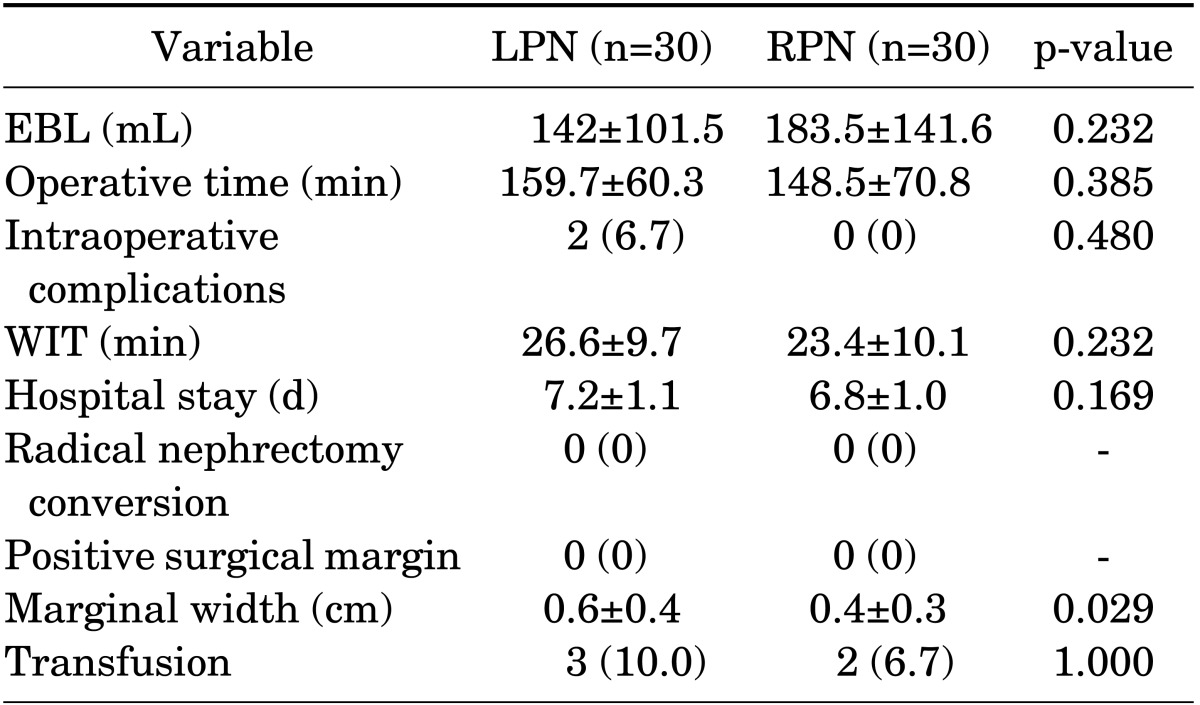
Values are presented as mean±standard deviation or number (%)
LPN, laparoscopic partial nephrectomy; RPN, robot-assisted laparoscopic partial nephrectomy; EBL, estimated blood loss; WIT, warm ischemic time.
DISCUSSION
The number of renal cell carcinomas diagnosed each year is on the rise owing to developments in and increased availability of imaging modalities. At the same time, the number of cases of small localized renal cell carcinoma has also risen. With recent developments in surgical techniques, partial nephrectomy has become the method of choice for the management of clinical stage T1 renal masses, because a number of studies have shown that there is no difference in cancer-specific survival between partial nephrectomy and radical nephrectomy [15,16]. Nephron sparing through partial nephrectomy has many advantages, not only for preservation of renal function, but also for overall survival in cases of T1a lesions [5].
Of the available nephron-sparing surgeries, LPN offers a shorter convalescence, reduced need for analgesia, and comparable outcomes to open partial nephrectomy, at least in the hands of expert surgeons [17]. However, LPN can be particularly challenging for more complex tumors, such as large, completely endophytic, or hilar masses. RPN has emerged as a viable alternative to mitigate the technical challenges of LPN, demonstrating perioperative outcomes at least comparable to those of LPN. Recent data have focused on the importance of minimizing WIT and preserving renal parenchyma in an attempt to avoid chronic renal disease and associated morbidity [18,19].
In our study, LPN and RPN had comparable results with respect to perioperative and functional outcomes. RPN was associated with shorter operative time and WIT, but these differences were not significant. LPN and RPN were completed successfully in all patients. In matching data, intraoperative complications occurred in two LPN patients (6.7%; one transfusion due to bleeding, one pleural tearing). There were no intraoperative complications in the RPN patients.
Obtaining negative surgical margins is the primary focus of partial nephrectomy. In a previous single institutional study comparing 186 LPN and 75 RPN cases, there were no significant differences with respect to WIT (18.2 minutes vs. 20.3 minutes, respectively, p=0.27) [14]. In the series of Long et al. [1], as in the present series, WIT was not significantly different between LPN and RPN (23.2 minutes vs. 22.4 minutes, respectively, p=0.469). Margin status was similar among the groups, although there was one positive margin in the LPN group.
Recent studies have shown that functional volume preservation is the primary determinant of long-term functional outcomes in patients whose ischemia time is within acceptable limits [20]. Although all patients in the RPN group had negative surgical margins, the surgical margin width in the RPN group was smaller than in the LPN group. This may reflect the fact that the resected normal parenchymal volume was smaller in the RPN group. Although the reason for this result is not obvious, it may be due to the technical advantages offered by the articulated robotic instruments and three-dimensional, high-definition magnified vision, making it possible for the surgeon to excise closer to the tumor and preserve more renal parenchymal tissue with RPN. Moreover, the technical limitations of LPN may increase surgeon anxiety about positive surgical margins, resulting in a larger margin of normal tissue surrounding the tumor. Additionally, surgeons tend to excise more renal parenchymal tissue to obtain an optimal angle for intracorporeal suturing in LPN.
Hospital stay was not significantly different between the two groups. Most patients were discharged 5 to 7 days or later after their operation, regardless of their actual medical demands, because the national medical insurance system of Korea is quite different from those of Western countries.
Generally, more complex tumors are associated with longer operative times, longer WIT, and greater blood loss, as well as higher complication rates. Overall, 29 patients (22.8%) had postoperative complications. There were 11 (28.9%) and 18 postoperative complications (20.2%) in LPN and RPN patients, respectively. Most complications were minor (21.3%) and were managed by conservative therapy, pharmacotherapy, or blood transfusion. In each group there were only two (1.6%) major complications (Clavien-Dindo grade III or greater). One of the major complications of each group was pseudoaneurysm, for which the patient was managed by angioembolization of a renal artery for gross hematuria. There were no life-threatening grade 4 complications. Postoperative complications were not significantly different between LPN and RPN. RPN, as well as LPN, seemed to be a safe procedure for renal tumors because there were no Clavien-Dindo grade IV complications.
We found that the extent of postoperative changes in eGFR was similar between the groups. LPN and RPN had comparable results associated with functional outcomes. Although overall functional reduction was similar in both groups, renal functional results may have been misperceived because of the compensatory role of the contralateral kidney. Radionucleotide scans provide more comparable absolute estimates of the function of each renal unit than do MDRD equations. Preoperative and postoperative radionucleotide scans can help to avoid potential confounders affecting renal function. In our study, a radionucleotide scan (diethylenetriamine pentaacetic acid GFR) was performed for 77 consecutive patients. There were no significant differences in eGFR change value or eGFR change percentage between the LPN (n=24) and RPN (n=53) groups (-14.0 mL/min/1.73 m2 vs. -16.4 mL/min/1.73 m2, p=0.634, and -28.8% vs. -34.2%, p=0.779, respectively).
Patients undergoing RPN had larger tumors (3.0 cm vs. 2.5 cm, p=0.044) but had a slightly lower CCI (3.7 vs. 4.4, p=0.017) than did patients in the LPN group. The slightly lower CCI in the RPN group may be associated with the younger age of these patients compared with the LPN group.
Propensity-based matching was performed to adjust for potential baseline differences. WIT and operative time were not significantly different between groups. For experienced laparoscopic surgeons, this result may reflect that LPN has comparable outcomes with a robotic approach with respect to WIT and operative time. In addition, all patients in both groups had negative surgical margins, but the surgical margin width in the RPN group was smaller than that in the LPN group. We believe that RPN is superior to LPN for healthy parenchymal preservation, because the mean resected safe surgical margin length was significantly greater in the LPN group than in the RPN group (0.6 cm vs. 0.4 cm, respectively, p=0.029). However, additional long-term comparative studies are needed to evaluate the actual impact of reduced resected safe surgical margin length on long-term postoperative renal functional outcomes. It is possible to save more renal parenchymal tissue with RPN. Therefore, parenchymal suturing at the hilar area can be done more easily because there is sufficient tissue for renorrhaphy. We believe this is one of the advantages of RPN. We believe that the greatest advantage of RPN is not the significant improvements in operative time or WIT, but rather the extended indications of minimally invasive partial nephrectomy.
The retrospective nonrandomized study design was the main limitation of this study. A second limitation is that our results are based on a single surgeon's experiences. Additional long-term comparative studies are needed to confirm our results. A third limitation is the bias associated with sequential series, in that RPN was performed after the surgeon was more experienced in LPN. To more objectively elucidate comparisons between RPN and LPN, there is a need for a randomized controlled trial gathering more long-term data.
CONCLUSIONS
RPN provides perioperative outcomes comparable to those of LPN and has the advantage of healthy parenchymal preservation for complex renal tumors (RENAL score≥7). Therefore, we believe that RPN can extend the indications for minimally invasive partial nephrectomy, especially hilar-located complex renal tumors. Also, RPN may provide better overall renal functional preservation through the preservation of more healthy tissue.
Footnotes
The authors have nothing to disclose.
References
- 1.Long JA, Yakoubi R, Lee B, Guillotreau J, Autorino R, Laydner H, et al. Robotic versus laparoscopic partial nephrectomy for complex tumors: comparison of perioperative outcomes. Eur Urol. 2012;61:1257–1262. doi: 10.1016/j.eururo.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Patard JJ, Shvarts O, Lam JS, Pantuck AJ, Kim HL, Ficarra V, et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004;171(6 Pt 1):2181–2185. doi: 10.1097/01.ju.0000124846.37299.5e. [DOI] [PubMed] [Google Scholar]
- 3.Barlow LJ, Korets R, Laudano M, Benson M, McKiernan J. Predicting renal functional outcomes after surgery for renal cortical tumours: a multifactorial analysis. BJU Int. 2010;106:489–492. doi: 10.1111/j.1464-410X.2009.09147.x. [DOI] [PubMed] [Google Scholar]
- 4.Weight CJ, Larson BT, Fergany AF, Gao T, Lane BR, Campbell SC, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol. 2010;183:1317–1323. doi: 10.1016/j.juro.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Zini L, Perrotte P, Capitanio U, Jeldres C, Shariat SF, Antebi E, et al. Radical versus partial nephrectomy: effect on overall and noncancer mortality. Cancer. 2009;115:1465–1471. doi: 10.1002/cncr.24035. [DOI] [PubMed] [Google Scholar]
- 6.Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR, Jr, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41–46. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 7.Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166:6–18. [PubMed] [Google Scholar]
- 8.White MA, Haber GP, Autorino R, Khanna R, Hernandez AV, Forest S, et al. Outcomes of robotic partial nephrectomy for renal masses with nephrometry score of ≥7. Urology. 2011;77:809–813. doi: 10.1016/j.urology.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Wang AJ, Bhayani SB. Robotic partial nephrectomy versus laparoscopic partial nephrectomy for renal cell carcinoma: single-surgeon analysis of >100 consecutive procedures. Urology. 2009;73:306–310. doi: 10.1016/j.urology.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 10.Benway BM, Bhayani SB, Rogers CG, Dulabon LM, Patel MN, Lipkin M, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol. 2009;182:866–872. doi: 10.1016/j.juro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 11.Rogers CG, Metwalli A, Blatt AM, Bratslavsky G, Menon M, Linehan WM, et al. Robotic partial nephrectomy for renal hilar tumors: a multi-institutional analysis. J Urol. 2008;180:2353–2356. doi: 10.1016/j.juro.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aron M, Koenig P, Kaouk JH, Nguyen MM, Desai MM, Gill IS. Robotic and laparoscopic partial nephrectomy: a matched-pair comparison from a high-volume centre. BJU Int. 2008;102:86–92. doi: 10.1111/j.1464-410X.2008.07580.x. [DOI] [PubMed] [Google Scholar]
- 13.Ellison JS, Montgomery JS, Wolf JS, Jr, Hafez KS, Miller DC, Weizer AZ. A matched comparison of perioperative outcomes of a single laparoscopic surgeon versus a multisurgeon robot-assisted cohort for partial nephrectomy. J Urol. 2012;188:45–50. doi: 10.1016/j.juro.2012.02.2570. [DOI] [PubMed] [Google Scholar]
- 14.Haber GP, White WM, Crouzet S, White MA, Forest S, Autorino R, et al. Robotic versus laparoscopic partial nephrectomy: single-surgeon matched cohort study of 150 patients. Urology. 2010;76:754–758. doi: 10.1016/j.urology.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 15.Antonelli A, Cozzoli A, Nicolai M, Zani D, Zanotelli T, Perucchini L, et al. Nephron-sparing surgery versus radical nephrectomy in the treatment of intracapsular renal cell carcinoma up to 7 cm. Eur Urol. 2008;53:803–809. doi: 10.1016/j.eururo.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Becker F, Siemer S, Humke U, Hack M, Ziegler M, Stockle M. Elective nephron sparing surgery should become standard treatment for small unilateral renal cell carcinoma: Long-term survival data of 216 patients. Eur Urol. 2006;49:308–313. doi: 10.1016/j.eururo.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Riggs SB, Larochelle JC, Belldegrun AS. Partial nephrectomy: a contemporary review regarding outcomes and different techniques. Cancer J. 2008;14:302–307. doi: 10.1097/PPO.0b013e31818675ae. [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 19.Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol. 2010;58:340–345. doi: 10.1016/j.eururo.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 20.Simmons MN, Fergany AF, Campbell SC. Effect of parenchymal volume preservation on kidney function after partial nephrectomy. J Urol. 2011;186:405–410. doi: 10.1016/j.juro.2011.03.154. [DOI] [PubMed] [Google Scholar]


