Abstract
Objective
S-Nitrosothiols (RSNOs) are bioactive forms of nitric oxide which are involved in cell signalling and redox regulation of vascular function. Circulating S-nitrosothiols are predominantly in the form of S-nitrosoalbumin. In this study plasma concentrations of S-nitrosothiols were measured in patients with systemic sclerosis (SSc) where NO metabolism is known to be abnormal.
Patients and methods
Venous blood was collected from 16 patients with Raynaud's phenomenon (RP), 45 with systemic sclerosis (SSc) (34 patients had limited SSc (IcSSc) and 11 diffuse cutaneous disease (dcSSc)). Twenty six healthy subjects were used as controls. Plasma S-nitrosothiol concentrations were measured by chemiluminescence. The measurements were related to the extent of biological age, capillary/skin scores and disease duration.
Results
Plasma RSNO levels in patients with Raynaud's phenomenon (RP) and in those with SSc was significantly lower compared to the concentrations in control subjects. In SSc, plasma S-nitrosothiols were often below the level of detection (1nM).
Conclusions
Low S-nitrosothiol concentrations were observed in the blood of patients with SSc and patients with RP indicating a profound disturbance of nitric oxide metabolism.
Chemical compounds studied in this article: N-ethylmaleimide (PubChem ClD:4362), Acetic acid (PubChem: ClD:176), Potassium iodide (PubChem ClD: 4875), Nitric oxide (PubChem: ClD: 145068), Sulfanilamide (PubChem: ClD 5333), Copper sulphate (PubChem: ClD 24462), Dithiothreitol (PubChem: ClD 19001), Ethylenediaminetetracetic acid (Pub Chem ClD 6049)
Keywords: S-nitrosothiols, Systemic sclerosis, Skin score
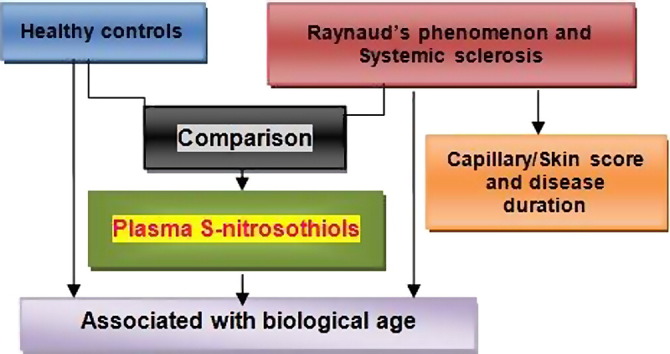
Graphical abstract
Chemiluminescence techniques were used to show that plasma levels of S-nitrosothiols were low in patients with Raynaud's phenomenon and in systemic sclerosis and in the latter were negatively related to age, capillary/skin scores and disease duration.
1. Introduction
Systemic sclerosis (SSc) is a rheumatic and connective tissue disorder that affects the skin and the internal organs (lungs, heart, kidney and gut) leading to tissue fibrosis. The majority of SSc patients exhibit Raynaud's phenomenon (RP) [1], a vasospastic response to cold which induces ischemic attacks and vasospasm of the digits [2]. The pathophysiology of RP includes abnormality of blood vessels, abnormal neural control mechanisms and platelet activation [3]. Patients with RP do not show clinical features of SSc. However, some cases may evolve to SSc and exhibit abnormal nail fold capillaries and develop autoantibodies that are typically observed in patients with SSc [4]. The clinical subgroups of SSc are classified into limited SSc (lcSSc) and diffuse cutaneous SSc (dcSSc), according to their degree and distribution of skin sclerosis, their immunological profile and extent of microvascular dysfunction [5], [6].
Several studies have shown that patients with SSc exhibit many features suggestive of decreased levels of antioxidant vitamins and enzyme activities related to defence against oxidative stress [7]. The fibroblasts show increased release of reactive oxygen species [8] and exhibit stress in their vascular and immune systems and extracellular matrix, which may contribute to progressive fibrosis [9].
The formation of vasodilator nitric oxide (NO) in normal endothelial cells [10] has been the focus of study in the pathogenesis of SSc [11], [12], [13]. Decreased endothelial NO formation has been reported in SSc patients [14], [15], [16]. However, not all of the evidence points in the same direction [17]. In macrophages, NO produced by the inducible form of the enzyme NO synthase (iNOS) is a cytotoxic agent released as part of the primary defence mechanism against microorganisms. Pulmonary production of NO is increased as lung macrophages express higher amounts of iNOS in SSc patients [18]. Elevated levels of nitrite/nitrate have also been reported in serum of SSc patients [19], [20], [21]. The excess NO reacts with superoxide anions to produce peroxynitrite, an oxidant that nitrates tyrosine residues in proteins to form nitrotyrosine. The levels of nitrated proteins in the skin and plasma are increased, especially in patients with diffuse SSc at early stages of the disease [13].
S-nitrosothiols (RSNOs) are more stable forms of bioactive NO in vivo. RSNO formation occurs by several pathways including oxidation of thiols with peroxynitrite [22], or their reaction with N2O3 a product from oxygen and NO [23]. RSNO act as NO donors to specific thiol-regulatory effector sites including enzymes and signalling proteins by transnitrosation [24]. RSNOs bind covalently to SH groups of proteins and low molecular weight thiols in vivo and signal through the modification of cysteine (CYS) residues by covalent addition of a NO moiety, (formally as NO+), which regulates the function of a broad spectrum of proteins in cells by S-nitrosylation [25]. The reaction of NO with the free thiols of serum albumin, cysteine and, to a lesser extent, glutathione results in the formation of the respective S-nitrosothiols in vivo [26], [27], [28]. RSNOs are reduced to NO and nitrite when degraded [29].
We hypothesized that the circulating concentration of RSNOs in SSc would decrease at an early stage of the disease resulting in endothelial damage and impaired NO biosynthesis. These changes may contribute to both the presence of Raynaud's phenomenon and also the fibrotic changes that ensue in SSc.
2. Materials and methods
2.1. Patients
Patients with RP and systemic sclerosis were enrolled in this study with the approval of the Royal Free Hospital Ethical Practices subcommittee. RP patients included are those with normal capillaries (NC) and negative antinuclear–antibody (ANA) and those with NC or ANA or both, but without evidence of connective tissue disease. Out of 16 RP patients 6 patients had positive ANA and 4 had abnormal capillaroscopy and no patient with ANA and with capillaroscopy. The patients with SSc fulfilled the American College of Rheumatology (formerly the American Rheumatism Association) preliminary diagnostic criteria for SSc [30] and included patients with lcSSc and dcSSc.
The study was carried out with a total of 61 patients selected randomly and who attended the Rheumatology clinic at the Royal Free Hospital exhibiting either Raynaud's phenomenon (RP) or SSc with subgroups of lcSSc and dcSSc. Both male and female patients were included and none of whom smoked. Twenty six healthy non-smoking subjects (male and female) were randomly selected and used as controls. Informed consent was obtained from all subjects enrolled in the study. Measurement of RSNO concentrations of patients was carried out blind and was compared to the skin thickness assessments for SSc patients measured within three months of the blood samples being taken. RSNO levels were also related to patient age, capillary/skin scores and duration of disease.
2.2. Bloods
Venous blood was taken from patients and healthy controls into pre-chilled tubes containing EDTA (ethylenediaminetetracetic acid final concentration 2 mM) and NEM (N-ethylmaleimide final concentration 5 mM) and centrifuged for 10 min at 1300 g at 4 °C. NEM prevented RSNO degradation by transnitrosation reactions [31] through alkylation of thiol groups. RSNOs were determined on the day of sample collection.
2.3. Determination of plasma S-nitrosothiols
The assay is based upon the detection of nitric oxide released by copper, iodine–iodide mediated cleavage of RSNOs to form NO, with subsequent quantification by its gas phase chemiluminescence reaction with ozone [32]. 8 ml glacial acetic acid and 2 ml KI (50 mg/ml) was kept in a reaction chamber at 70 °C (custom designed to accommodate larger sample volume or identical in design to a commercially available glass chamber (Sievers Radical Purger™)). One minute prior to injection of the sample (200 μl), CuSO4 (200 mM) was added and the solution purged with N2 and used once per sample. The gas stream was passed into Sievers Instruments Model 280 Nitric Oxide Analyser (NOA™ Sievers, Boulder, Colorado, USA). Data collection and analysis were performed using the NO Analysis™ software (Sievers, Boulder, Colorado, USA). To remove the background nitrite in plasma samples, sulfanilamide 0.5% (w/v) in HCl (final concentration 0.2 M) was added. This forms a stable diazonium salt and prevents NO formation. Antifoam™ (Sievers, Boulder, Colorado, USA) in 1:30 dilution in deionised water was added to the plasma at a ratio of 1:9, immediately before injection of the sample. 100–1000 μl plasma extracts were injected to the reaction chamber, before and after addition of CuSO4 in order to calculate the level of S-nitrosothiol and exclude N-nitrosamine or iron nitrosyl. The NO2− measurements were taken by chemiluminescence before the addition of CuSO4, whereas concentrations of S-nitrosothiols were determined after the addition of CuSO4 to the reaction chamber with sulfanilamide. RSNO concentrations were calculated by subtraction of the value of NO2− from the value received after injection in CuSO4.
2.4. Preparation of standard (SNOalb)
Standard curves for SNO-albumin (SNO-alb) were obtained over the range of 1nM to 5 μM by addition of known amounts of SNO-alb to PBS with 5 mM NEM. To prepare SNO-albumin human serum albumin (20 mg/ml) was treated with dithiothreitol in phosphate buffer saline (PBS) together with 100 μM diethyleneaminepenta-acetic acid (DTPA) and gently stirred at 20 °C for 2 h to reduce the Cys-34 thiol group. The sample was dialysed extensively in Visking tubing ((Medicell, London, UK) molecular weight cut off 14,000) at 4 °C against 3 × 3 L. PBS, pH 7.4 containing 100 μM DTPA. Any untreated thiol was alkylated with NEM (1 mM) at room temperature and dialysed against PBS/DTPA. The stock solution of SNO-alb was stored at − 20 °C. SNO-alb was measured prior to use by the Saville reaction [33].
2.5. Skin scores
Tightness and thickening of skin were measured in the majority of SSc patient using the modified Rodnan skin score method [34]. The total skin surface area was divided into 17 different areas: the skin score evaluated in each by manual palpation. The skin score grading was 0 for uninvolved skin, 1 for mild thickening, 2 for moderate thickening, and 3 for severe thickening (hidebound skin). The total skin score was the sum of the skin scores of the individual areas, the maximum possible score being 51. The skin scores correlate with the extent of dermal fibrosis and the extent of fibrosis and dysfunction of internal organs.
2.6. Nailfold Capillary scores (NC) were measured as described in Ref. [35]
2.6.1. Data analysis
Data were expressed as mean ± SE where n was the number of individual samples assayed in duplicate or more. Comparisons of multiple means were performed using a one way ANOVA followed by the Student's Newman–Keuls test. Correlation and regression were performed using the ‘least squares’ method in the Microsoft Excel programme. P value of < 0.05 was considered as statistically significant.
3. Results
3.1. Demographic data of patients and controls
The characteristics of controls and patients are described in Table 1. The age range was similar in each group but the controls had a higher proportion of males.
Table 1.
Demographic data for patients and controls:
Mean age, age range and males/females ratio were reported for healthy controls (HC) and in patients with Raynaud's phenomenon (RP), systemic sclerosis (SSc), limited SSc (lcSSc) and diffuse cutaneous SSc (dcSSc).
| Subjects | Number studied (Individuals) |
Age range (Years) |
Mean age (mean ± SEM) |
Males/females |
|---|---|---|---|---|
| Healthy Controls | 26 | 22–60 | 41 ± 2 | 10/16 |
| Patients: | ||||
| RP | 16 | 15–78 | 46 ± 1 | 2/14 |
| SSc | 45 | 40–97 | 58 ± 2 | 8/37 |
| lcSSc | 34 | 40–97 | 63 ± 3 | 6/28 |
| dcSSc | 11 | 41–61 | 47 ± 2 | 2/9 |
| (B). Number of individuals (males/females) available for skin score data | ||||
| Patients: | Number studied (Individuals) |
Skin score range | Mean skin score (mean ± SEM) |
Males/females |
| SSc (skin score) | 36 | 0–47 | 17 ± 2 | 6/30 |
| lcSSc (skin score) | 25 | 0–23 | 10 ± 1 | 4/21 |
| dcSSc (skin score) | 11 | 12–47 | 31 ± 3 | 3/8 |
3.2. Plasma RSNOs in healthy controls: effects of age
The concentrations of RSNOs in healthy controls were relatively low compared to those reported using other methods, and rather below those measured for controls by the originators of this procedure [32]. However the mean age of this cohort is likely to have been higher and reflects a population with a wide age range which seems to influence RSNO concentration negatively as age increases (r2 = 0.22; correlation; P = 2.67 × 10− 6, n = 26) but the relationship was not linear.
At ages below 36 in the controls RSNO levels appeared to be higher (6.36 ± 1.1 nM; n = 11) and correlated negatively to age (r2 = 0.34; P = 0.015); whereas as age increased to 60, RSNO values were lower at 4.03 ± 1 nM; n = 15 and within this subgroup were not correlated with age (r2 = 0.04; P = 0.12).
3.3. Plasma RSNOs in RP and SSc patients
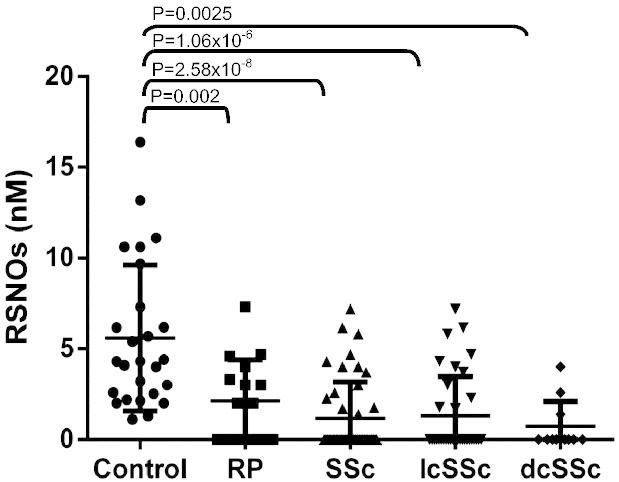
Plasma RSNO levels in healthy controls (6.0 ± 0.8 nM; n = 26) were significantly higher than those of all patients taken together (RP and SSc) (1.38 ± 0.4 nM; n = 61, P = 2.58 × 10− 8). RP patients alone had lower levels than those of controls (2.12 ± 0.6 nM; n = 16; P = 0.002). Even lower levels were found in SSc patients (1.17 ± 0.5 nM; n = 45, P = 2. 58 × 10− 8). Within the SSc subgroups, the RSNO levels were again significantly lower than controls (IcSSc 1.31 ± 0.37 nM; n = 34, P = 1.06 × 10− 6; dcSSc, 0.73 ± 0.41; n = 11; P = 0.00254) but not between each other (P = 0.34) (Fig. 1). There was no correlation between plasma RSNO and age or gender within any of the patient groups [not shown].
Fig. 1.
Individual levels of S-nitrosothiol (RSNO) in plasma of healthy controls, Raynaud's phenomenon (RP), SSc systemic sclerosis (SSc), lcSSc (limited SSc) and dcSSc (diffuse cutaneous disease) patients. RSNO levels were detected by a NO chemiluminescence detector (NOA 280, Sievers). Horizontal bars represent median values observed in each group. Results obtain in each group were compared to the results obtained in healthy controls (one way ANOVA followed by the students Newman–Keuls test). P < 0.05 was considered statistically significant.
3.4. Correlation of RSNO levels with capillary/skin scores and disease duration
RSNO values of the RP group correlated negatively to the capillary scores (r2 = 0.18, P = 0.03). The skin scores for all SSc patients were on average 16.5 ± 2.02 (n = 36), in the lcSSc group alone 10.1 ± 1 (n = 25) and in dcSSc patients 30.9 ± 3.1 (n = 11) respectively. The RSNO levels correlated negatively to the skin scores (r2 = 0.20, P = 0.002, n = 25) in lcSSc. In the total SSc cohort, the correlation was also significant (r2 = 0.08, P = 0.001, n = 36) but a large number of patients had undetectable RSNOs making a generalizable relationship difficult to sustain. RSNOs were negatively correlated to the duration (in years) of the disease in the SSc patients (r2 = 0.26; P = 0.001 n = 36).
4. Discussion
In this study S-nitrosothiol levels in plasma of patients with systemic sclerosis and those with Raynaud's phenomenon were reported. As in most previous studies, the patient cohort had an excess of females to males. The onset of disease is more common after the fifth decade of life, although it is also found in younger patients [36]. The prevalence of lSSc and diffuse subsets were in a similar proportion to those found in other studies [37]. RSNO levels in both controls and patients were low but consistent with those where chemiluminescence detection [32] was employed. Elevated levels of S-nitrosoalbumin and total S-nitrosothiol concentrations in pre-eclampsia plasma were reported using this technique [38]. A variety of techniques have been used to measure RSNO [39], [40] but there is no consensus about which is the best. However, earlier estimates placing the levels of these compounds in the micromolar range are unlikely to be accurate, because of the profound effects they would have on blood pressure [41].
In these studies we found that RSNO levels were higher in the healthy controls compared to those over the age of thirty six, especially if they were young. The finding is consistent with earlier reports that NO biosynthesis in endothelial cells was reduced with age [42], [43] an effect associated with increasing apoptosis [44]. Age may contribute to the low levels of RSNO in patients with SSc and RP, but this appears not to be the only factor, since many patients have RSNOs below the measureable threshold. These very low values were not found in any of the controls. The low levels of RSNOs are consistent with previous studies showing severe disturbances in nitric oxide metabolism in these diseases e.g. increased nitrated proteins and ADMA [13] especially in diffuse SSc.
The reduction of RSNOs in patients with IRP is less consistent, but accords with reports that show disturbances in NO metabolism in these patients [13]. In contrast, in SSc, NO release may be increased transitorily because of the induction of NOSII [12].
The reduction in the level of RSNOs in SSc may track a gradual deterioration of endothelial function post the inflammatory phase of the disease, but low levels were also found in several of the RP patients suggesting that the fall may occur at an early stage in some cases. The decline of RSNOs was also parallel to increasing skin score, which highlights earlier observations where nitric oxide inhibits collagen secretion in human dermal fibroblasts through a cGMP-independent regulatory mechanism by activation of matrix metalloproteinase-1 [45]. The inhibition was less effective in SSc fibroblasts. However these correlations should be regarded with caution as there were many patients who had undetectable levels of RSNO.
The low plasma levels of ascorbic acid in SSc may also contribute to a reduction in NO biosynthesis or by reducing thiol availability [46]. Recent work suggests that S-nitrosothiols and products of S-nitrosylation play key roles in human health and disease.
An understanding of the factors that determine RSNO levels in vivo and the reasons why aberrant depletion of intracellular RSNOs contributes to disease are beginning to emerge [47].
5. Conclusion
The findings in this study strengthen the view that NO metabolism is profoundly disturbed in SSc and that low levels of RSNO may play a key role in its progress. This may influence not only the universal existence of RP in SSc patients, but also abnormalities in platelet activation, the regulation of collagen activity and defective angiogenesis. The evaluation and study of the concentration of RSNOs in plasma of patients in RP and SSc and correlation with some clinical markers may help to improve safe treatment with NO donor drugs.
Acknowledgement
We wish to acknowledge the Arthritis Research UK (19427, 144524, 14383) for their support, Prof K. Moore for his helpful suggestions and Dr S Guerra for her help with the figure.
References
- 1.Herrick A.L. Pathogenesis of Raynaud's phenomenon. Rheumatology. 2005;44(5):587–596. doi: 10.1093/rheumatology/keh552. [DOI] [PubMed] [Google Scholar]
- 2.Kahaleh B. The microvascular endothelium in scleroderma. Rheumatology. 2008;47:v14–v15. doi: 10.1093/rheumatology/ken279. [DOI] [PubMed] [Google Scholar]
- 3.Herrick A.L. The pathogenesis, diagnosis and treatment of Raynaud phenomenon. Nat Rev Rheumatol. 2012;8(8):469–479. doi: 10.1038/nrrheum.2012.96. [DOI] [PubMed] [Google Scholar]
- 4.Sunderkötter C., Riemekasten G. Pathophysiology and clinical consequences of Raynaud's phenomenon related to systemic sclerosis. Rheumatology. 2006;45:iii33–iii35. doi: 10.1093/rheumatology/kel280. [DOI] [PubMed] [Google Scholar]
- 5.Black C.M., Denton C.P. In: 2nd ed. Maddison P.J., Isenberg D.A., Woo P., Glass D.N., editors. Oxford University Press; New York, NY, USA: 1998. Scleroderma and related disorders in adults and children Oxford Textbook of Rheumatology; pp. 1217–1247. [Google Scholar]
- 6.Prescott R.J., Freemont A.J., Jones C.J., Holland J., Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol. 1992;166(3):255–263. doi: 10.1002/path.1711660307. [DOI] [PubMed] [Google Scholar]
- 7.Bruckdorfer K.R., Hillary J.B., Bunce T., Vankecheswaran R., Black C.M. Increased susceptibility to oxidation of low-density lipoproteins isolated from patients with systemic sclerosis. Arthritis Rheum. 1995;38:1060–1067. doi: 10.1002/art.1780380807. [DOI] [PubMed] [Google Scholar]
- 8.Murrel D.F. A radical proposal for the pathogenesis of scleroderma. J Am Acad Dermatol. 1993;28(1):78–85. doi: 10.1016/0190-9622(93)70014-k. [DOI] [PubMed] [Google Scholar]
- 9.Denton C.P., Black C.M., Abraham D. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2(3):134–144. doi: 10.1038/ncprheum0115. [DOI] [PubMed] [Google Scholar]
- 10.Bruckdorfer K.R. The basics about nitric oxide. Mol Asp Med. 2005;26:3–31. doi: 10.1016/j.mam.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Yamato T., Katayama l, Nishioka K. Nitric oxide production and inducible nitric oxide synthase expression in systemic sclerosis. J Rheumatol. 1998;25(2):314–317. [PubMed] [Google Scholar]
- 12.Cotton S.A., Herrick A.L., Jayson M.l.V., Freemont A.J. Endothelial expression of nitric oxide synthases and nitrotyrosine in systemic sclerosis skin. J Pathol. 1999;189(2):273–278. doi: 10.1002/(SICI)1096-9896(199910)189:2<273::AID-PATH413>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Dooley A., Gao B., Bradley N.D., Abraham J., Black C.M., Jacobs M. Abnormal nitric oxide metabolism in systemic sclerosis: increased levels of nitrated proteins and asymmetric dimethylarginine. Rheumatology. 2006;45(6):676–684. doi: 10.1093/rheumatology/kei276. [DOI] [PubMed] [Google Scholar]
- 14.Allanore Y., Borderie D., Hilliquin P., Hernvann A., Levacher M., Lemeréchal H. Low levels of nitric oxide (NO) in systemic sclerosis: inducible NO synthase production is decreased in cultured peripheral blood monocyte/macrophage cells. Rheumatology. 2001;40(10):1089–1096. doi: 10.1093/rheumatology/40.10.1089. [DOI] [PubMed] [Google Scholar]
- 15.Kharitonov S.A., Cailes J.B., Black C.M., du Bois R.M., Barnes J.P. Decreased nitric oxide in the exhaled air of patients with systemic sclerosis with pulmonary hypertension. Thorax. 1997;52:1051–1055. doi: 10.1136/thx.52.12.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menkès C.J., Allanore Y., Borderie D., Hilliquin P., Hernvann A., Ekinddijian O. Inducible nitric oxide synthase expression and nitric oxide production by monocytes in systemic sclerosis. Bull Acad Natl Med. 2001;185:509–522. [PubMed] [Google Scholar]
- 17.Sud A., Khullar M., Wanchu A., Bambery P. Increased nitric oxide production in patients with systemic sclerosis. Nitric Oxide. 2000;4(6):615–619. doi: 10.1006/niox.2000.0309. [DOI] [PubMed] [Google Scholar]
- 18.Rolla G., Colagrade P., Scappaticci E., Chiavassa G., Dutto L., Cannizzo S. Exhaled nitric oxide in systemic sclerosis: relationship with lung involvement and pulmonary hypertension. J Rheumatol. 2000;27(7):1693–1698. [PubMed] [Google Scholar]
- 19.Matucci M.C., Kahaleh M.B. Beauty and the beast. The nitric oxide paradox in systemic sclerosis. Rheumatology. 2002;41:843–847. doi: 10.1093/rheumatology/41.8.843. [DOI] [PubMed] [Google Scholar]
- 20.Failli P., Palmieri L., D'Alfanso C., Giovannelli L., Generini S., Rosso G.S., Matucci-Cerinic M. Effect of N-acetyl-cysteine on peroxynitrite and super oxide anion of lung alveolar macrophages in systemic sclerosis. Nitric Oxide. 2002;7:277–282. doi: 10.1016/s1089-8603(02)00120-9. [DOI] [PubMed] [Google Scholar]
- 21.Hua-Huv T.K., Tiev P., Chéreau C., Duong-Quvs S., Cabane J., Dinh-Xuan A.T. Increased alveolar concentration of nitric oxide is related to serum-induced lung fibroblast proliferation in patients with systemic sclerosis. J Rheumatol. 2010;37:1680–1687. doi: 10.3899/jrheum.090915. [DOI] [PubMed] [Google Scholar]
- 22.Vliet V.D., Howen P.A.C., Wong P.S.-Y., Bast A., Cross C.E. Formation of S-nitrosothiols via direct neucleophilic nitrosation of thiols by peroxynitrite with elimination of hydrogen peroxide. J Biol Chem. 1998;273(46):30255–30262. doi: 10.1074/jbc.273.46.30255. [DOI] [PubMed] [Google Scholar]
- 23.Gow A.J., Buerk D.G., Ischiropoulos H.A. A novel reaction mechanism for the formation of s-nitrosothiol in vivo. J Biol Chem. 1997;272(5):2841–2845. doi: 10.1074/jbc.272.5.2841. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z., Rudd M.A., Freedman J.E., Loscalzo J. S-transnitrosation reactions are involved in the metabolic fate and biological actions of nitric oxide. J Pharmacol Exp Ther. 1998;284:526–534. [PubMed] [Google Scholar]
- 25.Stamler J.S., Lamas S., Fang F.C. Nitrosylation: the prototypic redox-based. Signal Mech Cell. 2001;106(2):675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 26.Stamler J.S., Simon D.l., Osborne J.A., Mullins M.E., Jaraki O., Michel T. S-nitrosylation of proteins with nitric oxide: synthesis and characterisation of biologically active compounds. Proc Natl Acad Sci U S A. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Hogg N. S-nitrosothiols: cellular formation and transport. Free Rad. Biol Med. 2005;38:831–838. doi: 10.1016/j.freeradbiomed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Gaston B., Reilly J., Drazen J.M., Fackler J., Ramdev P., Arnelle D. Endogenous nitrogen oxides and bronchodilator S- nitrosothiols in human airways. Proc Natl Acad Sci U S A. 1993;90:10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ewing J.F., Janero D.R. Specific S-nitrosothiol (thinitrite) quantification as solution nitrite after vanadium (lll) reduction and ozone-chemiluminscent detection. Free Rad. Biol Med. 1998;25(4–5):621–628. doi: 10.1016/s0891-5849(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 30.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association. Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 31.Scorza G., Petraforte D., Minetti M. Role of ascorbate and protein thiols in the release of nitric oxide from S-nitroso-albumin and S-nitroso-glutathione in human plasma. Free Radic Biol Med. 1997;22:633–642. doi: 10.1016/s0891-5849(96)00378-4. [DOI] [PubMed] [Google Scholar]
- 32.Marley R., Feelish M., Holt S., Moore K. A chemilluminescence-based assay for S-nitrosothiols. Free Radic Res. 2000;32:1–9. doi: 10.1080/10715760000300011. [DOI] [PubMed] [Google Scholar]
- 33.Saville B. A scheme of the colorimetric determination of microgram amounts of thiols. Analyst. 1958;83:670–672. [Google Scholar]
- 34.Rodnan G.P., Lipinski E., Luksick J. Skin thickness and collagen content in progressive systemic sclerosis and localized scleroderma. Arthritis Rheum. 1970;22:130–140. doi: 10.1002/art.1780220205. [DOI] [PubMed] [Google Scholar]
- 35.Ohtsuka T. Quantitative analysis of nailfold capillary abnormalities in patients with connective tissue diseases. J Dermatol. 1999;38(10):757–764. doi: 10.1046/j.1365-4362.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 36.Manno R.L., Wigley F.M., Gelber A.C., Hummers L.K. Late-age onset systemic sclerosis. J Rheumatol. 2011;38:1317–1325. doi: 10.3899/jrheum.100956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pérez-Bocanegra C., Solans-Laqué R., Simeóne-Aznar C.P., Campillo M., Fonollosa-Pla V., Villardell-Tarrés M. Age-related survival and clinical features in systemic sclerosis patients older or younger than 65 at diagnosis. Rheumatology. 2010;49:1112–1117. doi: 10.1093/rheumatology/keq046. [DOI] [PubMed] [Google Scholar]
- 38.Tyurin V.A., Liu S., Tyurina Y.Y., Sussman N.B., Hubel C.A., Roberts J.M. Elevated levels of S-nitrosothiols in preeclampsia plasma. Circ Res. 2001;88:1201–1215. doi: 10.1161/hh1101.092179. [DOI] [PubMed] [Google Scholar]
- 39.Giustarini D., Milazani A., Dalle-Donne I., Rossi R. Detection of S-nitrosothiols in biological fluids: a comparison of most widely applied methodologies. J Chromatogr B. 2007;851:134–139. doi: 10.1016/j.jchromb.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 40.MacArther P.H., Shiva S., Gladwin M.T. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J Chromatogr B. 2007;851:93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Massy Z.A., Fumeron C., Borderie D., Tuppin P., Naguyen-khoa T., Benoit M. Increased plasma S-nitrosothiol concentrations predict cardiovascular outcomes among patients with end-stage renal disease: a prospective study. J Am Soc Nephrol. 2004;15:470–476. doi: 10.1097/01.asn.0000106716.22153.bb. [DOI] [PubMed] [Google Scholar]
- 42.Chou T.C., Yen M.H., Li C.Y., Ding Y.A. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension. 1998;31(2):643–648. doi: 10.1161/01.hyp.31.2.643. [DOI] [PubMed] [Google Scholar]
- 43.Tshudi M.R., Berton M., Bersinger N.A., Moreau P., Cosentino F., Noll G. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. Am. Soc Clin Investig. 1996;98(4):899–905. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffman J., Haendeler J., Aicher A., Rossig L., Vasa M., Zeiher A.M. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res. 2001;89:709–715. doi: 10.1161/hh2001.097796. [DOI] [PubMed] [Google Scholar]
- 45.Dooley A., Gao B., Shi-Wen X., Abraham D.J., Black C.M., Jacobs M. Effect of nitric oxide and peroxynitrite on type I collagen synthesis in normal and scleroderma dermal fibroblasts. Free Radic Biol Med. 2007;43:253–264. doi: 10.1016/j.freeradbiomed.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Saura M.F., Saijo F., Bryan N.S., Bauer S., Rodriquez J., Feelisch M. Nitroso-redox status and vascular function in marginal and severe ascorbate deficiency. Antioxid Redox Signal. 2012;17:937–950. doi: 10.1089/ars.2011.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haldar M.H., Stamler J.S. S-nitrosylation: integrator of cardiovascular performance and oxygen delivery. J Clin Invest. 2013;123:101–110. doi: 10.1172/JCI62854. [DOI] [PMC free article] [PubMed] [Google Scholar]