Abstract
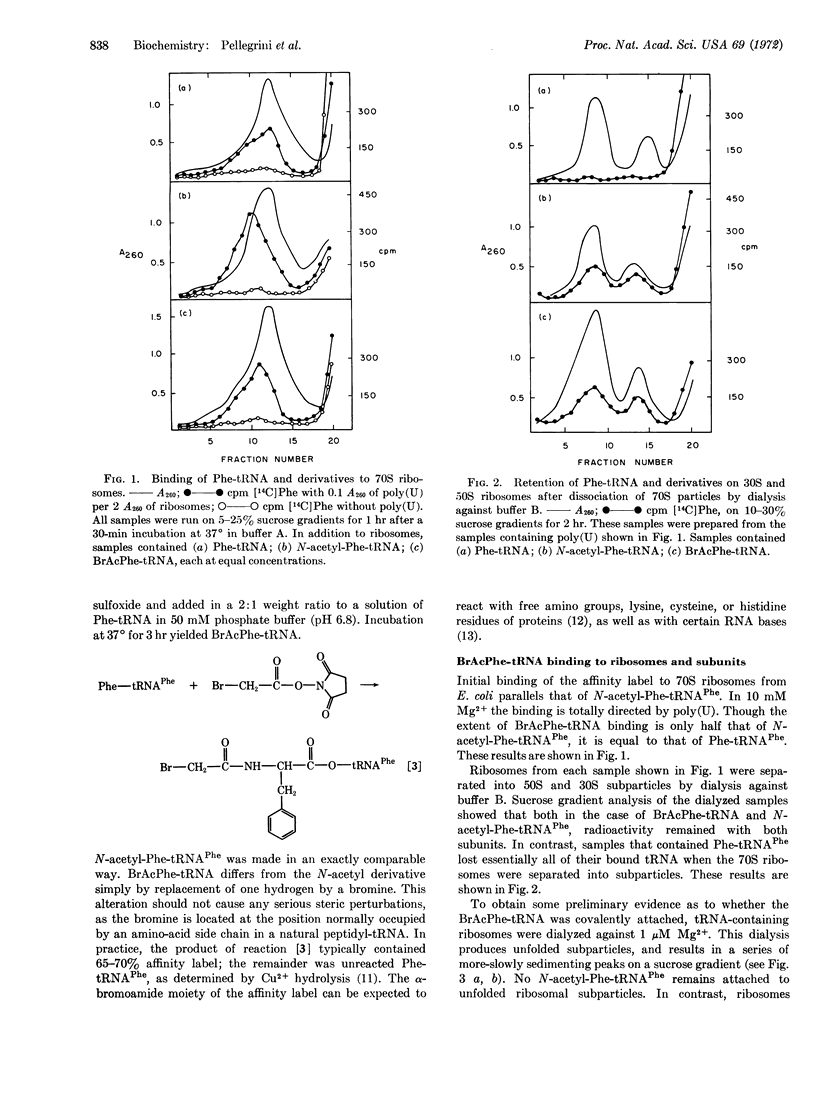
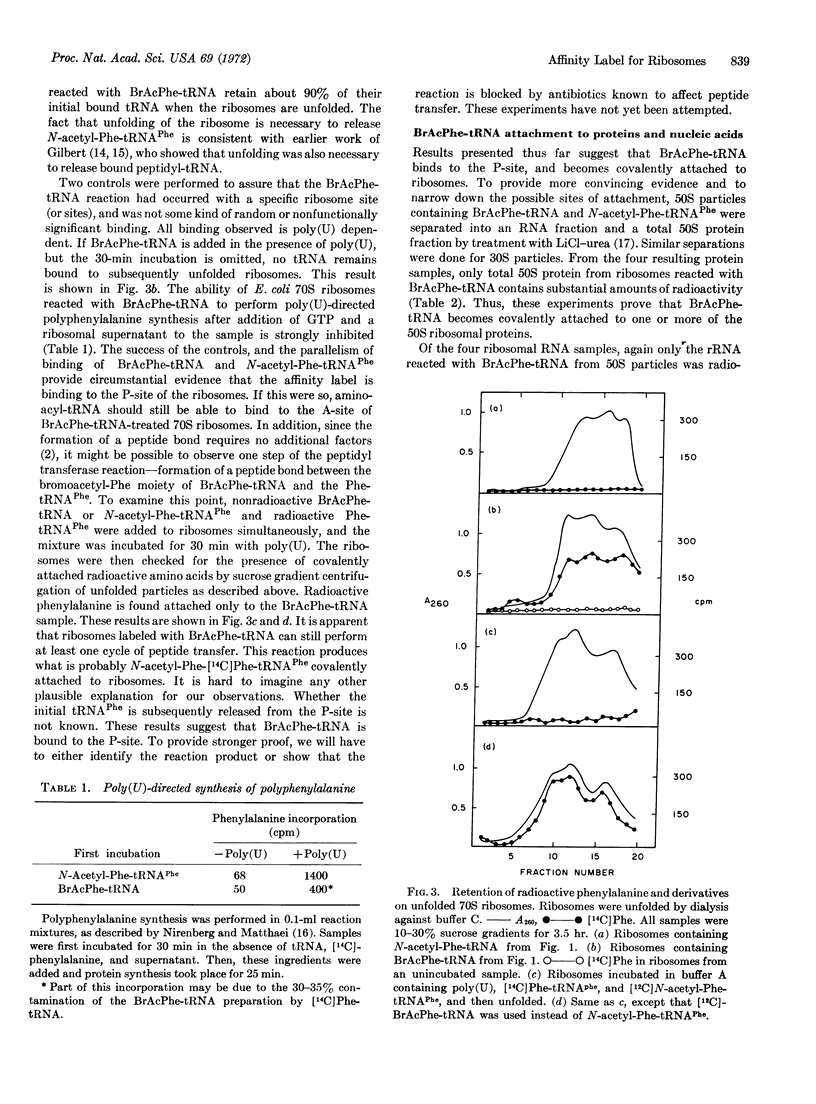
The peptidyl-tRNA analog, N-bromo-acetyl-Phenylalanyl-tRNAPhe has been prepared. Its binding to the 70S ribosome of E. coli is totally dependent upon polyuridylic acid. The analog becomes covalently attached to the 50S particle. It is associated with only one protein fraction after polyacrylamide-gel separation of total 50S proteins. The analog also reacts with 23S ribosomal RNA or a protein that remains tightly bound to this RNA after treatment with LiCl-urea and sodium dodecyl sulfate. The analog can function as a peptidyl-tRNA for at least one peptide transfer, but it then inhibits further chain elongation. This result strongly suggests that this analog becomes covalently bound at the P-site of the ribosome.
Keywords: protein synthesis, acrylamide gel, α-bromoamide, affinity label
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang F. N., Flaks J. G. Topography of the Escherichia coli ribosome. II. Preliminary sequence of 50 s subunit protein attack by trypsin and its correlation with functional activities. J Mol Biol. 1971 Oct 28;61(2):387–400. doi: 10.1016/0022-2836(71)90388-3. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- GILBERT W. Polypeptide synthesis in Escherichia coli. I. Ribosomes and the active complex. J Mol Biol. 1963 May;6:374–388. doi: 10.1016/s0022-2836(63)80050-9. [DOI] [PubMed] [Google Scholar]
- GILBERT W. Polypeptide synthesis in Escherichia coli. II. The polypeptide chain and S-RNA. J Mol Biol. 1963 May;6:389–403. doi: 10.1016/s0022-2836(63)80051-0. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Chapeville F. The behaviour of acetylphenylalanyl soluble ribonucleic acid in polyphenylalanine synthesis. Biochim Biophys Acta. 1966 Jan 18;114(1):135–148. doi: 10.1016/0005-2787(66)90261-9. [DOI] [PubMed] [Google Scholar]
- Lanks K. W., Sciscenti J., Weinstein I. B., Cantor C. R. Studies on rat liver phenylalanyl transfer ribonucleic acid synthetase. I. Purification, stabilization, and complex formation. J Biol Chem. 1971 Jun 10;246(11):3494–3499. [PubMed] [Google Scholar]
- Maden B. E., Traut R. R., Monro R. E. Ribosome-catalysed peptidyl transfer: the polyphenylalanine system. J Mol Biol. 1968 Jul 28;35(2):333–345. doi: 10.1016/s0022-2836(68)80028-2. [DOI] [PubMed] [Google Scholar]
- Monro R. E. Catalysis of peptide bond formation by 50 S ribosomal subunits from Escherichia coli. J Mol Biol. 1967 May 28;26(1):147–151. doi: 10.1016/0022-2836(67)90271-9. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto T., Kalokofsky D. A possible mechanism for initiation of protein synthesis. Proc Natl Acad Sci U S A. 1966 Mar;55(3):606–613. doi: 10.1073/pnas.55.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Bacterial ribosome. Bacteriol Rev. 1970 Sep;34(3):228–277. doi: 10.1128/br.34.3.228-277.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Erdmann V. A. Reconstitution of 50S ribosomal subunits from dissociated molecular components. Nature. 1970 Nov 21;228(5273):744–748. doi: 10.1038/228744a0. [DOI] [PubMed] [Google Scholar]
- Schofield P., Zamecnik P. C. Cupric ion catalysis in hydrolysis of aminoacyl-tRNA. Biochim Biophys Acta. 1968 Feb 26;155(2):410–416. doi: 10.1016/0005-2787(68)90185-8. [DOI] [PubMed] [Google Scholar]
- Shaw E. Selective chemical modification of proteins. Physiol Rev. 1970 Apr;50(2):244–296. doi: 10.1152/physrev.1970.50.2.244. [DOI] [PubMed] [Google Scholar]
- Smith I. C., Yamane T. Spin-labeled nucleic acids. Proc Natl Acad Sci U S A. 1967 Sep;58(3):884–887. doi: 10.1073/pnas.58.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin T., Maglott D. M., Monro R. E. On the catalytic center of peptidyl transfer: a part of the 50 S ribosome structure. Cold Spring Harb Symp Quant Biol. 1969;34:39–48. doi: 10.1101/sqb.1969.034.01.008. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A. 1968 Mar;59(3):777–784. doi: 10.1073/pnas.59.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut R. R., Delius H., Ahmad-Zadeh C., Bickle T. A., Pearson P., Tissières A. Ribosomal proteins of E. Coli: stoichiometry and implications for ribosome structure. Cold Spring Harb Symp Quant Biol. 1969;34:25–38. doi: 10.1101/sqb.1969.034.01.007. [DOI] [PubMed] [Google Scholar]
- WOOD W. B., BERG P. The effect of enzymatically synthesized ribonucleic acid on amino acid incorporation by a soluble protein-ribosome system from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:94–104. doi: 10.1073/pnas.48.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot N., Lapidot Y., Panet A., Wolman Y. The synthesis of N-acetylphenylalanyl-sRNA. Biochem Biophys Res Commun. 1966 Oct 5;25(1):17–22. doi: 10.1016/0006-291x(66)90633-4. [DOI] [PubMed] [Google Scholar]