Abstract
The colonial ascidian Botryllus schlosseri undergoes a histocompatibility reaction that can result in vascular fusion of distinct genotypes, creating a chimera. Chimerism has both potential benefits, such as an immediate increase in size that may enhance growth rates, and costs. For the latter, the presence of multiple genotypes in a chimera can lead to competition between genetically distinct stem cell lineages, resulting in complete replacement of somatic and germline tissues by a single genotype. Although fusion can occur at any point after metamorphosis, previous studies have focused on chimeras created from sexually mature adults, where no benefit to chimerism has been documented. Here we focus on the costs and benefits of fusion between juveniles, characterizing growth rates and patterns of somatic and germline chimerism after natural and controlled fusion events. We also compared outcomes between low- and high-density growth conditions, the latter more likely representative of what occurs in natural populations. We found that growth rates were density-dependent, and that only chimeras grew under high-density conditions. We also observed a positional component to a post-fusion event called resorption, indicating that extrinsic factors were important in this process. Patterns of germline and somatic chimerism and dominance in chimeras made from fused juveniles were equivalent to those after fusion of sexually mature adults, and there were no age-related differences in these processes. Finally, by using genetic markers that could retrospectively assign genotypes, we also found that the majority of individual testes in a chimera were clonally derived.
Introduction
Many marine invertebrates have the ability to combine tissues with conspecifics and form chimeras (Buss, 1990). This ability is usually accompanied by the presence of a polymorphic self/non-self recognition system that allows integration of closely related individuals, but blocks interactions between those more distantly related. The presence of a discriminatory allorecognition system implies that there are costs and benefits to chimerism that are correlated to relatedness, but the nature of these costs and benefits is still poorly understood (reviewed in Grosberg, 1988; Rinkevich and Weissman, 1992; De Tomaso, 2006)
The colonial ascidian Botryllus schlosseri (Pallas, 1766) is an excellent model for the study of allorecognition and chimerism. Ascidian embryogenesis results in a swimming chordate larva, which soon settles and metamorphoses into an invertebrate adult body plan. B. schlosseri is a colonial species and after metamorphosis begins a lifelong asexual budding process that results in a colony of genetically identical individuals (zooids) united by a common, extra-corporeal vasculature (reviewed in Manni et al., 2007). The budding cycle is a key component of allorecognition in this species, as each zooid can give rise to 1–4 new buds each week, depending on the growth conditions. At the end of the cycle, zooids die in a process called takeover, during which all their tissues undergo apoptosis and are resorbed by phagocytic cells in the blood, after which the new bud or buds complete development and become the adult zooids, and the process starts anew. Budding is synchronized throughout the colony, and takeover is an intrinsic property of the zooid, so if new buds do not develop, the colony resorbs itself and dies (Lauzon et al., 2002). In summary, growth in B. schlosseri is continuous, as each week all somatic and germline tissues are being regenerated, but it is not cumulative: maintenance of a constant size requires that at least one bud mature per zooid, while growth of a colony depends on multiple buds developing for each zooid.
Histocompatibility in Botryllus takes place in the adult body plan at the tips of the vasculature, called ampullae, and results in either a vascular fusion, creating a hematopoietic chimera, or a localized inflammatory reaction that blocks blood transfer. This reaction is regulated by the fusion histocompatibility locus (fuhc), which encodes a single, highly polymorphic, co-dominantly expressed gene (De Tomaso et al., 2005). Two individuals that share one or both fuhc alleles fuse, while those that share neither reject.
Fusion has both hypothesized benefits and well-documented costs. For the former, the immediate increase in size could affect survivability, as well as decrease the time to sexual maturity. In addition, multiple genotypes in a single individual may create genetic diversity that could help a chimera survive in a changing environment. However, while allorecognition in Botryllus appears to be promoting highly discriminatory fusion events, to date no clear advantage to chimerism has been documented (reviewed in Rinkevich and Weissman, 1992; De Tomaso, 2006).
In contrast, the costs of fusion can be severe. After the integration of two compatible colonies, one is often eliminated in a process called resorption, during which all zooid and bud tissues of one of the partners are phagocytosed, leaving behind the tunic, blood, and vasculature (Rinkevich and Weissman, 1987, 1992; Weissman et al., 1990; Rinkevich, 2002). In addition, vascular fusion allows mobile germline and somatic stem cells responsible for budding to naturally transplant between individuals, and these cells can contribute to asexual development in the parabiosed partner or partners This can result in genetic heterogeneity, where both genotypes are represented in somatic and germline tissues of the new zooids, or in parasitism, where stem cells of one genotype compete with another, and all resulting tissues are derived from a single genotype. Parasitism of germline and somatic tissues appears to be different: germline parasitism is a repeatable, hierarchical, and genetically determined process, while somatic parasitism is unlinked to that of the germline and is more random. In fact, the resorbed partner is often the only genotype found in the germline (Sabbadin and Zaniolo, 1979; Pancer et al., 1995; Stoner and Weissman, 1996; Stoner et al., 1999).
Although we are beginning to understand the molecular mechanisms that underlie the allorecognition process (De Tomaso et al., 2005; Nyholm et al., 2006), what remains unclear is the selective forces that maintain the extraordinary polymorphism of the fuhc, and how in turn allorecognition combined with extrinsic biological and environmental factors might affect the cellular and physiological development of Botryllus colonies. As the allorecognition system is competent immediately after metamorphosis, fusion can occur between adults, juveniles, or juveniles and adults.
While previous reports have focused on chimeras made between sexually mature adults (Sabbadin and Zaniolo, 1979; Pancer et al., 1995; Stoner and Weissman, 1996; Stoner et al., 1999), in this study we focused on chimeras made between juvenile individuals, in order to compare and contrast the costs and benefits of chimerism at these two ages. This choice was motivated by results from multiple studies which together suggest that the probability of a juvenile interacting with another juvenile is likely several orders of magnitude higher than an adult interacting with another adult (reviewed in De Tomaso, 2006). First, each week, each zooid gives rise to multiple motile larvae; however, the dispersal range is very short, on the order of 1 m (Grosberg, 1987). In addition, motile tadpoles have been shown to preferentially settle near compatible individuals (Grosberg and Quinn, 1986). In summary, juveniles are likely to settle in high-density aggregates of related (and hence compatible) individuals, suggesting some benefit of the chimerism originating at the juvenile stage of the life history. As the entire fuhc-based histocompatibility system seems to function to promote fusion between related individuals, we designed experiments to assess potential fitness costs and benefits of chimerism that was initiated at the juvenile stage, and also compared results between low and high densities: the former replicating conditions of previous studies; the latter attempting to reproduce natural aggregations.
Materials and Methods
Collection, mariculture and creation of chimeras
All mariculture procedures have been described previously (Boyd et al., 1986). Briefly, we collected 14 pregnant Botryllus schlosseri colonies from the marina in Monterey, California, and one from Santa Barbara, California. Individual colonies were tied to 3 × 5-cm glass slides and placed 5 cm opposite another glass slide (called the settlement slide) in a slide rack. The slide rack was placed into an aquarium, and within a few days the tadpoles hatched, swam to the settlement slide, and metamorphosed into the adult body plan (oozooid). Over 95% of the tadpoles hatch and immediately swim to and settle on the juxtaposed slide (Boyd et al., 1986). This produces high-density clutches of oozooids from a single mother (Table 1 shows densities for these experiments). As has been described previously, individuals from a single clutch fuse at a frequency of about 50%, indicating that they are half-siblings on average (Scofield et al., 1982); the same was seen here (data not shown). Each clutch was numbered and monitored daily as described. Animals are reared in 15-l tanks supplemented with food in suspension daily, and food is not limiting (Boyd et al., 1986).
Table 1.
Characterization of clutches used in this study
| Clutch # | Number of individuals |
Number of Chimeras |
Total number of oozooids |
Density (oozooids/cm2) |
# Chimeras/ # individual |
|---|---|---|---|---|---|
| 4450 | 44 | 20 | 99 | 2.64 | 0.45 |
| 4444 | 47 | 16 | 85 | 2.27 | 0.34 |
| 4497 | 47 | 19 | 85 | 2.27 | 0.40 |
| 4480 | 29 | 54 | 184 | 4.91 | 1.86 |
| 4477 | 118 | 45 | 223 | 3.55 | 0.41 |
| 4503 | 74 | 20 | 119 | 3.17 | 0.27 |
| 4509 | 166 | 51 | 281 | 7.49 | 0.31 |
| 4533 | 113 | 21 | 146 | 3.89 | 0.19 |
| 4542 | NA | 31 | 77 | 2.05 | NA |
| 4535 | NA | 35 | 80 | 2.13 | NA |
| 4577 | 69 | 5 | 81 | 2.16 | 0.07 |
| 4551 | 57 | 6 | 71 | 1.89 | 0.11 |
| 4274 | NA | 14 | 30 | 0.80 | NA |
| 4570 | NA | 6 | 15 | 0.40 | NA |
For each clutch the number of viable individuals, fused chimeras (bi- and multi-combined), and total number of oozooids (number of viable oozooids including those in chimeras) are presented. Density is calculated as number of oozooids per square centimeter of the settlement slides (total number/area of slide). Final column (# chimeras / # individuals) allows comparison of the number of chimeras fused to the number of oozooids remaining unfused on a slide of a particular density. NA indicates that data are not available for those samples.
Unmanipulated clutches were monitored for fusion or rejection over the oozooid stage (less than 7 days old) and in the week after takeover by the first asexual generation (called a blastozooid, herein referred to as a zooid) 8–15 days post–settlement. A total of 117 bichimeras and 51 trichimeras were formed in these first 2 weeks on 14 settlement slides (Table 1). These were deemed natural fusions. Un-fused individuals and fused chimeras left on the settlement slide were characterized as being grown at high density. After 14 days no further natural fusion occurred.
In addition, a subset of individuals from each clutch was transferred onto individual slides. In the first case we transferred three individuals and placed them on a 3 × 5-cm slide several centimeters from each other. These were individuals plated at low density (i.e., un-fused genotypes). In addition, we transferred several individuals from a single clutch and placed them adjacent to each other, then monitored them for fusion. This resulted in controlled fusions (36 bichimeras and 16 trichimeras were formed in this manner), which were also maintained at low density. One low-density chimera used in this study (SB23) was made in Santa Barbara.
From this point on colonies were monitored, and were referred to as either individuals (genotypes that were never parabiosed to another individual) or chimeras (i.e, in the first two weeks an individual was united in a parabiotic union with one [bichimera] or two [trichimera] other half-siblings). However, note that due to resorption or somatic/germline dominance following parabiosis, in some cases a chimera (as defined above) was no longer chimeric—that is, even though it was at some point parabiosed to another individual, one genotype did not contribute to the soma and germline that were sampled at the end of the observations. As we did only a single sampling of somatic and germline tissues, the time at which one genotype ceased to contribute to development is unknown.
Each individual and chimera was given a unique identifier and observed for up to 7 months. We recorded (1) age (days old); (2) health (poor or good); (3) number of zooids; (4) rejection, fusion, and resorption status; and (5) age at sexual maturity (visible presence of testes, eggs, or embryos where applicable). Rejection, fusion, and resorption were characterized visually. Colonies that survived were isolated and dissected.
Density-dependent growth rates
Growth
We calculated growth rates of individuals and chimeras from eight clutches (pooled within clutches) and analyzed them as a metric of fitness to compare (1) individual colonies and chimeras, and (2) colonies subjected to growth in high-density (>1.0 oozoids/cm2; 1.89 –7.49 oozoids/cm2; avg. 2.8 oozooids/cm2) settlement aggregations, as found on the original settlement slides, and low-density (<1.0 oozoids/cm2; 0.027–0.16 oozoids/cm2; avg. 0.076 oozooids/cm2) conditions, as artificially produced by placing 1–3 individuals on a single slide. The growth rates were calculated as the total increase in the number of zooids in a colony during the experiment, normalized to the number of days observed and the number of zooids fused. Using this metric, an average growth rate of zero represents no increase in the number of zooids from asexual budding, just weekly self-replacement. In other words, growth in B. schlosseri is not cumulative—in order for a colony to increase in size, each individual must have more than 1 bud/week.
High- to low-density growth comparisons
After growth at high density, we transferred a subset of individuals and chimeras to low-density conditions by removing them with a razor blade and transferring them individually onto a new 3 × 5-cm glass slide (Boyd et al., 1986). As a control for the transfer process itself, we replicated the transfer procedure on an equivalent number of individuals and chimeras by lifting them off the substrate, then placing them back down in the same spot. The transfer procedure did not affect growth when colonies were placed back at high density.
Statistical tests
Data was tested for normality using the Shapiro-Wilk W test. If the null hypothesis was not rejected, two-tailed, paired Student’s t-tests were used to compare individuals and chimeras to test two null hypotheses (α = 0.05): (1) growth rates of both individuals and chimeras are independent of density, and (2) individuals and chimeras grow at the same rate. However, if the Shapiro-Wilk W test rejected the null hypothesis, the data were analyzed using the Wilcoxon signed-rank test. All statistical analyses were performed in the computing program R, ver. 2.11 (R Development Core Team, 2010).
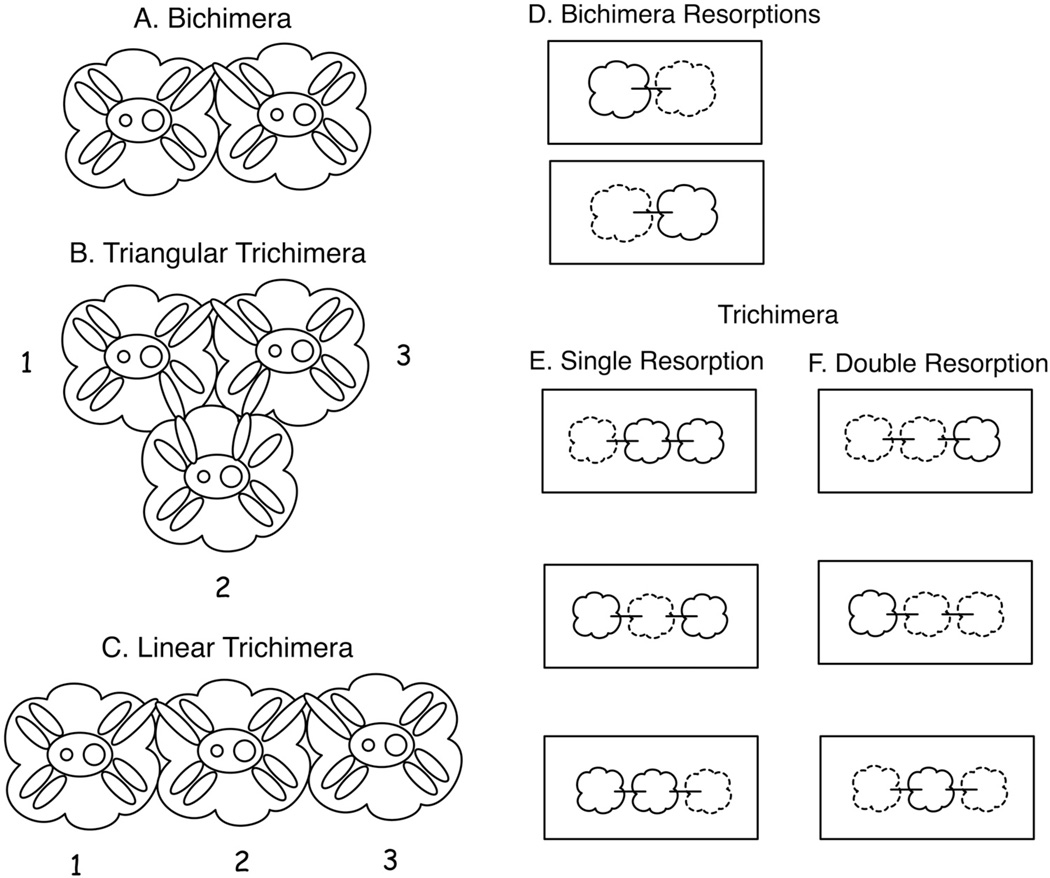
Positional resorption patterns
We observed resorption patterns and growth rates, and we statistically analyzed trends in the data. We characterized fusion and resorption patterns for chimeras as outlined in Figure 1, to test the dependence on resorption on spatial arrangement. Juveniles in each trichimera were numbered 1, 2, or 3 according to position. Two fusion patterns resulted from both the mechanical and natural trichimeras: triangular, where each juvenile was fused to both other juveniles (1 fused to 2, 2 fused to 3, and 3 fused to 1); and linear, where 1 fused to 2 and 2 fused to 3, but 1 and 3 were not fused. Resorptions of juveniles in trichimeric fusions were characterized by the number of individuals resorbed (zero, one, or two) and the position of the resorbed individuals (position 1, 2, or 3). For linear trichimeras, resorption data was compiled in a 3 × 2 table, with each column representing one of the three possible positions and the rows quantifying the number of oozooids in that position that were either resorbed or not resorbed. A chi-squared goodness of fit test (α = 0.05) was used to examine how the observed data fit the null hypothesis: if two of three individuals in the fused chimera were resorbed, there would be a 2/3 chance of resorption and 1/3 chance of non-resorption of individuals in each position. All statistical analyses were performed in the computing program R, ver. 2.11 (R Development Core Team, 2010).
Figure 1.
Characterization of fusion and resorption patterns for juvenile chimeras. The left panels depict natural and mechanical fusions of (A) bichimeras, (B) triangular trichimeras, and (C) linear trichimeras, numbering consistent with positional analysis (1 = left, 2 = middle, 3 = right). Connected ampullae depict fusion. Resorptions are shown in the right panels for (D) bichimeras and both (E) single and (F) double resorption of trichimeras. Horizontal lines between juveniles represent fusion. Individuals drawn with solid lines are unresorbed, and those drawn with dotted lines are resorbed.
Normality of the observations was confirmed using box-plots that showed slight skewedness of some data but no outliers, and the assumption of independence of observations can be made as (1) all tank treatments were identical and (2) slides were intermittently moved between tanks in order to avoid tank effects.
Analysis of somatic and germline chimerism
We extracted DNA from intestines (somatic) and testes (gametic) dissected from the sexually mature individuals and chimeras. Testes and intestines were removed from zooids and cleaned of all visible hematopoietic contamination (Laird et al., 2005). From 3 to 10 intestines were combined for each somatic sample to obtain enough DNA for reliable amplified fragment length polymorphism (AFLP) analysis. Testes were analyzed individually. All samples were immediately frozen in liquid nitrogen and then kept at −80 °C until DNA extraction.
DNA was extracted using a silica-based DNA extraction protocol (Stoner et al., 1999) and AFLP was performed as described (De Tomaso et al., 1998). AFLP fingerprints are 98% repeatable from independent assays on the same DNA, very resistant to contamination (Fig. 2), and generally amplify from 50 to more than 100 distinctive loci, of which inbred siblings have at least 8 Mendelianly inherited polymorphic regions. AFLP fingerprints were used to retrospectively assign genotypes to samples by comparing numerous distinctive loci simultaneously. We used previous results to set an initial threshold of 8 or more distinctive polymorphic regions to assign a sample as a unique genotype, while samples with 7 or fewer differences were assigned the same genotype (sample differences <8 were considered noise probably caused by contamination or low DNA concentration in the sample; De Tomaso et al., 1998). This was a very conservative threshold, as most genetically unique samples in this study had 10 or more differences (Figs. 2–4) and most genetically identical samples had 3 or fewer differences, making the retrospective assignment of genotypes more apparent than a single number cutoff would suggest. Importantly, each chimera had multiple independent samples that gave identical AFLP fingerprints, removing any ambiguity in the assignments (see Figs. 3–4). We also found that about 98% of the germline and 79% of the somatic samples were clonal (within the limit of AFLP detection), but some samples with genetic heterogeneity were observed. In that case we classified a sample as heterogeneous (i.e., derived from multiple genotypes within a chimera) only when all the polymorphic presence bands from two unique genotypes in the same chimera were observed.
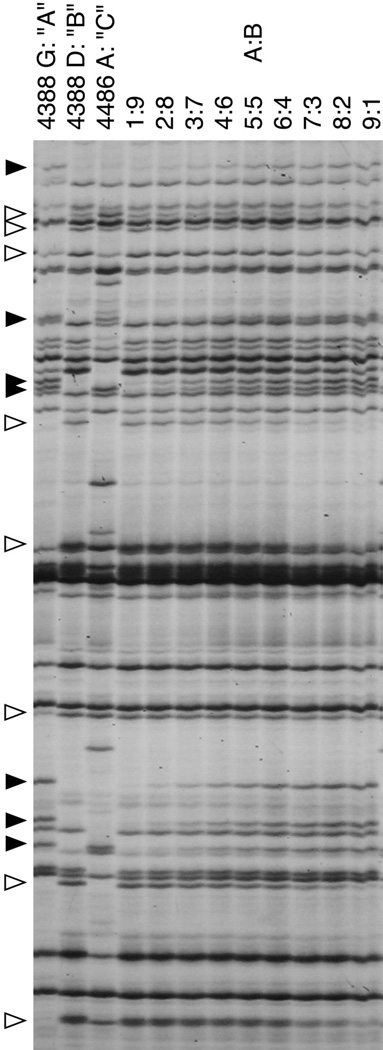
Figure 2.
Full AFLP gel showing fingerprints of three genotypes and a replicated bi-chimerism dilution series. Left three lanes: AFLP fingerprints of genotypes “A”, “B”, and “C”. Genotypes A and B are clutch-mates and likely half-siblings (see Methods). C is from an independent collection. Each individual fingerprint differs by at least 10 presence and absence bands from any other. Remaining lanes: AFLP fingerprints from a DNA dilution series between samples A and B, showing the detection of different polymorphic bands at different dilutions. Black arrowheads show bands unique to genotype A; white arrowheads depict bands unique to genotype B. As shown, each band shows a unique sensitivity and appears at a different dilution. Overall, multiple bands appear at about a 10% dilution, indicating the baseline sensitivity of this technique to detect chimerism.
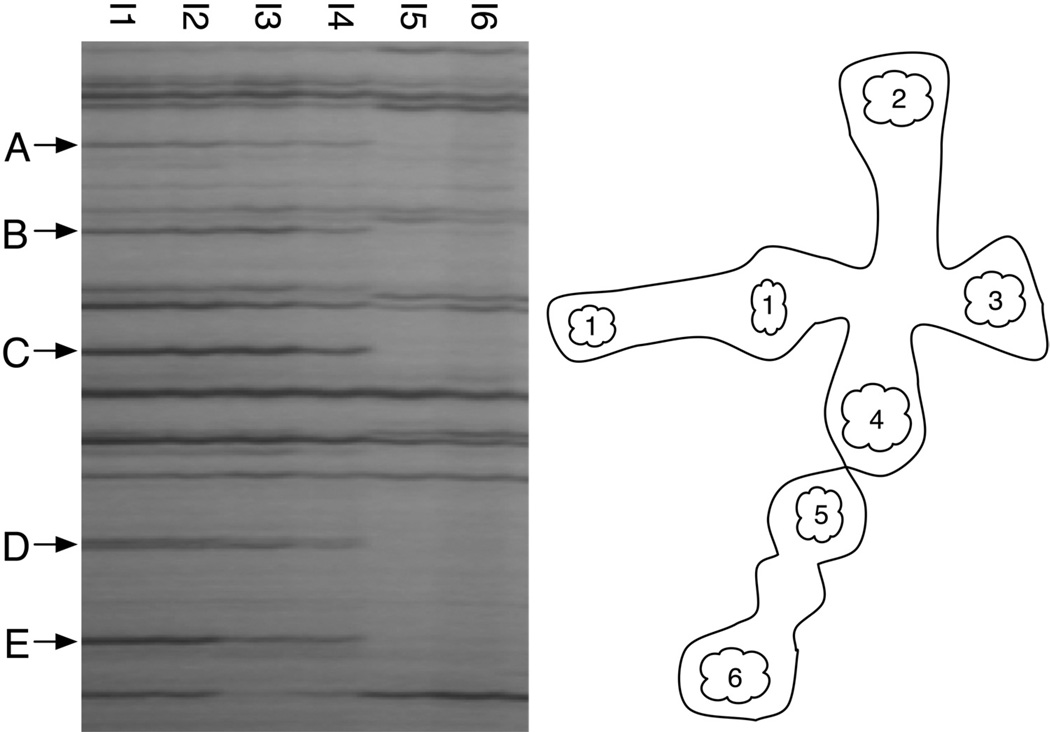
Figure 4.
AFLP fingerprints of somatic samples from bichimera 4497-7 show the two distinct genotypes are spatially segregated within the colony. Each lane of the gel corresponds to a somatic sample of pooled intestines (I1–I6); location of each sampling site in the chimera is illustrated on the right. Note the repeatability of independent samples I1-I4 (arrowheads outline unique presence bands in these samples), and I5 and I6, which are also equivalent.
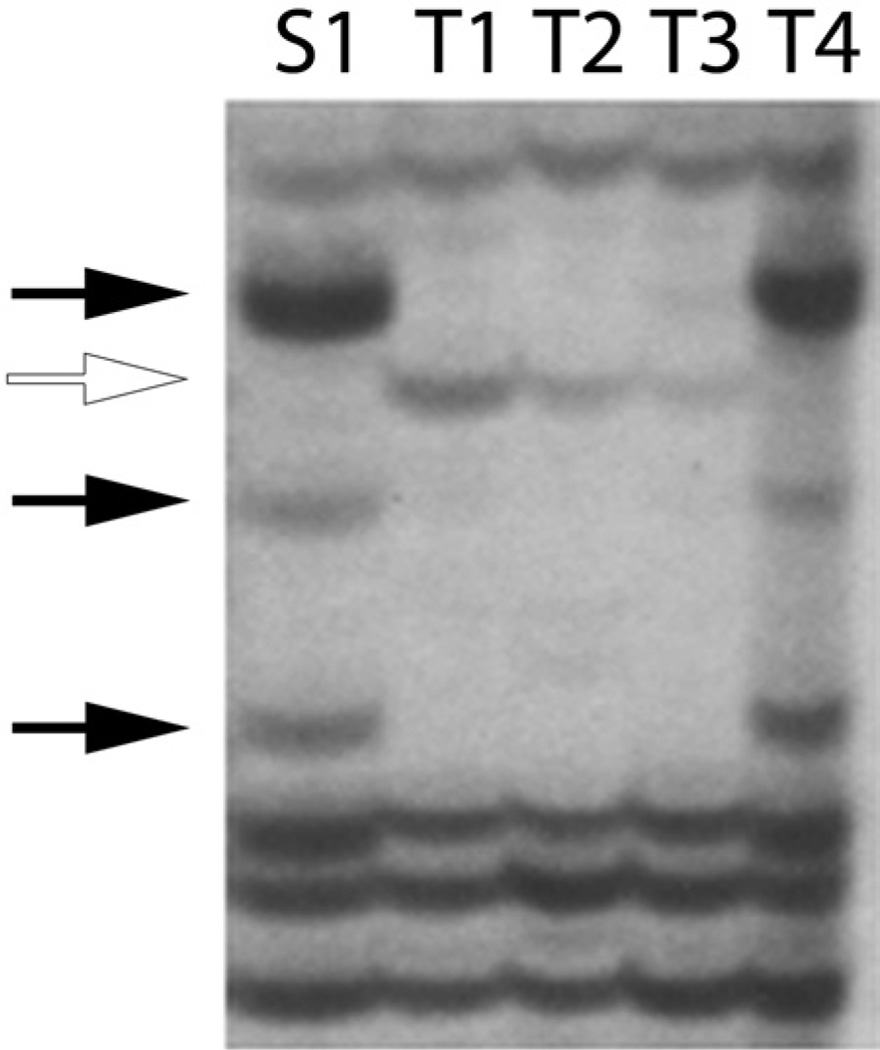
Figure 3.
Patterns of somatic and germline chimerism in Chimera SB23. This bi-chimera was sacrificed 142 days after fusion of two oozooids, and one somatic sample and four testes were isolated and analyzed by AFLP as described in the methods. Bands unique to each genotype are illustrated (solid and open arrows). Somatic (S1) and testis (T1-T4) fingerprints are outlined. AFLP fingerprints of one of the four testis samples exactly match the somatic sample, while three testis samples are divergent, but match each other. As shown, each independent testis is derived from either one or the other genotype present in the chimera. Thus within the detection level of the AFLP technique (Fig. 2), individual testis are clonal and derived from a single genotype within the chimera.
Results
Density-dependent growth rates
Previous studies on chimeric colonies have not found any increase in fitness (e.g., growth rates, time to sexual maturity, lifespan) when compared to unmanipulated individuals, but every one of these studies compared chimeras to single individuals when each sample was grown on an independent substrate and isolated from each other (Rinkevich and Weissman, 1987; Chadwick-Furman and Weissman, 1995, 2003). However, B. schlosseri tadpoles have a very limited dispersal range, and in addition have been shown to preferentially settle near kin: these characteristics could potentially establish high-density kinship aggregations in nature consisting of half-siblings and chimeras (Grosberg and Quinn, 1986; Grosberg, 1987). Thus we compared growth between individuals and chimeras reared at low and high density under controlled laboratory conditions from birth to sexual maturity to see if chimerism had any benefit under these conditions.
To assess growth under low- and high-density conditions, we compared growth rates of both individuals and chimeras that formed naturally on the settlement slides at high-density conditions to a subset of individuals and chimeras made by controlled fusions grown on individual slides at low density. Table 1 shows the number and densities of individuals and chimeras on each settlement slide. The growth rate results of chimeras and individuals are shown in Table 2.
Table 2.
Average growth rates of chimeras and individuals in each clutch
| Chimera growth rate |
Individual growth rate |
|||||
|---|---|---|---|---|---|---|
| Mother Colony |
Low-density | High-density | After transfer | High-density | After transfer | |
| 4450 | 0.189 | 0 | 0.054 | 0 | 0.061 | |
| 4444 | 0.177 | 0 | 0.14 | 0 | 0.054 | |
| 4497 | 0.200 | 0.017 | 0.14 | 0 | ND | |
| 4480 | ND | 0.023 | 0.069 | 0 | 0.065 | |
| 4477 | 0.223 | 0.013 | 0.068 | 0 | 0.068 | |
| 4503 | 0.164 | 0.007 | ND | 0 | 0.079 | |
| 4509 | 0.178 | 0 | ND | 0 | 0.06 | |
| 4533 | 0.166 | 0 | ND | 0 | 0.051 | |
| Average | 0.19 | 0.0076 | 0.10 | 0 | 0.069 | |
Columns represent the average growth rates of specimen chimeras on either settlement (high-density) or individual (low-density) slides, or after transfer between the two. Growth rates were calculated as number of additional asexually derived zooids in a colony per day, normalized to the original number of zooids. Values of 0 represent clutches in which no specimen grew to a size greater than one zooid. ND indicates that there were no specimens from a given clutch.
If individuals or chimeras were transferred from the settlement slide within the first 2 weeks after hatching and maintained at low density, they grew at an average rate of 0.19 zooids/zooid/day, and there was no noticeable difference between individuals and chimeras over the course of this study (Table 2 shows data for chimeras; results were equivalent for individuals [not shown]), and this result is consistent with previous studies (Rinkevich and Weissman 1987; Chadwick-Furman and Weissman, 1995, 2003). In contrast, if left under high-density conditions, none of the individuals grew but remained at one zooid (with only one bud developing per week) Other than this lack of increase in zooid number, these individuals looked healthy and robust.
In contrast, many (but not all) chimeras did increase in size when maintained at high-density conditions, although this was over 10 times slower than growth when maintained at low-density conditions (Table 2). The overall lack of growth under high-density conditions has been reported previously, and due to these observations it is standard mariculture practice to transfer newly settled colonies within a week or two of hatching (Boyd et al., 1986). However, we have never directly compared the two density conditions.
To assess the significance of the observed density-dependence to growth, we analyzed the data statistically with the null hypothesis that colony growth is not density-dependent (see Methods). This analysis started with chimeras.
When we compared the growth rates of chimeras under low-density conditions (controlled fusions on individual slides) to chimeras at high density (natural fusions on settlement slides), the null hypothesis was rejected (Wilcoxon signed-rank test; n = 7; df = 6; P = 0.016), rejecting the null hypothesis and suggesting that growth is density-dependent.
To test if the density effect was reversible, we compared the growth rates of a subset of chimeras that were initially grown under high-density settlement conditions to the growth rates of the same chimeras after their transfer to low-density growing conditions (<1.0 oozoids/cm2). Growth increased after transfer, and the null hypothesis was again rejected (paired t-test; n = 5; df = 4; P value = 0.0135), suggesting that growth rates are continually dependent on density, and inhibition of growth at high density can be reversed if the density is reduced. As a control for the transfer procedure itself, five colonies were lifted from the substrate and then placed back in their original location: no difference in growth rates versus unmanipulated chimeras was observed (not shown).
Next, we repeated the same tests on individuals. As nearly 100% of individuals in the high-density settlement aggregations showed zero growth (n = 100; Table 2), and growth only occurred under low-density conditions, this was not analyzed further. However, when individuals were transferred from high- to low-density conditions, growth rates increased significantly (paired t-test; n = 7; df = 6; P value = 2.05 × 10−6) to an average of 0.069 zooids/zooid/day (Table 2). The control for the transfer procedure (described above, not shown) did not stimulate growth, equivalent to results in chimeras.
In summary, growth in both individuals and chimeras was density-dependent, and the inhibition observed at high density was reversible, as growth would increase after transfer to low-density conditions.
Next, we compared growth of individuals to chimeras maintained at high-density conditions with the null hypothesis that growth between individuals and chimeras at high density was equivalent. A one-tailed Wilcoxon signed-rank test gave a P value of 0.062 (n = 8; df = 7), which is not significant and does not reject the null hypothesis. Finally, we did an equivalent test comparing growth of individuals versus chimeras after transfer. Again, the null hypothesis was not rejected (not shown).
In conclusion, growth of juvenile B. schlosseri is density-dependent for both individuals and chimeras. Density inhibits growth, but this effect is reversible, and growth will increase once an individual or chimera is transferred to low-density conditions. These observations were consistent between each clutch, and likely have no genetic basis. Under high-density conditions some chimeras grew slowly, while individuals did not grow at all, but there is no statistical support for chimeras having an advantage under high-density conditions. However, these latter interpretations have an additional caveat: it is likely that many of the chimeras were in reality dominated by a single genotype (discussed below).
Positional resorption patterns
In many cases, one colony disappears after fusion in a process called resorption, during which the tissues of one genotype in the chimera are eliminated by phagocytic blood cells. Resorption is a robust phenomenon in the laboratory that has been attributed to a second level of allorecognition, and is thought to have a genetic basis (Rinkevich et al., 1993). In this study we characterized resorption in both natural and experimental chimeras. Resorption of individuals (ranging in size from 1 to 7 zooids) largely occurred at 1 to 50 days post-fusion, although some chimeras did not show resorption before observation ceased (up to 6 months post-fusion). Only 16% of chimeras (7.4% bichimeras and 9.4% trichimeras) reached sexual maturity without visual resorption (Table 3). Resorption was independent of density and occurred almost equally in both treatments (not shown). Thus there are no density-dependent differences in this post-fusion event.
Table 3.
Resorption in chimeras
| Chimera type |
Non-resorption (%) |
Single resorption (%) |
Double resorption (%) |
Total number |
|---|---|---|---|---|
| Bichimera | 7.35 | 92.6 | NA | 204 |
| Trichimera | 9.38 | 17.2 | 71.9 | 64 |
| Linear | 5.88 | 13.5 | 80.4 | 52 |
| Triangular | 25.0 | 33.0 | 42.0 | 12 |
Shown are the aggregate percentages of each type of resorption in both bi- and trichimeras (both linear and triangular) for all clutches in this study. Percentages are calculated as the number of chimeras in the resorption category out of the total number of chimeras in that group (final column).
NA = not appllicable.
However, over the course of this study we observed a repeatable resorption pattern in trichimeras that suggested a positional component to this process and was independent of natural or controlled fusions (described below). As resorption is a major cost of chimerism, and we were observing an extrinsic effect to this process, we followed up on these observations, and the combined results are summarized in Tables 3 and 4.
Table 4.
Chi-square goodness of fit table for linear fusions with two resorbed individuals
| Position |
||||
|---|---|---|---|---|
| 1 | 2 | 3 | Total | |
| OBSERVED | ||||
| Resorbed | 35 | 17 | 30 | 82 |
| Not Resorbed | 6 | 24 | 11 | 41 |
| TOTAL | 41 | 41 | 41 | 123 |
| EXPECTED | ||||
| Resorbed | 27.3 | 27.3 | 27.3 | |
| Not Resorbed | 13.7 | 13.7 | 13.7 | |
Number of resorbed specimens observed in each location is reported. The expected numbers were calculated by hypothesizing a 2/3 chance of resorption and a 1/3 chance of non-resorption of an individual in a given position in a given chimera. Category totals are italicized below the observed values.
Two distinct types of trichimeras were followed in this study, a linear fusion, with a distinct middle individual fused to two outside individuals, and a triangular fusion, such that each individual is fused to both others (Fig. 1). For the purpose of this study, linear trichimeras provided us more information about the position-dependence of resorption, as we were clearly able to define the “middle” and “outside” individuals. In all, we followed 12 triangular and 52 linear trichimeras. In general, resorption occurred less frequently in the triangular fusions (75% resorption) than in the linear fusions (about 95% resorption); and when resorptions did occur, single resorptions were more frequent in triangular trichimeras (33% triangular vs. 13.5% linear), while double resorptions dominated the linear counterpart (42% triangular vs. 80% linear; Table 3).
We used a chi-square test to assess position specificity in the group of linearly fused trichimeras with double resorption, both because of the clear position distinction and the fact that it provided the largest data set (implying the greatest frequency of occurrence). The observed and expected values are shown in Table 4. The results reject the null hypothesis of equal resorption probability at all positions (P = 7.67 × 10−5), instead supporting the possibility of a position-specific resorption trend in the linear fusions. The data demonstrate that the observed occurrence of non-resorption differs significantly from the expected values (P = 0.05): specifically, the central zooid remains unresorbed more frequently than expected. We conclude that there are positional components to resorption, and as discussed below, this has major implications for the cost/benefit analysis of fusion and chimerism.
Analysis of somatic and germline chimerism
Previous studies on somatic and germline chimerism have mainly focused on parabiosis between equivalent-sized, sexually mature adults. Under these conditions a number of different outcomes can occur after fusion— including resorption, chimerism, and parasitism of the germline or soma, and combinations of all three processes (Pancer et al., 1995; Stoner and Weissman, 1996; Stoner et al., 1999).
We wondered whether the patterns of germline and somatic chimerism would differ if the parabiosis occurred when the genotypes were juveniles, months prior to sexual maturity. If they are different, then an age component may be necessary when assessing the costs and benefits of fusion. In addition, these experiments have an interrelated developmental component because they provide insight into the nature of the somatic and germline stem cells responsible for regeneration. For example, if patterns of germline chimerism are equivalent between chimeras made between juveniles and adults, this would suggest that mobile germline progenitors are specified early in development and contribute to germline development when the individual or chimera reaches sexual maturity. In contrast, if patterns of germline chimerism are different, for example, if the resorbed juvenile could no longer contribute to germline development in the sexually mature adult, this would suggest that germline progenitors arise later in development. If the latter situation was correct, this developmental characteristic would have a major effect on the costs of fusion for juveniles, but not adults.
Detection of somatic and germline chimerism
Previous studies of chimeras relied on a small number of molecular markers, restricting studies to genetically distinguishable, fusible adults. In this study we randomly created chimeras from half-sibling groups and assigned genotypes retrospectively, using AFLPs as molecular genetic markers. AFLPs can discriminate between highly inbred siblings (De Tomaso et al., 1998, 2003, 2005) and in addition are semiquantitative (Fig. 2), potentially allowing us to assess contribution of multiple genotypes in a chimera (see Methods).
Initially, we determined the ability of the AFLP technique to detect multiple genotypes in a mixed sample, specifically assessing the amount of chimerism that could be detected (Fig. 2). In this experiment, AFLP fingerprints of individuals isolated from three laboratory colonies (4383G = “A”, 4388D = “B”, 4388A = “C”; B and C are siblings; Fig. 2) were compared, and it was found that each individual can be discriminated by at least eight polymorphic bands regardless of parentage, consistent with previous results (De Tomaso et al., 1998). We next simulated a range of genetic heterogeneity that could be observed in a bi-chimera by combining DNA from colonies A and B in standard ratios (1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1). This allowed us to assess the sensitivity of AFLPs in detecting differing amounts of DNA from each genotype in a chimera (Fig. 2). In all dilutions, AFLP fingerprints revealed 5 to 10 bands unique to each genotype in the DNA mixture, down to a 1:9 ratio; however, some lighter bands did disappear as early as at 50% dilution. These results confirm the ability of AFLP analysis to detect low-level presence of minority genotypes in DNA, with the caveat that not all bands of the minority progenitor will definitely be visualized in the chimera. In summary, Figure 2 demonstrates that by using AFLPs we can retrospectively assign individual genotypes with 90% confidence and detect chimerism within a DNA sample when it reaches 10% or more of the total sample mass.
Patterns of chimerism in adults after fusion as juveniles
After fusion of juveniles, chimeras were grown to sexual maturity and then sacrificed. Somatic and gametic tissue samples were isolated (Table 5) and 395 were analyzed. These included samples from 10 un-fused control specimens (5 with both somatic and gametic tissue amplifications), 22 bichimeras, and 7 trichimeric fusions. The genetic breakdown of all samples for each colony is provided in Table 5; Table 6 summarizes the overall frequencies of these results. In no case did the AFLPs assign more genotypes to the chimeras then the number of individuals that fused, and all samples from an unmanipulated individual had only a single genotype. In addition, each chimera had multiple independent testes or somatic samples with identical fingerprints, and the few heterogeneous samples identified were consistent with results shown in Figure 2, as they contained all polymorphic bands of multiple independent samples within the same chimera (not shown).
Table 5.
Genotypes of multiple somatic and gametic samples from un-fused individuals and chimeras
| Specimen ID | Genotype | Age at dissection (days) |
Resorption type |
Genotype of somatic samples |
Genotype of individual testes |
|---|---|---|---|---|---|
| A | |||||
| 4551-8 | 1 | 110 | NA | A^,A | A,A,A,A,A,A |
| 4577-I1 | 1 | 110 | NA | A | NA |
| 4509-4 | 1 | 113 | NA | A^ | A,A,A,A,A,A,A,A,A,A,A,A |
| 4503-9 | 1 | 116 | NA | A | A,A,A,A,A,A |
| 4509-I1 | 1 | 119 | NA | A | A,A,A,A |
| 4450-2A | 1 | 148 | NA | A,A | NA |
| 4444-ind | 1 | 153 | NA | NA | A,A,A,A,A,A,A |
| 4480-ind. | 1 | 153 | NA | A^ | A,A,A,A,A,A |
| A,B | |||||
| 4450-1A | 2 | 66 | L | A,B*,B* | B,B,B,B,B,B,B,B,B,B,B |
| 4577-6 | 2 | 85 | R | A | B,B,B,B,B,B,B,B |
| 4450-2 | 2 | 90 | L | A*,A/B*,B**, A** | A,A,A,A |
| 4551-6 | 2 | 90 | L | A | B,B |
| 4577-7 | 2 | 90 | L | A,B | A,A,A,A,A,A,A,A,A,A,A,A,A,A,A |
| 4497-2 | 2 | 91 | R | A,A | B,B,B,B,B,A/B,B,B,B |
| 4551-1 | 2 | 93 | R | A^,A | B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B |
| 4533-2 | 2 | 95 | R | A | A,A |
| 4533-4 | 2 | 95 | L | A^,A | B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B |
| 4509-1 | 2 | 113 | R | A | A,A,A,A,A,A |
| 4509-8 | 2 | 113 | R | A | A,A,A,A,A,A,A,A,A,A,A,A,A,A |
| 4477-22 | 2 | 129 | L | A^ | A,A,A |
| 4497-1 | 2 | 139 | R | A,A,A^ | A,A,A |
| 4497-8 | 2 | 139 | R | A | A,A,A,A |
| 4497-4 | 2 | 144 | L | A^,A | B,B,B,B,B,B,A,B |
| 4477-26(s) | 2 | 149 | R | A | A,A,A,A,A,A |
| 4480-25(s) | 2 | 153 | R | NA | A,A,A,A,A,A,A |
| 4477-20 | 2 | 154 | L | A,A^ | A,A,A,A,B,A,B |
| 4497-14(s) | 2 | 154 | R | A,A | A,A,A,A,A,A,A,A,A,A,A,A,A |
| 4497-16(s) | 2 | 154 | L | A | B,B,B,B,B,B,B,B,B,B,B,B,B,B,B |
| 4450-6(s) | 2 | 162 | R | A | B,B,B,B |
| 4503-13(s) | 2 | 171 | R | A,A | A,A,A,A |
| SB23 | 2 | 142 | R | A | B,B,B,A |
| A,B,C | |||||
| 4450-3 | 3 | 66 | Double: 1,3 | A*,A* | A,A,A,B,A,A,A,A,A |
| 4444-4 | 3 | 87 | Single: 2 | A,A*,A* | A,A |
| 4577-8 | 3 | 90 | Double: 1,3 | NA | A,A,A,A,A,A,A,A,A |
| 4551-2 | 3 | 93 | Single: 2 | A^,A | A,A,A,A,A,A,A,A,A,A,A,A,A,A,A,A |
| 4477-30(s) | 3 | 149 | Double: 1,3 | A | A,A,A,A,A,A,A,A |
| 4497-3 | 3 | 158 | Single: 2 | A,A,B^,A^ | B,B,B,B,C,B,B,B,B,B,B,B,B,A/B,B,B |
| 4497-7 | 3 | 158 | Single: 3 | A^, A, A, A, B, B | B,B,C,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B,B |
Information regarding the individual specimen ID, including the genotype (1 = un-fused, 2 = bichimera, 3 = trichimera); the age (days from hatch) of the specimen when isolated; which and how many of the individuals within a chimera were resorbed (NA = not applicable for the individuals, L = left, R = right [bichimeras], 1 = left, 2 = middle, 3 = right [trichimeras]); and the AFLP fingerprints for the somatic and gametic tissue samples (A = genotype 1, B = genotype 2, C = genotype 3 for each respective colony; A/B = chimeric mix of genotypes A and B, NA = data not available). Each genotype letter represents an individual somatic and gametic sample, and the order of the letters reflects the relative position in the colony. Somatic samples are noted as containing intestines collected from multiple systems (^), as multiple samples taken from the same system (sample pairs from same system marked correspondingly as *,* or **,**), or as containing intestines from only one system (no mark).
Table 6.
Summary of the frequencies of chimerism and parasitism of somatic and gametic tissues from juvenile bi- and trichimeras
| Bichimeras | Trichimeras | Total chimeric specimen |
|
|---|---|---|---|
| Number of Specimens | 23 | 7 | 30 |
| Somatic Patterns | |||
| Only one genotype present | 20 (87%) | 4 (57%) | 23 (77%) |
| Both genotypes present: discrete | 2 (9%) | 1 (14%) | 3 (10%) |
| Both genotypes present: gradient | 1 (4%) | 1 (14%) | 2 (7%) |
| No intestine samples | 1 (4%) | 1 (14%) | 2 (7%) |
| Gametic Patterns | |||
| All testes match all soma | 10 (43%) | 3 (43%) | 13 (43%) |
| All testes match one of multiple soma signature present | 3 (13%) | 1 (14%) | 4 (13%) |
| All testes different than all soma | 5 (22%) | 0 (0%) | 5 (17%) |
| Multiple genotypes present | 3 (13%) | 1 (14%) | 3 (10%) |
| Chimeric testes present | 1 (4%) | 1 (14%) | 2 (7%) |
| No intestine samples; all | 1(4%) | 1(14%) | 2(7%) |
The number of chimeras observed in each category is followed in parentheses by the frequency of the result (with respect to each of the three columnar categories). The total chimeric specimen column represents the overall chimera trends, derived as the numerical combination of bichimeras and trichimeras in each category.
Overall, four combinations of somatic and germline chimerism were observed in this study (Tables 5 and 6): (1) all gametic and somatic samples within a chimera had the same signature, indicating either complete stem cell parasitism in both somatic and germline tissues or equal contribution from both genotypes in all sampled tissues; (2) all gametic samples matched one of multiple somatic signatures (representing either a combination of clonally derived intestines from multiple genotypes, or single intestines derived from both genotypes) within a chimera—these are examples of somatic co-dominance and germline parasitism; (3) all gametic samples matched but were different from the somatic signature present—thus a single genotype was the contributor to the gamete tissue, but was not represented in any somatic samples; or (4) a single somatic genotype was observed, but there was chimerism in the gametic samples. This could be due to somatic dominance or resorption of one of the genotypes coupled to contribution from both genotypes in the germline.
An example of a bichimera with pattern (4) is shown in Figure 3. This chimera (SB23; Table 5) shows somatic dominance coupled to germline chimerism. One somatic genotype is observed in intestines sampled throughout the adult colony, and this fingerprint repeated in one of the four testis sampled. The other three testis samples show a completely different AFLP fingerprint. Thus one genotype contributed all the sampled somatic tissue, but both genotypes contributed to the gametic tissue.
Figure 3 is also an example of a striking observation from these studies: within the detection level of the AFLPs, >99% of the testes examined in this study were clonal— that is, derived from a single genotype within the chimera. In Figure 3 there are four polymorphic presence bands, three from one genotype (solid arrows), and one from the other (open arrow). The AFLP fingerprint of the somatic sample (S1) is exactly the same as that of one of the testis (T4), while the remaining three testes (T1–T3) have a completely distinct but repeatable fingerprint. Thus, within the detection level of the AFLPs (Fig. 2), an individual testis is derived from one genotype or the other within the chimera. In only two samples we did see AFLP ladders that included a combination of both genotypes (Table 5).
A second observation is that germline and somatic chimerism and parasitism act independently of each other, and this was independent of the clutch the chimera was derived from. Although both of these observations were made previously (Stoner and Weissman, 1996; discussed below), adults in those experiments were fused to other adults, whereas in this study fusions occurred months prior to sexual maturity. This suggests that somatic and germline progenitors are separate lineages that segregate prior to metamorphosis, and moreover that parasitic abilities are unique to each lineage and autonomous to the cells themselves (Laird et al., 2005).
One outstanding question regards the nature of the somatic progenitor(s)—namely, whether there is a pluripotent somatic stem cell, or multiple, lineage-restricted populations, and in turn whether these progenitors are mobile. For example, long-term chimerism of the mobile pigment cells can be visualized after natural or experimental transplantation between two color morphs (Laird et al., 2005). However, this only indicates the presence of a precursor to the pigment cells..
In this study, our somatic samples were dissected intestines from which hematopoietic contamination was removed as well as possible, and these were pooled from individual systems within a chimera (see Methods). In many cases we found that each system provided a single fingerprint, which was identical to those of other systems within the chimera. An example is shown in Figure 4, where two distinct genotypes (I1-I4 and I5-I6) within this bichimera are present in multiple somatic samples that originate from distinct regions of the colony. This suggests that at least for the gut, new buds originate from somatic precursors in pre-existing zooids. In contrast, if all progenitors were mobile (like pigment and germline precursors), then we would expect to see a mixture of contributions scattered throughout the colony, with fingerprints more akin to the mixtures seen in Figure 2. Somatic samples that were mixtures of two unique signatures were observed, but only in a few cases (Table 5). However, due to the pooling required for reliable AFLP analysis, we were unable to discriminate between individual intestines that were derived from different genotypes and a mixture of intestines each derived from a different genotype. Nevertheless, the chimera in Figure 4 is stable and expresses somatic tissue from both genotypes, yet we observe spatial segregation in the expression patterns. This suggests that, at least for the intestine, the precursors are not mobile and regenerated tissue comes from the preexisting individual.
Discussion
Density-dependent growth rate
Previous studies on chimeric colonies have not found any increase in fitness (e.g., growth rates, time to sexual maturity, lifespan) when compared to individuals (Rinkevich and Weissman, 1987; Chadwick-Furman and Weissman, 1995, 2003), although in those studies each sample was grown on an individual substrate and separate from other colonies (equivalent to the low-density conditions in this study). However, juveniles have been shown to preferentially settle near kin, potentially establishing high-density kinship aggregations (Grosberg, 1987). Thus, this study approached growth rates as a factor of density-dependence, considering what could happen to juvenile individuals and chimeras in an unmanipulated, high-density setting, which may be more akin to the aggregation conditions found in many natural populations. Our results show a negative correlation between the density of colony aggregations and growth rate for both individuals and chimeras. This finding could confound the results of previous allogeneic contact comparison studies if aggregation density does affect growth rate and was not controlled for in the studies. For example, previous results demonstrating a reduced fitness of colonies following either a fusion or rejection reaction could be confounded by the unaccounted for density factor to growth (Chadwick-Furman and Weissman, 2003).
The inhibition of growth documented under high-density growth conditions has three interesting characteristics: (1) inhibition is mutual (all genotypes are growth-limited)—as other resources, such as food, are not limiting (Boyd et al., 1986), it appears that all juveniles in a hatch may have the capacity to inhibit others in dense aggregations; (2) juveniles in dense aggregations were often visually in excellent health, but only a single bud developed per generation (self-replacement); and (3) the inhibition is reversible, as colonies transferred from high-to low-density conditions increased their growth rate significantly (Table 4). If Botryllus larvae preferentially settle near kin (Grosberg and Quinn, 1986), would a system have evolved to inhibit growth of nearby individuals? The long-term inhibition of growth on high-density settlement plates could be a complete artifact of rearing in the laboratory; however, given the similarity between laboratory-generated and natural kinship aggregations (Grosberg and Quinn, 1986), fitness variability could indicate unknown allogeneic processes that are present in natural populations under high-density conditions. In this study, only chimeras grew under these high-density conditions, although this was not statistically significant. Despite a lack of statistical support, it may be that chimerism is slightly favored under high-density conditions, as long as the well-documented costs are ameliorated by restricting fusion to kin via fuhc-mediated allorecognition.
One caveat to these conclusions is that at the end of these experiments, colonies were sacrificed and tissues dissected for genetic analysis. At that point, about 43% of bichimeras and 46% of trichimeras appeared to be composed of only a single genotype (within the limit of AFLP detection; Table 6; discussed below). Unfortunately, we cannot monitor chimerism in real-time; thus we have no estimate of when one genotype was eliminated from the chimera, and in turn, whether this had any effect on growth. However, this points to how dynamic the interactions between growth, population density, and chimerism must be in natural populations.
Positional resorption patterns
Previous studies hypothesized that resorption is a repeat-able, hierarchal, and heritable trait with winner and loser genotypes (Rinkevich et al., 1993). In contrast, these same studies document variability in fusion patterns due to differences in growth conditions and show that the hierarchy is directly correlated to the level of inbreeding, which reduces fitness. Another study showed that resorption patterns are reversible by stress on the dominant genotype (Rinkevich et al., 1994).
In this study we found an extrinsic, position-specific effect on resorption in chimeras derived from half-sibling juveniles. Specifically, in the linear fusion trichimeras, the middle individual was resorbed less frequently than expected and the outside individuals were resorbed more frequently than expected. These combined findings indicate that resorption events are potentially influenced by extrinsic or intraspecific biological factors that are independent of putative intrinsic hierarchies. In this study we assigned genotypes retrospectively; thus we cannot rule out the influence of underlying genotype-specific patterns on these results. And while this study was focused on interactions between juveniles, future studies in adults using clonal replicates rotated in all positions would be informative.
If this observed phenomenon holds true, it might imply some sort of behavioral component to settlement and downstream costs of fusion. Since it is hardly conceivable that a larva could choose to be in the middle of a linear chimera, we speculate that the effect would be either to cause the larvae to settle first, so others would by definition be on the outside, or more likely, to settle into a large aggregate, so fusion to multiple individuals could occur.
In contrast, other studies have concluded that resorption is an artifact of a laboratory environment, as replicate chimeras made with isogeneic subclones showed resorption when reared in the laboratory, but not in the field (Chadwick-Furman and Weissman, 1995, 2003). When growth rates of isogeneic subclones are compared between the laboratory and field, growth in the laboratory does not even approach that in the field (Boyd et al., 1986; Chadwick-Furman and Weissman, 1995). Thus it may be that, in reality, laboratory-reared colonies are always under stress, and resorption is a means of allocating resources under these conditions, analogous to studies applying experimental stress (Rinkevich et al., 1994). If the linear resorption patterns found in this study are indicative of resource allocation trends, we propose two hypotheses as to why the middle juvenile most often remains un-resorbed: (1) because the middle individual is fed by the most vessels, it is simply easiest for the chimera to concentrate resources by converging into the center; or (2) by resorbing into the central individual, the chimera is potentially providing itself a buffer zone of empty space for future growth, reducing immediate contact with nearby individuals. Future studies could follow multi-chimeras (four or more individuals) in various (e.g., linear or circular) fusion patterns to confirm this intra-chimeric, position-dependent resorption pattern.
Finally, this is at least the fourth study to observe chimeras in which the resorbed genotype is the only one found in the germline (Tables 5, 6; Pancer et al., 1995; Stoner and Weissman, 1996; Stoner et al., 1999). However, unlike previous studies, resorption in these experiments occurred weeks to months prior to sexual maturity in the colony. This is further evidence that if resorption is a level of histocompatibility and is supposed to eliminate the tissues of another individual in a chimera, it completely fails to acquire the most important cells—that is, self-renewing germline progenitors that make a large tissue (a testis) that sits directly in the open circulatory system. Coupled to the potential environmental factors documented in this and previous studies (Rinkevich et al., 1994) and the absence of this phenomenon in the field between two adults (Chadwick-Furman and Weissman, 2003), it appears that resorption is more likely to be a physiological process than another level of histocom-patibility (Rinkevich et al., 1993).
Analysis of somatic and germline chimerism
From a developmental standpoint, the characterization of chimerism after fusion of juveniles could provide insight into the ontogeny of different stem cell populations in B. schlosseri, specifically whether they are pluripotent or lineage-restricted. This in turn could have major effects on the cost and benefits of fusion. For example, if germline progenitors arise from pluripotent stem cells after metamorpho-sis—for example, at the onset of sexual maturity—then a resorbed juvenile may not be able to contribute to germline development in a chimera once it reaches sexual maturity, in direct contrast to a sexually mature adult, which can contribute to germline development in succeeding generations of buds after resorption (Sabbadin and Zaniolo, 1979; Pancer et al., 1995; Stoner and Weissman, 1996; Stoner et al., 1999).
We found that chimeras formed in the juvenile stage were equivalent to results in chimeras made with adults, with cases of co-dominance and complete replacement of both somatic and germline tissues, in various permutations. Thus, despite the difference in the age of fusion, there are no major differences in the overall patterns of chimerism. In addition, there is no correlation between germline chimerism and somatic chimerism—that is, somatic losers are often germline winners, which also are equivalent to results in chimeras made from adults (Pancer et al., 1995; Stoner and Weissman, 1996; Stoner et al., 1999).
From the perspective of the source of the germline, these findings have two implications: (1) the fact that germline and somatic parasitism occurred independently in juvenile chimeras and remained independent months after fusion and resorption suggests that germline progenitors are a separate lineage of cells set aside early in embryogenesis; (2) they are further evidence that parasitic abilities are autonomous to those early-lineage cells and are genetically determined (Stoner et al., 1999; Laird et al., 2005; Brown et al., 2009). While these data are correlative, they are further evidence that long-lived functional germline progenitors are found in juvenile individuals (Brown et al., 2009).
The second finding is that within the limit of resolution of the AFLP technique, over 99% of the testes examined in this study were clonal—that is, derived from a single genotype within the chimera. Clonality of testes has been detected in other studies in our laboratory (Brown et al., 2009) and was also noted previously (Stoner and Weissman, 1996) in several cases using microsatellites as molecular markers, which are much more sensitive to DNA contamination. However, those studies required that individuals in a chimera were both fusible and polymorphic at 1–4 microsatellite loci, and only certain combinations of alleles could unambiguously determine clonality (e.g., Stoner and Weissman, 1996, fig. 3). In contrast, the AFLPs can easily discriminate between any two individuals and have revealed the extent of this clonal aspect of male germline formation. In only two cases did we see a single testis made up of both genotypes present in the soma (Table 5). As shown in Figure 2, this would be observed only if > 10% of the DNA was derived from another genotype, and this suggest that testes develop in situ from a small number of cells that migrate to the secondary bud (Sabbadin and Zaniolo, 1979). This clonal nature of testes will allow the quantification of chimerism in the future and higher resolution cost/benefit analyses.
In analyzing somatic contribution to the chimeras, one notable difference from previous studies is that we observed a much higher rate of single genotype dominance in the soma (Tables 5, 6). When multiple somatic genotypes were present, two patterns were observed: (1) distinct and non-overlapping genotypes represented in the soma; and (2) a mixture of genotypes suggesting overlapping presence of genotypes in the soma. As our somatic samples were derived from pooled intestines, most often from a single system, it appears that in the former case zooids derived from the two individuals stayed in separate systems, while in the latter there seems to have been some mixture of the two genotypes. The reason for this is unknown, but is most likely a result of how new systems form during the takeover process. Although we did not compare the level of genetic heterogeneity in the soma to the free-flowing hemocytes (which do not provide enough DNA for reliable AFLP analysis), the results suggest that the soma of new buds comes from preexisting buds rather than from blood-based mobile progenitors. We are currently doing a thorough analysis of genetic heterogeneity in blood and intestine samples collected from multiple chimeras to determine if they are equivalent, suggesting the presence of a pluripotent somatic progenitor giving equal contribution in multiple tissues; or different, which would be evidence for multiple progenitors, each with their own contribution to development within a chimera.
Acknowledgments
We thank an anonymous reviewer for suggestions on statistics; Tanya McKitrick, Marie Nydam, and Alessandro De Maio for critical reading of the manuscript; and three anonymous reviewers of the first version for multiple comments/suggestions. This work was supported by grants from the NSF (IOS-0842138), NIH (DK405762/AI041588/AG037966), and the Ellison Foundation (AG-NS-0399-07) to AWD.
Literature Cited
- Boyd HC, Brown SK, Harp JA, Weissman IL. Growth and sexual maturation of laboratory-cultured Monterey Botryllus schlosseri. Biol. Bull. 1986;170:91–109. [Google Scholar]
- Brown FD, Tiozzo S, Roux MM, Ishizuka KJ, Swalla BJ, De Tomaso AW. Early lineage specification of long-lived germline precursors in the colonial ascidian, Botryllus schlosseri. Development. 2009;136:3485–3494. doi: 10.1242/dev.037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss LW. Competition within and between encrusting clonal invertebrates. Trends Ecol. Evol. 1990;5:352–356. doi: 10.1016/0169-5347(90)90093-S. [DOI] [PubMed] [Google Scholar]
- Chadwick-Furman NE, Weissman IL. Life history plasticity in chimeras of the colonial ascidian Botryllus schlosseri. Proc. R. Soc. Lond. B. 1995;262:157–162. doi: 10.1098/rspb.1995.0190. [DOI] [PubMed] [Google Scholar]
- Chadwick-Furman NE, Weissman IL. Effects of allogeneic contact on life-history traits of the colonial ascidian Botryl-lus schlosseri in Monterey Bay. Biol. Bull. 2003;205:133–143. doi: 10.2307/1543234. [DOI] [PubMed] [Google Scholar]
- De Tomaso AW. Allorecognition polymorphism versus parasitic stem cells. Trends Genet. 2006;22:485–590. doi: 10.1016/j.tig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- De Tomaso AW, Saito Y, Ishizuka KJ, Palmeri KJ, Weissman IL. Mapping the genome of a model protochordate. I. A low resolution genetic map encompassing the fusion/histocompatibility (Fu/HC) locus of Botryllus schlosseri. Genetics. 1998;149:277–287. doi: 10.1093/genetics/149.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tomaso AW, Nyholm SV, Palmeri KJ, Ishizuka KJ, Ludington WB, Mitchel K, Weissman IL. Isolation and characterization of a protochordate histocompatibility locus. Nature. 2005;438:454–459. doi: 10.1038/nature04150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosberg RK. Limited dispersal and proximity-dependent mating success in the colonial ascidian Botryllus schlosseri. Evolution. 1987;41:372–384. doi: 10.1111/j.1558-5646.1987.tb05804.x. [DOI] [PubMed] [Google Scholar]
- Grosberg RK. The evolution of allorecognition specificity in clonal invertebrates. Q. Rev. Biol. 1988;63:377–411. [Google Scholar]
- Grosberg RK, Quinn JF. The genetic control and consequences of kin recognition by the larvae of a colonial marine invertebrate. Nature. 1986;322:456–459. [Google Scholar]
- Laird DJ, De Tomaso AW, Weissman IL. Stem cells are units of natural selection in a colonial ascidian. Cell. 2005;123:1351–1360. doi: 10.1016/j.cell.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Lauzon RJ, Ishizuka KJ, Weissman IL. Cyclical generation and degeneration of organs in a colonial urochordate involves crosstalk between old and new: a model for development and regeneration. Dev. Biol. 2002;249:333–348. doi: 10.1006/dbio.2002.0772. [DOI] [PubMed] [Google Scholar]
- Manni L, Zaniolo G, Cima F, Burighel P, Ballarin L. Botryllus schlosseri: a model ascidian for the study of asexual reproduction. Dev. Dyn. 2007;236:335–352. doi: 10.1002/dvdy.21037. [DOI] [PubMed] [Google Scholar]
- Nyholm SV, Passegue E, Ludington WB, Voskoboynik A, Mitchel K, Weissman IL, De Tomaso AW. fester, a candidate allorecognition receptor from a primitive chordate. Immunity. 2006;25:163–173. doi: 10.1016/j.immuni.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Pancer Z, Gershon H, Rinkevich B. Coexistence and possible parasitism of somatic and germ cell lines in chimeras of the colonial urochordate Botryllus schlosseri. Biol. Bull. 1995;189:106–112. doi: 10.2307/1542460. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria; 2010. ISBN 3-900051-70-0. [Online]. Available: http://www.R-pro-ject.org [2009, October]. [Google Scholar]
- Rinkevich B. Germ cell parasitism as an ecological and evolutionary puzzle: hitchhiking with positively selected genotypes. OIKOS. 2002;96:25–30. [Google Scholar]
- Rinkevich B, Weissman IL. A long-term study of fused subclones in the ascidian Botryllus schlosseri: the resorption phenomenon (Protochordata: Tunicata) J. Zool. 1987;213:717–733. [Google Scholar]
- Rinkevich B, Weissman IL. Chimeras vs. genetically homogenous individuals: potential fitness costs and benefits. OIKOS. 1992;63:119–124. [Google Scholar]
- Rinkevich B, Saito Y, Weissman IL. A colonial invertebrate species that displays a hierarchy of allorecognition responses. Biol. Bull. 1993;184:79–86. doi: 10.2307/1542381. [DOI] [PubMed] [Google Scholar]
- Rinkevich B, Weissman IL, Shapira M. Alloimmune hieracrchies and stress-induced reversals in the resorption of chimeric protochordate colonies. Proc. R. Soc. Lond. B. 1994;258:215–220. [Google Scholar]
- Sabbadin A, Zaniolo G. Sexual differentiation and germ cell transfer in the colonial ascidian Botryllus schlosseri. J. Exp. Zool. 1979;207:279–301. [Google Scholar]
- Scofield VL, Schlumpberger JM, West LA, Weissman IL. Protochordate allorecognition is controlled by MHC-like gene system. Nature. 1982;295:499–502. doi: 10.1038/295499a0. [DOI] [PubMed] [Google Scholar]
- Stoner DS, Weissman IL. Somatic and germ cell parasitism in colonial ascidian: possible role for a highly polymorphic allorecognition system. Proc. Natl. Acad. Sci. USA. 1996;93:15254–15259. doi: 10.1073/pnas.93.26.15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner DS, Rinkevich B, Weissman IL. Heritable germ and somatic cell lineage competitions in chimeric colonial protochordates. Proc. Natl. Acad. Sci. USA. 1999;96:9148–9153. doi: 10.1073/pnas.96.16.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman IL, Saito Y, Rinkevich B. Allorecognition histocompatibility in a protochordate species: Is the relationship to MHC somatic or structural? Immunol. Rev. 1990;113:227–241. doi: 10.1111/j.1600-065x.1990.tb00043.x. [DOI] [PubMed] [Google Scholar]