Abstract
Purpose
Fuchs’ endothelial corneal dystrophy (FECD), which affects approximately 5% of the population over 40 in the U.S.A., is a major cause of corneal transplantation. FECD is associated with mutations of a variety of unrelated genes: SLC4A11, COL8A2, TCF8, and LOXHD1. The current pathological description of the dystrophy includes deficiency of corneal endothelium (CE) pump function and induction of the unfolded protein response (UPR). This study aims to determine the contribution of the two mechanisms by assessing the expression levels of (1) seven endothelial ion transporters known to regulate stromal hydration and (2) UPR related genes in a set of six CE samples obtained from FECD patients compared to that of normal controls.
Methods
CE samples collected during FECD keratoplasty or from an eye bank (normal control) were transferred into an RNA stabilizing agent and refrigerated. Total RNA from each CE specimen was individually extracted. The expression levels of ion transporters and UPR genes were tested using quantitative real-time (RT) PCR and a UPR specific PCR array, respectively.
Results
In normal CE, the comparative expression levels of ion transporters in decreasing order were SLC4A11, Na+/K+ ATPase, pNBCe1, and NHE1, followed by the isoforms of monocarboxylate transporters (MCTs). In FECD samples, Na+/K+ ATPase and MCTs 1 and 4 were significantly downregulated compared to normal controls (p<0.05). The PCR array tested 84 UPR related genes. Data analysis showed upregulation of 39 genes and downregulation of three genes, i.e., approximately 51% of the tested genes had their expression altered in FECD samples with a difference greater than ± twofold regulation. Thirteen of the altered genes showed significant changes (p<0.05). The PCR array results were validated by quantitative RT-PCR.
Conclusions
FECD samples had evident UPR with significant changes in the expression of the protein processing pathway genes. The significant downregulation of ion transporters indicates simultaneous compromised CE pump function in Fuchs’ dystrophy.
Introduction
The corneal endothelium (CE), the posterior monolayer of cells, maintains corneal transparency through its active ion-transport properties [1,2]. Corneal endothelial dystrophy is a major issue in the aging population, resulting in corneal edema with vision loss, and is a leading cause for corneal transplantation [3]. Fuchs’ endothelial corneal dystrophy (FECD), which affects 5% of the population over 40 in the U.S.A., is a late onset phenotype of corneal endothelial dystrophy [4]. Occurring as both sporadic and familial cases, FECD is identified by the bilateral progressive loss of endothelial cells, which features endothelial polymorphism with a concomitant decrease in function, resulting in the corneal edema. Other distinct characteristics include Descemet’s membrane thickening and a high incidence of guttata (posterior excrescences), which progresses in number.
The pathological mechanism resulting in FECD has not been established. Studies have shown that FECD is associated with mutations in SLC4A11 (Gene ID 83959, OMIM 610206) [5,6], LOXHD1 (Gene ID 125336, OMIM 613072) [7], TCF8/ZEB1 (Gene ID 6935, OMIM 189909) [8,9], and COL8A2 (Gene ID 1296, OMIM 120252) [10-12] and loci on chromosomes 13, 18, 5, and 9, where the genes have not yet been identified. Mutations within COL8A2, encoding the alpha 2 subunit of collagen VIII, a component of Descemet’s membrane, causes an early onset less frequent variant of Fuchs endothelial dystrophy [13]. Similarly, mutations in SLC4A11 also cause an early onset variant, congenital hereditary endothelial dystrophy 2 (CHED2), or Harboyan syndrome, which includes hearing deficits [14-17]. The genes associated with FECD have distinct unrelated functions, suggesting multiple mechanisms for disease development.
The functionality of the CE, which maintains stromal hydration and corneal transparency, is challenged in FECD. A constant leak of fluid into the cornea is driven by the hydrophilic stromal glycosaminoglycans. This is countered by fluid secretion from the stroma to the aqueous humor by the active ion transport mechanisms of the CE. Fluid secretion is a Na+/K+ ATPase dependent process involving bicarbonate and lactate transport accompanied by localized osmotic fluid transfer [1]. The major ion transporters that significantly regulate fluid transport are sodium bicarbonate cotransporter-1; Na+: 2HCO3- (pNBCe1) and sodium proton exchanger-1; Na+/H+ (NHE1). Also postulated to affect fluid secretion are monocarboxylate transporters (MCTs): lactate:H+) 1, 2, and 4 [18,19] and SLC4A11, a recently characterized intracellular pH regulator [20,21]. Decreased membrane expression of Na+/K+ ATPase [22,23] and downregulation of SLC4A11 [24] in FECD have been reported previously. Loss of ion transport due to the decreased expression or loss of cell density leads to fluid retention, stromal edema, and loss of corneal transparency. This study found altered expression of Na+/K+ ATPase, pNBCe1, NHE1, and the MCTs in FECD, which could provide potential approaches for overcoming the fluid transport deficit.
Unfolded protein response (UPR) as a common sign in late onset FECD is supported by histopathological indications of enlarged rough endoplasmic reticulum in endothelial cells [25]. A marked decrease in corneal endothelial cell density with the progression of dystrophy occurs via apoptosis [26], which can be activated by UPR. Endoplasmic reticulum (ER) stress in FECD could result from mutated genes like SLC4A11, a transmembrane protein contributing to the regulation of intracellular pH [20,21], which was shown to have posttranslational modifications and failed to localize to the cell surface [5,6]. Mutations in LOXHD1, a postulated chaperone targeting protein to the plasma membrane, induce cytoplasmic precipitation of the protein in FECD and could contribute to UPR [7]. Knock-in mice expressing a COL8 missense mutation homologous to that found in human patients show a phenotype similar to FECD and activation of the UPR response [27]. In addition, CE is comparatively highly metabolic, making it prone to oxidative damage and leading to ER stress and UPR [28,29].
UPR signaling linked to metabolic pathways is a component implicated in various diseases [30,31]. ER stress and UPR pathways, e.g., PERK, ATF6, and IRE1, are being tested as pharmacological targets for cancer, obesity, and metabolic, inflammatory, and neurodegenerative disorders [28,32]. UPR signaling involves both pro-survival and pro-apoptotic effectors; thus, regulating the components of these pathways can either lead to ER stress management or apoptosis. Pharmacologically targeting ER stress/UPR initiators either using inhibitors or gene therapy has been promising. Since modulating ER stress and UPR could be useful, determining the related genes affected by FECD can provide important insight. UPR related proteins have been demonstrated in FECD CE by immunostaining [25]. A comprehensive assessment is performed in the present study using a PCR array with the determination of 84 genes involved in the protein processing pathways that are altered due to UPR response in FECD.
CE tissues obtained from FECD patients undergoing keratoplasty were used to simultaneously determine expression of UPR genes and ion transporters. The study standardizes a method to carry out both estimations: a UPR PCR array including validation and RNA quantitation of seven ion transporters. This provides an estimation of the two mechanisms in each of the samples rather than using different corneas for each set of experiments. The results show UPR in FECD, including a significant decrease in the RNA levels for Na+/K+ ATPase and MCT 1 and 4. Thus, FECD CE has both UPR and a loss of ion transporters, which could both contribute to a loss of endothelial pump activity, cell density, and stromal edema.
Methods
Collection of human corneal endothelial specimens
Collection of Human endothelial specimens for the study was approved by the Indiana University Institutional Review Board. Table 1 presents information regarding six FECD patients selected for the study at Midwest Eye, Indianapolis, IN. Corneal Endothelium Descemet’s membrane strips from Fuchs patients were collected immediately post-keratoplasty (DSEK) and transferred into RNA stabilizing agent until the time of RNA extraction. Six Control CE were similarly obtained from corneas with normal CE (average specular cell count was 2918 ± 439 cell/mm2) but unsuitable for transplantation (Lions Eye Bank Indianapolis, IN). Control corneas obtained were from an average age group of 69.8±8 and death to procurement interval was less than 20 h.
Table 1. Characteristics of FECD corneas from which corneal endothelial tissue was obtained.
| Tissue # | Age | Race | Guttata | Stroma | History |
|---|---|---|---|---|---|
| 1 |
73 |
White |
3+ |
mild edema |
cataract surgery |
| 2 |
77 |
Caucasian |
4+ |
- |
R/L cataract |
| 3 |
84 |
Caucasian |
4+ |
trace edema |
none |
| 4 |
70 |
White |
4+ |
mild edema |
cataract |
| 5 |
75 |
White/Caucasian |
4+ |
edema |
cataract, glaucoma |
| 6 |
64 |
Caucasian |
4+ |
edema |
cataract surgery |
| Average age |
73.83 |
||||
| SD | 6.73 |
Total RNA extraction
Total RNA from Control and FECD samples were individually isolated using an RNeasy Micro Kit (Qiagen, Valencia, CA) as per the manufacturer’s protocol. Briefly, each of the CE tissue samples were extracted for total RNA using RLT buffer, the lysate was applied to a column, washed with ethanol to purify after treating with DNase I. Total RNA was eluted from the column using 14µl of RNase free water. Quantity and quality of the extracted RNA was measured by spectrophotometry (NanoDrop 2000; Thermo Scientific, Whatman, MA). Only RNA with A260/280: 1.8 to 2.0 was subjected to analysis.
Primer design and validation
Primers were designed using Primer 3 software. Table 2 and Table 3 give the primer sequences that were used. Primer efficiency was tested by performing a standard curve using serial dilutions of a representative cDNA sample from 10 ng to 0.1 ng. Only primers with high efficiency (≥90% and ≤110%) and the amplification of a single product and no primer dimers (tested by dissociation curve and gel electrophoresis) were used for comparative quantitation and PCR array validation.
Table 2. provides the list of UPR genes and the primer sequences for PCR array validation.
| UPR gene | Sequence | Product size (b.p) |
|---|---|---|
| EDEM3 |
F: 5′-GGAAGATGCATCCGAGTATTGTGG-3′
R: 5′-AGTCACAGCTTTCCCTATGGAAGG-3′ |
390 |
| SCAP |
F: 5′-CTGAGAGTGTTCCGTCTGGA-3′
R: 5′-CAGGTCCTGCTGAATGGAGT-3′ |
300 |
| SEL1L |
F: 5′-GTTCATGGGACATGGTACCTAAGC-3′
R: 5′-AGACAGTCGGCTAACCAACAAACC-3′ |
333 |
| ERN2 |
F: 5′-CGCTGTGGACATCTTCTCTG-3′
R: 5′-ACTCCTTCTCCAGCCAGTCA-3′ |
299 |
| HSPA5 |
F: 5′-GCTCTCTGGTGATCAAGATACAGG-3′
R: 5′-AAGGTGACTTCAATCTGTGGGACC-3′ |
291 |
| PFDN5 | F: 5′-GAGACTTCCTCTTCGTTAAGTCGG-3′ R: 5′-CTCTACATAGTACCCAGTTCCCAC-3′ | 313 |
Table 3. provides the list of ion transporters and the primer sequences for quantitative RT–PCR.
| Ion transporter | Sequence | Product size (b.p) |
|---|---|---|
| Na/K/ATPase |
F - 5′- ATGTGCCCAGTGAACCGAAA −3′
R - 5′- TGAACGGGAAGGACATTTGG - 3′ |
253 |
| NHE1 |
F – 5′- GACTACACACACGTGCGCACCCC-3′
R – 5′ – TCCAGGATGATGGGCGGCAGCAGGAAGAGGAA – 3′ |
233 |
| pNBC |
F – 5′-CCAAGAAATCCAACCTTCGG-3′
R- 5′-GTCATTCAGACTGGAGGAAG-3′ |
134 |
| MCT1 |
F-5′-TGACCATTGTGGAATGCTGT-3′
R: 5′-TTTCTGGTCCGGAGATTCTG-3′ |
281 |
| MCT2 |
F: 5′- TGAAACTCTCATGGACCTCGT-3′
R: 5′- ACACGCTTGCTGCTACCAC-3′ |
182 |
| MCT4 |
F: 5′-AAACCCTCCTGCCTCCACCAGA-3′
R: 5′-TGACGAAACAGCCGAAGAGCACG-3′ |
145 |
| SLC4A11 |
F: 5′-TGCTCTATGGCCTCTTCCTC-3′
R: 5′-CCCTCCGGATGTAGTGTGTC-3′ |
123 |
| β-actin | F: 5′-GCAAAGACCTGTACGCCAAC-3′ R: 5′-AGTACTTGCGCTCAGGAGGA-3′ | 144 |
Quantitative RT-PCR
Total RNA was converted to cDNA using a high capacity RNA to cDNA kit (4,390,777, Applied Biosystems, Carlsbad, CA) in a final volume of 20 µl. Real-time (RT) PCR with SYBR® Green dye (Qiagen, Valencia, CA) was performed in a 25 µl mixture containing 12.5 ul of Brilliant II SYBR® Green QPCR Master Mix, 200 nM of each primer, and 1 ul of cDNA template using Mx3005P Cycler (Agilent, Wilmington, DE). Each of the 12 samples was run in triplicate reactions. The threshold cycles (Ct) were calculated with MxPro software (Agilent). The cycling parameters used for amplification of targets were as follows: initial denaturation for 10 min at 95 °C, followed by 40 cycles of 30 s denaturation at 95 °C, 1 min annealing at 55 °C, and 30 s extension at 72 °C. The expression of ion transporters was normalized against the housekeeping gene β-actin. Relative quantitation was performed using the 2-∆∆Ct comparative Ct method to calculate the mRNA fold of change in FECD CE relative to the normal control. A procedure similar to quantitative RT-PCR was used to validate UPR PCR array results.
RT2 Profiler PCR array
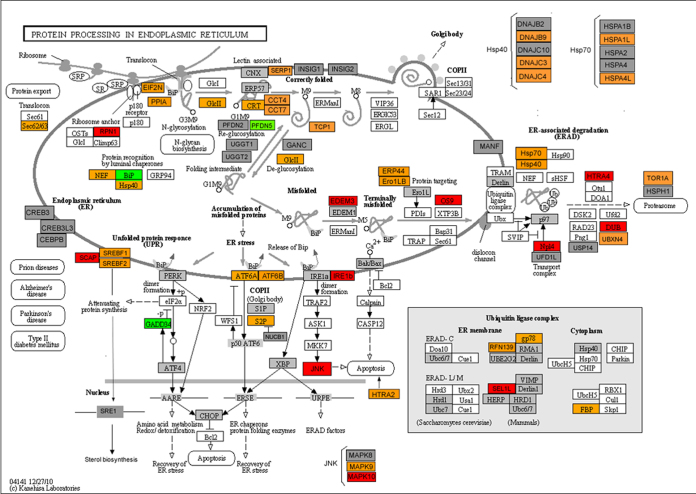
cDNA was synthesized using a RT2 PreAMP cDNA synthesis kit (330,451, SABiosciences, Qiagen Valencia, CA). cDNA was then preamplified for a UPR RT2 Profiler PCR array using RT2 PreAMP Pathway Primer Mix (PBH-5089, SABiosciences, Qiagen) specific for the UPR pathway. The amplification of cDNA specific for UPR genes targeted in the RT2 Profiler PCR array provided significant amounts of cDNA from smaller amounts (20 ng) of total RNA. Preamplified cDNA was mixed with a RT2 SYBR Green Master mix (330,520, Qiagen) and aliquoted into 96 wells of an RT2 Profiler PCR array (PAHS-089A, SABiosciences, Qiagen) containing UPR specific primer pairs. PCR was run on the Mx3005P Cycler (Agilent, Wilmington, DE, USA). The relative expression was determined using the ∆∆Ct method with web-based PCR array data analysis software. Briefly, the Ct values obtained from each sample (plate) were organized in columns and uploaded into the software. Next, each sample was tested for PCR array reproducibility, RT efficiency, and genomic DNA contamination using the controls included in the plate. All 12 samples passed this test. Next, from the panel of five housekeeping genes, RPL13A was selected as showing the lowest ∆Ct between the Fuchs’ and control groups. Finally, fold change analysis and scatter plot data were exported from the software to Excel spreadsheets. The pathway—protein processing in the endoplasmic reticulum, obtained from the Kyoto Encyclopedia of Genomes and Genes (KEGG) website [33,34]—was modified and colored by different criteria.
Statistical analysis
Statistical significance was determined using the Student t test. A p value <0.05 was considered significant. Histogram data are presented as mean values ± standard error of the mean.
Results
Determination of expression levels of ion transporters in FECD CE compared to normal controls
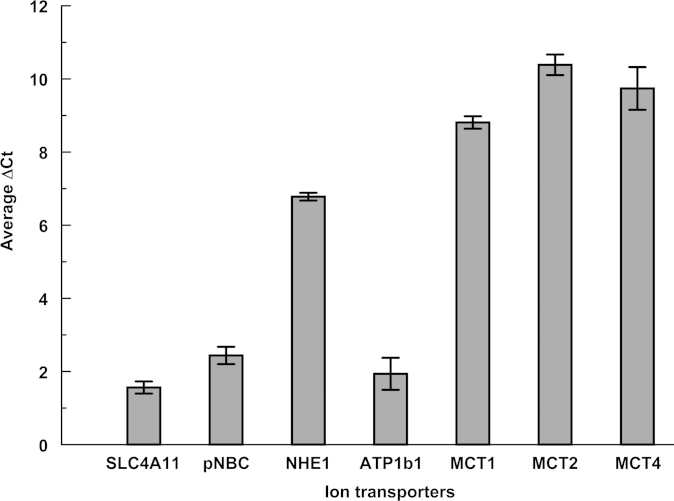
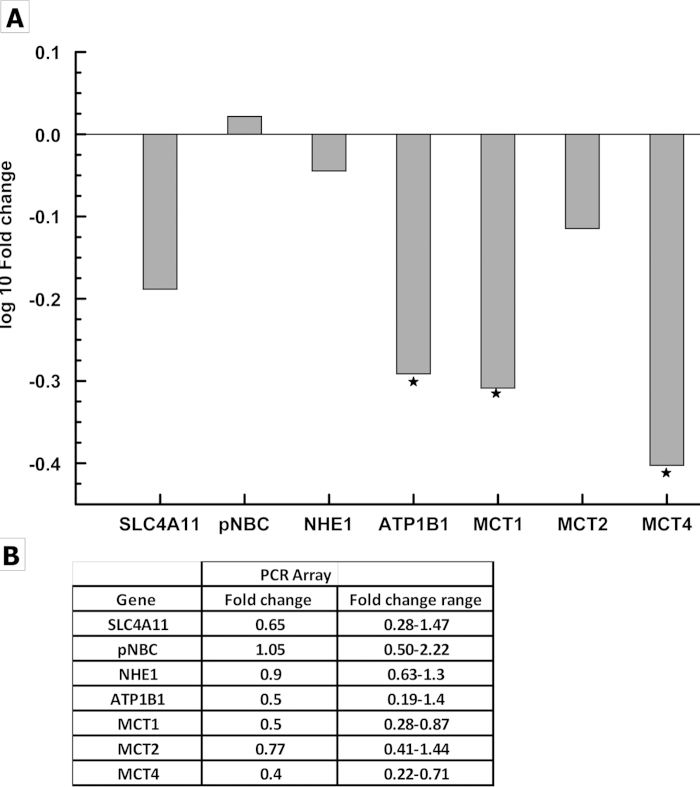
CE pump function was dependent on primary (Na+/K+ ATPase) and secondary active transporters, e.g., sodium bicarbonate co-transporter, Na+/H+ Exchanger, and monocarboxylate (lactate:H+) co-transporters. The presence of bicarbonate and the activity of carbonic anhydrases buffered protons and facilitated the efflux of lactate [18,19]. The expression levels of these ion transporters in FECD provided a qualitative estimate of the endothelial pump potential. The study also determined the expression levels of SLC4A11, an intracellular pH regulator whose role in the pump function is not completely understood but is relevant due to the association of various SLC4A11 mutations with FECD [5,6]. Figure 1 shows the comparative expression levels of the ion transporters in normal CE. The plot of ion transporters against ΔCt (Ct values normalized to that of β-actin) indicates that SLC4A11 was expressed at comparatively higher levels, followed by Na+/K+ ATPase (ATP1B1), pNBCe1, and NHE1. The three isoforms of MCTs had lower expression levels. Figure 2 shows the expression in FECD normalized to controls (normal CE). The expression of Na+/K+ ATPase, MCT1, and MCT4 were significantly decreased, with no significant alteration of the remaining ion transporters. The decreased RNA levels of these important ion transporters in FECD point toward possible loss of endothelial pump function, which leads to progressive loss of stromal hydration control and corneal edema.
Figure 1.
Expression levels of selected ion transporters in normal human corneal endothelium. Figure shows average normalized Ct value (Δ Ct value) for each ion-transporter. Gene with lowest ∆Ct has highest expression. solute carrier family 4 member 11 (SLC4A11), Na+/K+ ATPase (sodium potassium ATPase), sodium bicarbonate cotransporter-1 (pNBCe1), sodium proton exchanger-1 (NHE1), mono carboxylate transporters (MCTs).
Figure 2.
Quantitative expression of ion transporters in FECD CE. A: The log fold change for seven ion transporters in FECD CE relative to normal control by quantitative RT-PCR. * represents the transporters that had a significant change (p<0.05). B: The fold change for each ion transporter along with the fold change range calculated using ΔΔCt standard deviation.
Determination of expression levels of UPR genes in FECD CE compared to normal controls
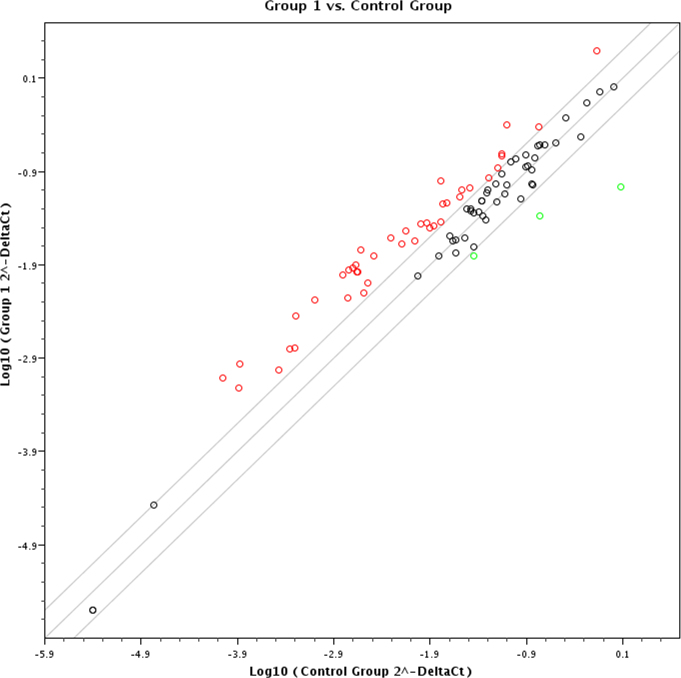
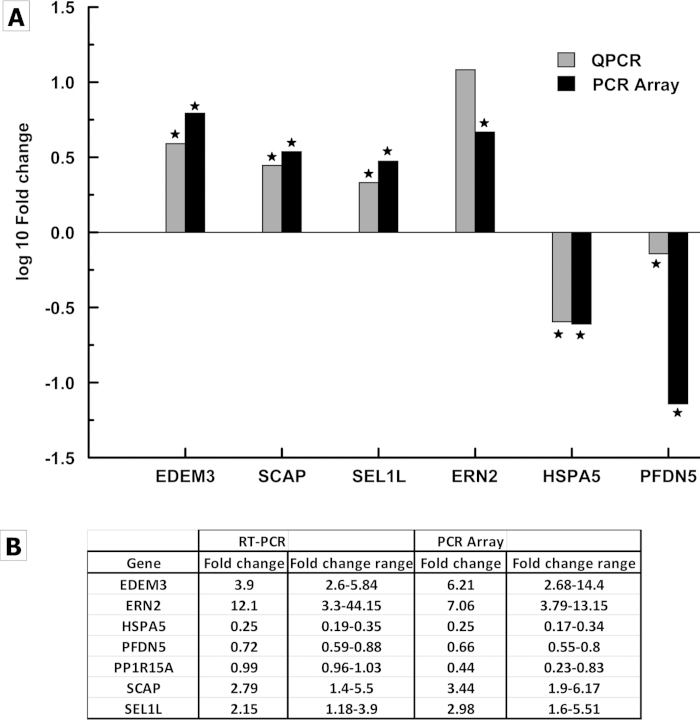
We used a UPR PCR array containing 84 genes covering the UPR pathways. Figure 3, a scatter plot indicating the distribution of all the genes tested, shows an average log fold change obtained from six individual FECD samples compared to normal control. The red and green open circles in the scatter plot indicate the genes that were upregulated and downregulated, respectively, with relative expression greater than ± twofold. FECD samples showed expression of 42 (approximately 51%) UPR related genes altered compared to normal controls (Appendix 1). Thirteen genes that are highlighted had significant change compared to control samples with p<0.05, ten of which were upregulated. The UPR PCR array results were validated by quantitative RT-PCR. The following significantly altered genes in FECD samples were tested: EDEM3, SCAP, SEL1L, ERN2, HSPA5, and PFDN5 (Figure 4). Due to limited amount of RNA, only the most highly regulated genes were included for validation. EDEM3, SCAP, and SEL1L were significantly upregulated, and HSPA5 and PFDN5 were significantly downregulated, similar to the PCR array results. ERN2 was increased in FECD with respect to control by RT-PCR, but did not reach statistical significance due to a large variance.
Figure 3.
Expression profile of all UPR related genes from the PCR array. The scatter plot shows expression levels of UPR related genes tested in FECD samples compared to normal controls. Of 84 UPR related genes, 42 (51%) were altered. The red circles represent the genes that had at least a twofold increase in expression, and the green circles are those that had a twofold decrease in expression compared to the control.
Figure 4.
Validation of UPR PCR array results by quantitative real-time PCR. A: The log fold change for six UPR related genes found to be significantly altered in FECD by PCR array were validated. The tested genes had their expression regulated similar to a PCR array. * indicates a significant fold change (p<0.05). B: The fold change range for each gene obtained by RT-PCR and PCR array calculated using ΔΔCt standard deviation.
Figure 5 shows a schematic of the pathways involving all the genes that were tested in the PCR array. The schematic contains color codes indicating relative change in expression in FECD. Two members of the IRE1 pathway of the unfolded protein response were upregulated in Fuchs’ patients: ERN2 (IRE1b) and MAPK10. The target of MAPK, the transcription factor JUN was increased in Fuchs’ patients and in the COL8A2 Q455K knock-in mouse model of early onset FECD [35]. Our data shows the expression of genes involved in protein folding quality control: EDEM3 and OS9 were increased in FECD. In addition, the following ER-associated degradation genes were upregulated: HTRA4, NPLOC4 (Npl4), and ATXN3 (DUB). Genes found to be down-regulated in Fuchs are HSPA5 (Bip), a regulator of UPR activation; PFDN5, a protein involved in re-glucosylation of folding intermediates, and PPP1R15A (GADD34), a regulator of the PERK UPR branch. These results strongly suggest that activation of UPR occurs in Fuchs’ patients.
Figure 5.
Protein processing pathways in endoplasmic reticulum. The figure [Modified with permission from Kyoto Encyclopedy of Genomes and Genes (KEGG) website [33,34]— shows various genes that are involved in the protein processing. For FECD samples, genes in Red boxes were up-regulated, p<0.05, Green box were down-regulated, p<0.05, Orange box: upregulated with p>0.05, Grey box: present in UPR array but not regulated, White box: not present in UPR array.
Discussion
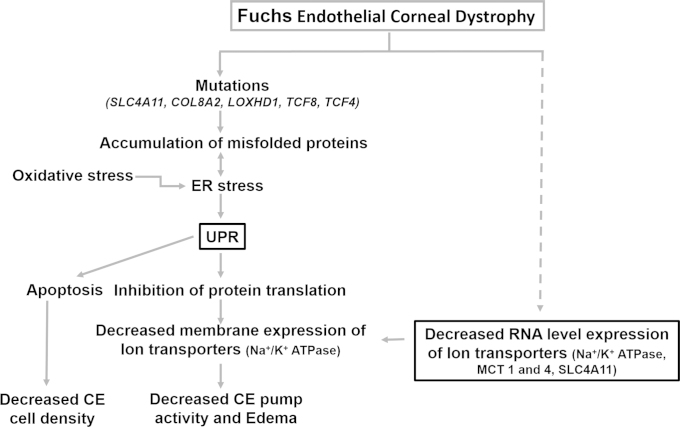
Multiple mechanisms have been suggested to describe the pathology of FECD, including apoptotic cell death due to oxidative stress or UPR and loss of CE pump function. This study aimed to analyze two of the proposed mechanisms: loss of corneal endothelial pump function and the induction of UPR by determining the expression levels of ion transporters and UPR related genes in CE from FECD patients. A comparative expression level analysis of the ion transporters of CE in normal CE showed higher expression of SLC4A11 among the seven ion transporters tested, underlining the importance of this transporter. FECD samples showed significant downregulation of Na+/K+ ATPase, which is critical for fluid secretion across CE, along with monocarboxylate transporters MCT1 and 4, which are postulated to have a role in corneal endothelial fluid secretion [1,2]. Simultaneous UPR PCR array analysis, performed for the first time using FECD CE total RNA, denoted significant UPR with more than twofold regulation of about 52% of the genes tested. Although the small amount of tissue available precluded protein level analysis, these results are an indication of compromised ion pump function, along with UPR in FECD. Thus, perhaps both UPR and the loss of ion transport function concurrently play a role in the pathology of FECD. Figure 6 shows a schematic summarizing the mechanisms that could contribute to the occurrence of the clinical symptoms observed in FECD. ER stress in FECD could occur due to identified mutations in associated genes and loci, along with oxidative stress [28]. Accumulation of unfolded protein as a result of ER stress can induce UPR [28,31,36], including the inhibition of protein translation, which could reduce the expression of membrane proteins like Na+/K+ ATPase, leading to inhibition of fluid secretion and corneal edema. In addition to UPR, pump function is also affected in FECD by the downregulation of ion transporters. Concomitantly, uncontrolled UPR induces a loss of endothelial cells due to apoptosis [36].
Figure 6.
Summary of the mechanisms possibly contributing to the clinical symptoms associated with FECD. Evidence of mutations and oxidative stress support the induction of UPR in FECD, which could cause decreased CE cell density and pump function followed by corneal edema. The clinical indications could also occur as a result of compromised pump function caused by the decreased RNA level expression of ion transporters demonstrated in this study, whose mechanism is not known.
Expression of SLC4A11 in normal CE was highest among the seven ion transporters of CE that were estimated. Higher expression of SLC4A11 was also reported in comparison to other members of the SLC4 family of bicarbonate transporters in murine CE [37]. Mutated SLC4A11, as seen in FECD, was retained in the ER [5], possibly affecting its transmembrane localization. Also, decreased expression of SLC4A11 has been reported by SAGE analysis in FECD [24]. Vilas et al. (2013) demonstrated corneal haze, increased corneal thickness, and abnormal endothelial cells in SLC4A11−/− mice [38], suggesting that the Fuchs’ phenotype can be generated without UPR. In our cohort of Fuchs’ patients, we observed a decreased expression of SLC4A11 with respect to normal controls, but the difference did not reach significance. The reduction in the RNA levels of Na+/K+ ATPase in FECD is supported with previous reports of the decreased membrane density of Na+/K+ ATPases [22,23]. Decreased expression of SLC4A11 and Na+/K+ ATPase was also consistently observed in the COL8A2 Q455K model of FECD [35]. These studies suggested a loss of corneal endothelial pump function. The expression of pNBCe1 and NHE and MCT1, 2, and 4 in FECD have not been determined previously. We found that MCT 1 and 4 expression were significantly diminished. Loss of these MCTs will decrease lactic acid efflux across the endothelium and cause corneal edema. Interestingly, an increase in the expression of ion transporters in the surviving cells did not occur. Nevertheless, the expression levels of ion transporters could be increased in the early stages of FECD (characterized with guttae and no corneal edema) [39]. CE specimens in our study can be characterized as end-stage due to the presence guttae and corneal edema. Although the mechanism and stage at which essential ion transporters are decreased are not known, increasing their expression in FECD CE via gene therapy is a possible means of restoring fluid secretion to either prevent or attenuate edema.
Oxidative stress and apoptosis are indicated as the underlying mechanism for the progressive loss of endothelial cells in FECD [26,40-42]. Engler et al showed UPR in FECD CE, by enlarged rough endoplasmic reticulum using TEM and increased UPR markers HSPA5 (Bip or GRP78), EIF2α, CHOP (C/EBP) [25], that could result in apoptotic cell death [43]. Transgenic mice carrying the human mutations of COL8A2 show corneal endothelial dystrophy and UPR induction with the upregulation of HSPA5 (Bip or GRP78) and CHOP [27]. However, in our study, HSPA5 (Bip or GRP78) was shown to be downregulated in FECD; EIF2α was upregulated, but did not reach significance; and CHOP (C/EBP beta) showed less than a twofold (0.8) change.
Activation of the IRE1 pathway of the unfolded protein response in Fuchs’ patients is indicated by the upregulation of ERN2 (IRE1b) and MAPK10. Although the role of ubiquitously expressing IRE1a is well characterized and its inhibitors are investigated in cancer therapy, the role of IRE1b, which is selectively expressed in the lungs and intestine and is upregulated in FECD CE, is not completely understood [31]. Upregulation of the genes involved in protein folding quality control and ERAD indicates a response to overcoming ER stress. HSPA5 (BiP), a chaperone that induces the activation of UPR pathways, is downregulated in Fuchs’ patients. Augmentation of BiP activity by increasing its expression to reduce ER stress using BiP inducer X or adeno-associated virus delivery of BiP is demonstrated in the protection of photoreceptors from light-induced cell death and restoration of visual acuity in retinitis pigmentosa, respectively [32]. PPP1R15A (GADD34), whose expression is decreased in Fuchs’ patients, causes inhibition of translation by the phosphorylation of eIF2alpha, a process necessary to control ER stress. Inhibition of eIF2alpha phosphorylation by lentiviral expression of GADD34 attenuated neural degeneration in mice [32]. These reports suggest UPR pathways as potential therapeutic targets for reducing CE cell death in FECD. Experiments to determine whether a reduction of ER stress could reduce dystrophic conditions and restore corneal transparency would provide insight into therapeutic strategy. For example, lithium, which can inhibit UPR and oxidative stress, promotes endothelial cell survival in the col8a2 knock-in mouse model of FECD [44].
In conclusion, simultaneous determination in the same set of samples indicates that apart from UPR, endothelial ion transport, which regulates pump function, is also inhibited in FECD. In addition, downregulation of MCTs opens up an additional mechanism in its pathological development, and the role of MCTs in FECD needs to be investigated. Specific elements of the fluid transport function as well as UPR are altered in FECD CE and are potential targets to pharmacologically decrease endothelial apoptosis and corneal edema in FECD, which is suggestive of combination therapy as a treatment strategy.
Acknowledgments
The authors would like the thank Dr. Clark L. Springs and Dr. Robert D. Deitch from School of Medicine, Indiana University, Indianapolis and Midwest Eye Institute, Indianapolis respectively, for collecting corneal endothelial tissue post-keratoplasty for the purpose of the study. We also thank Lions Eye Bank, Indianapolis for providing normal corneal endothelial tissue for comparative analysis. The study was supported by NIH R01EY008834 & Core P30EY019008 grants.
Appendix 1. List of UPR related genes altered in FECD CE.
Genes that are highlighted have significant change in expression compared to control samples with p value <0.05. Ten of the 39 genes overexpressed and all 3 of the under-expressed genes were significantly altered. To access the data, click or select the words “Appendix 1.”
References
- 1.Bonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res. 2012;95:2–7. doi: 10.1016/j.exer.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonanno JA. Identity and regulation of ion transport mechanisms in the corneal endothelium. Prog Retin Eye Res. 2003;22:69–94. doi: 10.1016/s1350-9462(02)00059-9. [DOI] [PubMed] [Google Scholar]
- 3.Klintworth GK. Corneal dystrophies. Orphanet J Rare Dis. 2009;4:7. doi: 10.1186/1750-1172-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzetti DW, Uotila MH, Parikh N, Kaufman HE. Central cornea guttata. Incidence in the general population. Am J Ophthalmol. 1967;64:1155–8. [PubMed] [Google Scholar]
- 5.Vithana EN, Morgan PE, Ramprasad V, Tan DT, Yong VH, Venkataraman D, Venkatraman A, Yam GH, Nagasamy S, Law RW, Rajagopal R, Pang CP, Kumaramanickevel G, Casey JR, Aung T. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum Mol Genet. 2008;17:656–66. doi: 10.1093/hmg/ddm337. [DOI] [PubMed] [Google Scholar]
- 6.Riazuddin SA, Vithana EN, Seet LF, Liu Y, Al-Saif A, Koh LW, Heng YM, Aung T, Meadows DN, Eghrari AO, Gottsch JD, Katsanis N. Missense mutations in the sodium borate cotransporter SLC4A11 cause late-onset Fuchs corneal dystrophy. Hum Mutat. 2010;31:1261–8. doi: 10.1002/humu.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riazuddin SA, Parker DS, McGlumphy EJ, Oh EC, Iliff BW, Schmedt T, Jurkunas U, Schleif R, Katsanis N, Gottsch JD. Mutations in LOXHD1, a recessive-deafness locus, cause dominant late-onset Fuchs corneal dystrophy. Am J Hum Genet. 2012;90:533–9. doi: 10.1016/j.ajhg.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riazuddin SA, Zaghloul NA, Al-Saif A, Davey L, Diplas BH, Meadows DN, Eghrari AO, Minear MA, Li YJ, Klintworth GK, Afshari N, Gregory SG, Gottsch JD, Katsanis N. Missense mutations in TCF8 cause late-onset Fuchs corneal dystrophy and interact with FCD4 on chromosome 9p. Am J Hum Genet. 2010;86:45–53. doi: 10.1016/j.ajhg.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta JS, Vithana EN, Tan DT, Yong VH, Yam GH, Law RW, Chong WG, Pang CP, Aung T. Analysis of the posterior polymorphous corneal dystrophy 3 gene, TCF8, in late-onset Fuchs endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2008;49:184–8. doi: 10.1167/iovs.07-0847. [DOI] [PubMed] [Google Scholar]
- 10.Mok JW, Kim HS, Joo CK. Q455V mutation in COL8A2 is associated with Fuchs' corneal dystrophy in Korean patients. Eye (Lond) 2009;23:895–903. doi: 10.1038/eye.2008.116. [DOI] [PubMed] [Google Scholar]
- 11.Gottsch JD, Zhang C, Sundin OH, Bell WR, Stark WJ, Green WR. Fuchs corneal dystrophy: aberrant collagen distribution in an L450W mutant of the COL8A2 gene. Invest Ophthalmol Vis Sci. 2005;46:4504–11. doi: 10.1167/iovs.05-0497. [DOI] [PubMed] [Google Scholar]
- 12.Biswas S, Munier FL, Yardley J, Hart-Holden N, Perveen R, Cousin P, Sutphin JE, Noble B, Batterbury M, Kielty C, Hackett A, Bonshek R, Ridgway A, McLeod D, Sheffield VC, Stone EM, Schorderet DF, Black GC. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet. 2001;10:2415–23. doi: 10.1093/hmg/10.21.2415. [DOI] [PubMed] [Google Scholar]
- 13.Gottsch JD, Sundin OH, Liu SH, Jun AS, Broman KW, Stark WJ, Vito EC, Narang AK, Thompson JM, Magovern M. Inheritance of a novel COL8A2 mutation defines a distinct early-onset subtype of fuchs corneal dystrophy. Invest Ophthalmol Vis Sci. 2005;46:1934–9. doi: 10.1167/iovs.04-0937. [DOI] [PubMed] [Google Scholar]
- 14.Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DT, Mohamed MD, Anand S, Khine KO, Venkataraman D, Yong VH, Salto-Tellez M, Venkatraman A, Guo K, Hemadevi B, Srinivasan M, Prajna V, Khine M, Casey JR, Inglehearn CF, Aung T. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2). Nat Genet. 2006;38:755–7. doi: 10.1038/ng1824. [DOI] [PubMed] [Google Scholar]
- 15.Desir J, Moya G, Reish O, Van Regemorter N, Deconinck H, David KL, Meire FM, Abramowicz MJ. Borate transporter SLC4A11 mutations cause both Harboyan syndrome and non-syndromic corneal endothelial dystrophy. J Med Genet. 2007;44:322–6. doi: 10.1136/jmg.2006.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desir J, Abramowicz M. Congenital hereditary endothelial dystrophy with progressive sensorineural deafness (Harboyan syndrome). Orphanet J Rare Dis. 2008;3:28. doi: 10.1186/1750-1172-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aldave AJ, Yellore VS, Bourla N, Momi RS, Khan MA, Salem AK, Rayner SA, Glasgow BJ, Kurtz I. Autosomal recessive CHED associated with novel compound heterozygous mutations in SLC4A11. Cornea. 2007;26:896–900. doi: 10.1097/ICO.0b013e318074bb01. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen TT, Bonanno JA. Lactate-H(+) transport is a significant component of the in vivo corneal endothelial pump. Invest Ophthalmol Vis Sci. 2012;53:2020–9. doi: 10.1167/iovs.12-9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen TT, Bonanno JA. Bicarbonate, NBCe1, NHE, and carbonic anhydrase activity enhance lactate-H+ transport in bovine corneal endothelium. Invest Ophthalmol Vis Sci. 2011;52:8086–93. doi: 10.1167/iovs.11-8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalimarada SS, Ogando DG, Vithana EN, Bonanno JA. Ion transport function of SLC4A11 in corneal endothelium. Invest Ophthalmol Vis Sci. 2013;54:4330–40. doi: 10.1167/iovs.13-11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogando DG, Jalimarada SS, Zhang W, Vithana EN, Bonanno JA. SLC4A11 is an EIPA-sensitive Na(+) permeable pHi regulator. Am J Physiol Cell Physiol. 2013;305:C716–27. doi: 10.1152/ajpcell.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCartney MD, Wood TO, McLaughlin BJ. Immunohistochemical localization of ATPase in human dysfunctional corneal endothelium. Curr Eye Res. 1987;6:1479–86. doi: 10.3109/02713688709044512. [DOI] [PubMed] [Google Scholar]
- 23.McCartney MD, Robertson DP, Wood TO, McLaughlin BJ. ATPase pump site density in human dysfunctional corneal endothelium. Invest Ophthalmol Vis Sci. 1987;28:1955–62. [PubMed] [Google Scholar]
- 24.Gottsch JD, Bowers AL, Margulies EH, Seitzman GD, Kim SW, Saha S, Jun AS, Stark WJ, Liu SH. Serial analysis of gene expression in the corneal endothelium of Fuchs' dystrophy. Invest Ophthalmol Vis Sci. 2003;44:594–9. doi: 10.1167/iovs.02-0300. [DOI] [PubMed] [Google Scholar]
- 25.Engler C, Kelliher C, Spitze AR, Speck CL, Eberhart CG, Jun AS. Unfolded protein response in fuchs endothelial corneal dystrophy: a unifying pathogenic pathway? Am J Ophthalmol. 2010;149:194–202. doi: 10.1016/j.ajo.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borderie VM, Baudrimont M, Vallee A, Ereau TL, Gray F, Laroche L. Corneal endothelial cell apoptosis in patients with Fuchs' dystrophy. Invest Ophthalmol Vis Sci. 2000;41:2501–5. [PubMed] [Google Scholar]
- 27.Jun AS, Meng H, Ramanan N, Matthaei M, Chakravarti S, Bonshek R, Black GC, Grebe R, Kimos M. An alpha 2 collagen VIII transgenic knock-in mouse model of Fuchs endothelial corneal dystrophy shows early endothelial cell unfolded protein response and apoptosis. Hum Mol Genet. 2012;21:384–93. doi: 10.1093/hmg/ddr473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schonthal AH. Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica. 2012;2012:857516. doi: 10.6064/2012/857516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buddi R, Lin B, Atilano SR, Zorapapel NC, Kenney MC, Brown DJ. Evidence of oxidative stress in human corneal diseases. Journal of the Histochemistry Society. 2002;50:341–51. doi: 10.1177/002215540205000306. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–67. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Ozcan U. Unfolded protein response signaling and metabolic diseases. J Biol Chem. 2014;289:1203–11. doi: 10.1074/jbc.R113.534743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12:703–19. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 33.Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 2014;42(Database issue):D199–205. doi: 10.1093/nar/gkt1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthaei M, Hu J, Meng H, Lackner EM, Eberhart CG, Qian J, Hao H, Jun AS. Endothelial cell whole genome expression analysis in a mouse model of early-onset Fuchs' endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2013;54:1931–40. doi: 10.1167/iovs.12-10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–58. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 37.Shei W, Liu J, Htoon HM, Aung T, Vithana EN. Differential expression of the Slc4 bicarbonate transporter family in murine corneal endothelium and cell culture. Mol Vis. 2013;19:1096–106. [PMC free article] [PubMed] [Google Scholar]
- 38.Vilas GL, Loganathan SK, Liu J, Riau AK, Young JD, Mehta JS, Vithana EN, Casey JR. Transmembrane water-flux through SLC4A11: a route defective in genetic corneal diseases. Hum Mol Genet. 2013;22:4579–90. doi: 10.1093/hmg/ddt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCartney MD, Wood TO, McLaughlin BJ. Moderate Fuchs' endothelial dystrophy ATPase pump site density. Invest Ophthalmol Vis Sci. 1989;30:1560–4. [PubMed] [Google Scholar]
- 40.Jurkunas UV, Bitar MS, Funaki T, Azizi B. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am J Pathol. 2010;177:2278–89. doi: 10.2353/ajpath.2010.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li QJ, Ashraf MF, Shen DF, Green WR, Stark WJ, Chan CC, O'Brien TP. The role of apoptosis in the pathogenesis of Fuchs endothelial dystrophy of the cornea. Arch Ophthalmol. 2001;119:1597–604. doi: 10.1001/archopht.119.11.1597. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Seet LF, Koh LW, Venkatraman A, Venkataraman D, Mohan RR, Praetorius J, Bonanno JA, Aung T, Vithana EN. Depletion of SLC4A11 causes cell death by apoptosis in an immortalized human corneal endothelial cell line. Invest Ophthalmol Vis Sci. 2012;53:3270–9. doi: 10.1167/iovs.11-8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diehl JA, Fuchs SY, Koumenis C. The cell biology of the unfolded protein response. Gastroenterology. 2011;141:38–41. doi: 10.1053/j.gastro.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim EC, Meng H, Jun AS. Lithium treatment increases endothelial cell survival and autophagy in a mouse model of Fuchs endothelial corneal dystrophy. Br J Ophthalmol. 2013;97:1068–73. doi: 10.1136/bjophthalmol-2012-302881. [DOI] [PMC free article] [PubMed] [Google Scholar]