Abstract
Purpose of review
The purpose of this review is to summarize evidence for the presence of two pathways of lipid absorption and their regulation.
Recent findings
Lipid absorption involves hydrolysis of dietary fat in the lumen of the intestine followed by the uptake of hydrolyzed products by enterocytes. Lipids are re-synthesized in the endoplasmic reticulum and are either secreted with chylomicrons and high density lipoproteins or stored as cytoplasmic lipid droplets. Lipids in the droplets are hydrolyzed and are secreted at a later time. Secretion of lipids by the chylomicron and HDL pathways are critically dependent on MTP and ABCA1, respectively, and are regulated independently. Gene ablation studies showed that MTP function and chylomicron assembly is essential for the absorption of triglyceride and retinyl esters. Ablation of MTP abolishes triglyceride absorption and results in massive triglyceride accumulation in enterocytes. Although majority of phospholipid, cholesterol and vitamin E are absorbed through the chylomicron pathway, a significant amount of these lipids are also absorbed via the HDL pathway. Chylomicron assembly and secretion is increased by the enhanced availability of fatty acids, whereas HDL pathway is upregulated by LXR agonists. Intestinal insulin resistance increases chylomicron and might reduce HDL production.
Summary
Triglycerides are exclusively transported via the chylomicron pathway and this process is critically dependent on MTP. Besides chylomicrons, absorption of phospholipids, free cholesterol, retinol, and vitamin E also involves high density lipoproteins. These two pathways are complementary and are regulated independently. They may be targeted to lower lipid absorption in order to control hyperlipidemia, obesity, metabolic syndrome, steatosis, insulin resistance, atherosclerosis and other disorders.
Keywords: Chylomicron, triglyceride, cholesterol, vitamin E, MTP, ABCA1, ACAT2, intestine, lipid absorption
INTRODUCTION
Dietary lipids are hydrolyzed in the intestinal lumen and products are taken up by enterocytes. Enterocytes re-synthesize lipids and package them into chylomicrons for secretion. These lipoproteins are hydrolyzed during circulation and products are taken by peripheral tissues. Remaining remnant particles are removed from circulation by the liver. Besides chylomicrons, recent studies indicate that high density lipoprotein assembly and secretion by the intestine also plays a role in intestinal lipid absorption. This review will summarize evidence for the existence and contribution of these pathways in the absorption of different lipids.
Intestinal Lipid Absorption
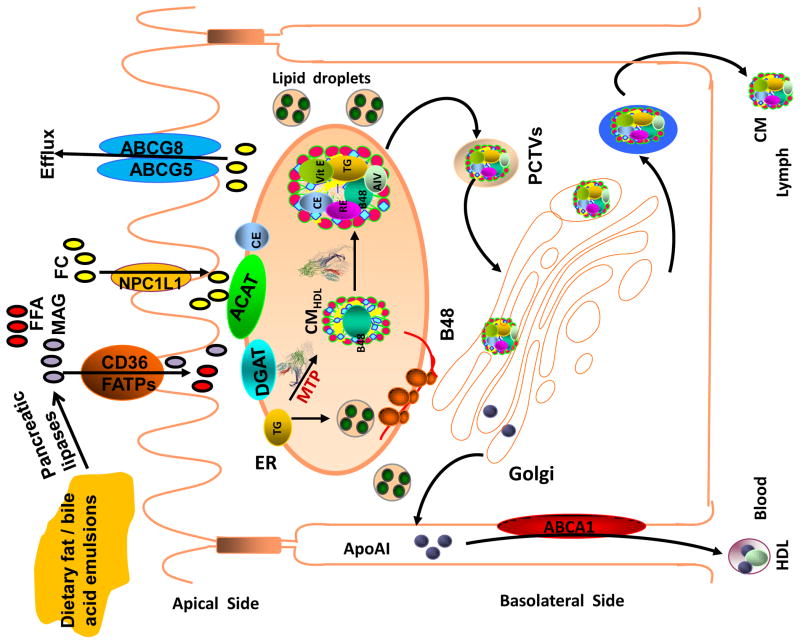
Triacylglycerols, phospholipids, and cholesterol esters are the predominant dietary lipids. An important step in the intestinal digestion of these lipids is their emulsification with bile salts [1–4]. Emulsification makes lipids better substrates for hydrolysis by various lipases found in the lumen of the intestine (Figure). Triglyceride hydrolysis releases free fatty acids (FFAs) and monoacylglycerols. Phospholipid hydrolysis yields FFAs and lysophospholipids. Cholesterol esters are hydrolyzed to free cholesterol and FFAs. Enterocytes use diffusion and protein- mediated transport mechanisms to take up monoacylglycerols and FFAs [3–5]. Diffusion across these epithelial cells occurs when FFA concentrations in the lumen exceed those inside the cell. When extracellular concentrations are lower, then protein-mediated uptake mechanisms might become more important in the uptake of monoacylglycerols and FAs. Several proteins including cluster of differentiation 36 (CD36) and various FA transport proteins have been shown to be involved in this process [3–6]. After their entry into cells, FFAs are transported to various organelles for further processing by FA-binding proteins [7;8]. An important step for their secretion is their transport to the ER.
Figure.
Intestinal lipid absorption: Dietary lipids are emulsified with bile salts and are hydrolyzed by different pancreatic lipases resulting in the generation of free fatty acids (FFA), monoacylglycerols (MAG) and free cholesterol (FC). These products are taken up by enterocytes involving various transporters and transported to the endoplasmic reticulum where they are used for the synthesis of phospholipids, triacylglycerols and cholesterol esters. These lipids are assembled into chylomicron particles using apoB48 as a scaffolding protein with the help of MTP. Alternatively, they are stored in cytosol as lipid droplets. FC can be either excreted back to the lumen via ABCAG5/ABCG8 transporters are effluxed to blood circulation by ABCA1 and apoAI.
The Niemann-Pick C1-like 1 (NPC1L1) protein plays a major role in the uptake of cholesterol by enterocytes [9;10]. NPC1L1 is enriched in the plasma membrane microdomains called rafts. Under normal condition, the N-terminal domain of NPC1L1 that harbors cholesterol binding site is exposed and the C-terminal domain interacts with the inner leaflet of plasma membrane. Binding of cholesterol to NPC1L1 at the N-terminus, exposes the C-terminal domain allowing it to interact with Numb to facilitate recruitment of clathrin [11]. These microdomains are then internalized and transported to endocytic recycling compartment where cholesterol is released and transported to other organelles, including endoplasmic reticulum, by unknown mechanisms.
In the endoplasmic reticulum (ER), monoacylglycerols are esterified with FFAs by monoacylglycerol acyltransferases to form diacylglycerols, which are converted to triacylglycerols by diacylglycerol acyltransferases [3;4;12]. Diacylglycerol can also be combined with choline and ethanolamine to synthesize phospholipids by choline and ethanolamine transferases. Free cholesterol taken up by the enterocytes is esterified in the ER by membrane- bound acyl-CoA:cholesterol acyltransferases. Esterification of free cholesterol and FFA into cholesteryl esters and glycerolipids may prevent deleterious effects associated with their excess accretions. Further, synthesis of these lipids may favor the entry of hydrolyzed intralumenal products into the cell by reducing their intracellular concentrations.
The lipids synthesized in the ER membrane have two fates. They can either become part of cytosolic lipid droplets and stored, or transported to the ER for secretion. The cytosolic lipid droplets [13–19] are large spherical particles surrounded by a phospholipid monolayer. They mainly accumulate toward the apical side in the cytosol of enterocytes. Their surface contains several proteins; perilipin family members being the prominent. The core consists of neutral lipids (triglycerides, cholesterol esters). Their biogenesis starts in the ER and mechanisms involved in their formation are being elucidated [13–19]. In most cells, these droplets bud off toward the cytosol. However, they may partition toward ER lumen in cells that assemble lipoproteins such as hepatocytes and enterocytes.
The cytosolic lipid droplets are subsequently mobilized and secreted during fasting state in enterocytes. Mobilization involves hydrolysis of triglycerides, mobilization of FAs to the ER and re-synthesis of triglycerides at the ER and subsequent secretion with lipoproteins. Their accumulation may provide a mechanism for the storage of fat during meal consumption and to mobilize it a later time. The mobilization of fat from these stores might contribute to plasma triglyceride increases seen just before the digestion of food [20]. Thus, the transient storage of fat as cytosolic lipid droplets might help optimize lipid absorption during food consumption and provide sustained lipid supply during fasting.
Besides the cytosolic droplets, lipid droplets are also seen in the ER lumen of hepatocytes and enterocytes. These lumenal droplets accumulate in the absence of apoB but not in the absence of MTP [21;22]. Further inhibition of MTP lowers accretions of lipids in the ER lumen [23;24]. How MTP assists in lumenal lipid droplet formation is not known. However, it is known that MTP can associate with lipid vesicles and can transfer lipids [15;25;26]. It is possible that MTP can associate and stabilize lipid droplets. Alternatively, it may transfer lipids to an unknown acceptor present on the lumenal droplet. Further, the fate of these lumenal lipid droplets is unknown. It is generally assumed that they fuse with apoB-containing lipoproteins, however very little experimental evidence is present for such a mechanism. It has been suggested that hydrolysis and re-esterification of lumenal lipid droplets might provide lipids for the second step core expansion during lipoprotein assembly [15].
Lipids are packaged into large, spherical triacylglycerol-rich lipoproteins called chylomicrons. The chylomicron surface is covered with phospholipid monolayer and contains free cholesterol and is surrounded by a large protein, apolipoprotein B48 (apoB48). In addition to the nonexchangeable apoB48, several exchangeable apolipoproteins can be found on the surface of the chylomicron, including apoAI, apoAIV, and apoCs. Chylomicron core is rich in triacylglycerols and cholesteryl esters. The apoB48-containing lipoproteins are synthesized in the intestine in a constitutive manner, but the amount of lipid transported with these particles changes dramatically during the postprandial state as a result of increased amounts of lipids being packaged into larger lipoprotein particles [3;27].
Chylomicron assembly begins with the translation of apoB48. In the absence of a sufficient supply of lipids or MTP, this nascent polypeptide is degraded intracellularly. MTP can interact physically with and transfer lipids to the nascent apoB [25]. This helps apoB fold into a structural configuration that is receptive to accepting more lipids [28]. The result is the formation of a smaller “primordial” particles of the size of a high-density lipoprotein (HDL) [27;29]. During the second step of “core expansion,” a large bolus of lipids, preferably newly synthesized triacylglycerols, is added to produce a larger lipoprotein [30]. The mechanisms underlying the addition of the lipid bolus and the origin of these lipid droplets have not been elucidated.
Chylomicron particles are then transported to the cis-Glogi by pre-chylomicron transport vesicles (PCTVs) [8;8;31–33]. These vesicles are larger than protein transport vesicles and contain the unique vesicle-associated membrane protein 7 [34]. Liver FA-binding protein and protein kinase C isoform ζ are involved in the budding of these PCTVs from the surface of the ER [35;36]. Chylomicrons also contain coat protein complex II-interacting proteins that are important for the subsequent fusion of PCTVs with the cis-Golgi [37]. These particles undergo further modifications in the Golgi such as the addition of apoAI and glycosylation of apoB48. Chylomicrons are then released from the basolateral side and enter the general circulation at the thoracic duct. Increases in blood triacylglycerol levels in the postprandial state might be due to the increased rate of entry of these particles into the general circulation.
The HDL pathway
Using differentiated Caco-2 cells and isolated enterocytes, it was shown that triglycerides are secreted with apoB-containing chylomicrons [38;39]. However, similar studies showed that secreted cholesterol was in two different lipoproteins; chylomicrons and HDL that contained apolipoprotein B and apoAI, respectively [40]. Chylomicrons contained both free and esterified cholesterol whereas HDL mainly contained free cholesterol. These studies indicated that, as opposed to triglycerides that are exclusively secreted with chylomicrons, cholesterol can be secreted by two mechanisms.
Secretion of cholesterol with chylomicrons, but not with HDL, was inhibited by MTP inhibitors. In contrast, cholesterol secretion with chylomicrons was increased when cells were supplemented with oleic acid. This treatment had no effect on the secretion of cholesterol with HDL. But, secretion of cholesterol with HDL increased when cells were treated with LXR/RXR agonists. LXR agonists increase the expression of ABCA1 [41]. Inhibition of ABCA1 by glyburide reduced secretion of cholesterol with HDL [38]. Therefore, cholesterol absorption by the intestine is a highly regulated process involving apoB-and MTP-dependent chylomicron and apoB-independent HDL pathways. Chylomicrons transport free and esterified cholesterol. Their assembly is induced by oleic acid, and is inhibited by MTP inhibitors. ApoB-independent HDL pathway is mediated by ABCA1. There could be two mechanisms by which ABAC1 could participate in the transport of cholesterol from enterocytes. It could increase the intercellular transport of cholesterol to the plasma membrane [42]. Further, it could facilitate the efflux of this cholesterol to extracellular acceptors. Increased expression of ABCA1 by LXR agonist enhances ABCA1-mediated cholesterol efflux pathway.
These studies were then extended to mice to evaluate the roles of apoAI and ABCA1 in the absorption of cholesterol via the HDL pathway [40;43]. Wildtype mice absorbed cholesterol involving both chylomicrons and HDL. Cholesterol secretion was significantly decreased in apoAI and ABCA1 deficient enterocytes compared to controls. Cholesterol transport studies using isolated enterocytes revealed that individual deficiencies of apoAI and ABCA1 had no significant effect on the uptake of cholesterol. However, enterocytes deficient in apoAI or ABCA1 secreted significantly lower amounts of cholesterol. Further lipoprotein analysis showed that deficiencies in these proteins specifically reduce the amounts of cholesterol secreted with HDL with no effect on cholesterol secreted with chylomicrons. These studies suggest that intestinal HDL is involved in the secretion of cholesterol.
Since HDL is mainly involved in the transport of free cholesterol, studies have been performed to ask whether increasing intestinal free cholesterol levels enhance secretion via this pathway. ACAT2 deficient mice absorb less cholesterol. Mice deficient in both ABCA1 and ACAT2 absorb lower cholesterol compared to mice that are individually deficient in these mice [44]. Authors have concluded that, in the absence of ACAT2, ABCA1 is involved in cholesterol absorption.
The studies summarized above suggest that cholesterol absorption by the intestine involves chylomicron and HDL pathways. We recently determined the contribution of these two pathways and asked whether there exist other mechanisms for cholesterol absorption using mice deficient in both MTP and ABCA1 [45]. Individual deficiencies of MTP and ABCA1 reduced cholesterol secretion by 68% and 27%, respectively, whereas their combined deficiency decreased cholesterol secretion by ~80%. MTP deficiency reduced cholesterol by the chylomicron pathway with no effect on HDL pathway. ABCA1 deficiency reduced cholesterol secretion with the HDL pathway with no effect on chylomicron pathway. Combined deficiencies of MTP and ABCA1 reduced cholesterol secretion with both chylomicrons and HDL. Thus, these two pathways act in concert to optimize cholesterol absorption.
Insulin resistance and intestinal lipoprotein production
Insulin resistance is accompanied with dyslipidemia and increase risk for the pathogenesis of cardiovascular disease. Dyslipidemia in insulin resistance is characterized by elevated concentrations of triglyceride-rich lipoproteins and reduced plasma HDL cholesterol. Several studies have shown that chylomicron production is increased in insulin resistance, type 2 diabetes and obesity [46]. Veilleux et al. [47] obtained duodenal biopsies from obese subjects undergoing bariatric surgery and divided them into insulin sensitive and insulin resistant groups. The duodenal explants from insulin resistant patients synthesized more lipids, apoB48 and secreted more lipoproteins. Further, they had higher mRNA levels of FA binding proteins and MTP. These studies indicate that insulin resistance enhances chylomicron production. In contrast, insulin resistant subjects had lower levels of ABCA1. It is possible that insulin resistance may increase cholesterol transport with chylomicrons and reduce cholesterol secretion with HDL. These changes might contribute to dyslipidemia seen in insulin resistant subjects.
Couture et al. [48] studied the kinetics of triglyceride-rich lipoprotein production and catabolism, as well as expression of key intestinal genes in 14 obese, insulin resistant, non- diabetic men and compared them with 10 insulin sensitive, obese subjects. They observed that production rates of intestinal apoB48-containing triglyceride-rich lipoprotein were 87% higher in insulin resistant men compared with insulin sensitive men. Surprisingly, they found lower expression of SREBP-2, HNF4a and MTP. Authors suggest that increased lipoprotein production with reduced MTP might be related increased lipid availability. Nevertheless, majority of studies indicate that increased intestinal lipoprotein production is associated with increased MTP expression in insulin resistance state [for review, [46]].
Vitamin A absorption
Dietary vitamin A includes retinyl esters and provitamin carotenoids. In the intestinal lumen, retinyl esters are hydrolyzed to retinol and fatty acids whereas carotenoids are converted to retinol. Retinol is taken up by enterocytes most likely involving transport proteins as this process is saturable in cell culture studies. It appears that STRA6 can mediate retinol uptake in intestinal cells. SR-B1 has been shown to be involved in the uptake of carotenoids such as lutein, β-carotene, zeaxanthin and lycopene. Intracellular transfer proteins such as CRBPII transfer retinol within cells. The uptake of retinol by enterocytes is enhanced by subsequent intracellular esterification of retinol by LRAT to retinyl esters. Retinyl esters are incorporated into chylomicrons by unknown mechanism and are exclusively secreted with these lipoproteins. Free retinol can be effluxed via the HDL pathway [49–51].
Vitamin E absorption
In differentiated Caco-2 cells, vitamin E (α-tocopherol) is secreted with both apoB-containing chylomicrons and apoAI-containing HDL [52]. Secretion of α-tocopherol with chylomicrons was increased when cells were supplemented with oleic acid and was inhibited by MTP inhibitors and inhibitors of secretory pathways e.g., Brefeldin A and monensin. The secretion of vitamin E with HDL was higher when cells were not supplemented with oleic acid and was inhibited by glyburide, an inhibitor of ABCA1. Further, secretion of vitamin E was increased when HDL was provided as an acceptor. Further studies by Nicod and Parker [53] showed secretion of vitamin E by differentiated Caco-2 cells with HDL was increased when cells were treated with LXR agonist, T0901317. They also showed that secretion via the HDL pathway may have selectivity towards different vitamers. These studies showed that differentiated Caco-2 cells use independently regulated chylomicron and HDL pathways to secrete vitamin E.
Studies in primary enterocytes isolated from rats also showed that vitamin E was secreted with chylomicrons and HDL [54]. Secretion with chylomicrons was increased with increasing concentrations of oleic acid indicating that lipid availability and apoB-lipoprotein formation is essential. This is supported by the observation that secretion with chylomicrons is inhibited by MTP inhibitors. Secretion of vitamin E by enterocytes was unaffected by lipid supplementation, MTP inhibition, and MTP gene deletion; however, it was increased when HDL was provided as an extracellular acceptor.
Conclusion
The data reviewed above suggest that chylomicron is the only pathways for triglyceride absorption. However, there are two pathways for the absorption of cholesterol; chylomicrons and HDL. Similarly, fat-soluble vitamins are also absorbed by these two different pathways. Ablation of chylomicron pathway reduces lipid absorption but is associated with massive triglyceride accumulation in enterocytes. It remains to be determined whether the HDL pathway can be inhibited to lower plasma cholesterol levels.
Table 1.
Characteristic features of lipid absorption by chylomicron and HDL pathways
| Chylomicron | HDL | |
|---|---|---|
| Triglyceride | Yes | No |
| Retinyl esters | Yes | No |
| Retinol | Yes | Yes |
| Phospholipids | Yes | Yes |
| Cholesterol | Yes | Yes |
| Vitamin E | Yes | Yes |
| Oleic acid supplementation | Increase | No effect |
| MTP | Required | Not required |
| ABCA1 | No role | Important |
| LXR agonist | No effect | Increases |
| ApoAI | No effect | Increases |
Acknowledgments
This work was supported in part by the NIH grant (DK-46900) and the VA Merit Award (BX001728).
Abbreviations used
- ABCA1
ATP binding cassette family A protein 1
- apoB
apolipoprotein B
- ACAT
acyl-CoA:cholesterol acyltransferase
- CD36
cluster of differentiation 36
- ER
endoplasmic reticulum
- FFA
free fatty acids
- HDL
high density lipoprotein
- MTP
microsomal triglyceride transfer protein
- NPC1L1
Niemann-Pick C1-Like 1
- PCTVs
pre-chylomicron transport vesicles
Reference List
- 1.Mu H, Hoy CE. The digestion of dietary triacylglycerols. Prog Lipid Res. 2004;43:105–133. doi: 10.1016/s0163-7827(03)00050-x. [DOI] [PubMed] [Google Scholar]
- 2.Phan CT, Tso P. Intestinal lipid absorption and transport. Front Biosci. 2001;6:D299–D319. doi: 10.2741/phan. [DOI] [PubMed] [Google Scholar]
- 3.Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296:E1183–E1194. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan X, Hussain MM. Gut triglyceride production. Biochim Biophys Acta. 2012;1821:727–735. doi: 10.1016/j.bbalip.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abumrad NA, Davidson NO. Role of the gut in lipid homeostasis. Physiol Rev. 2012;92:1061–1085. doi: 10.1152/physrev.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abumrad N, Harmon C, Ibrahimi A. Membrane transport of long-chain fatty acids: evidence for a facilitated process. J Lipid Res. 1998;39:2309–2318. [PubMed] [Google Scholar]
- 7.Storch J, Thumser AE. Tissue-specific functions in the fatty acid-binding protein family. J Biol Chem. 2010;285:32679–32683. doi: 10.1074/jbc.R110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansbach CM, Siddiqi SA. The biogenesis of chylomicrons. Annu Rev Physiol. 2010;72:315–333. doi: 10.1146/annurev-physiol-021909-135801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis HR, Jr, Tershakovec AM, Tomassini JE, Musliner T. Intestinal sterol transporters and cholesterol absorption inhibition. Curr Opin Lipidol. 2011;22:467–478. doi: 10.1097/MOL.0b013e32834c7c28. [DOI] [PubMed] [Google Scholar]
- 10.Wang LJ, Song BL. Niemann-Pick C1-Like 1 and cholesterol uptake. Biochim Biophys Acta. 2012;1821:964–972. doi: 10.1016/j.bbalip.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Li PS, Fu ZY, Zhang YY, Zhang JH, Xu CQ, Ma YT, Li BL, Song BL. The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1. Nat Med. 2014;20:80–86. doi: 10.1038/nm.3417. [DOI] [PubMed] [Google Scholar]
- 12.Hussain MM, Leung TM, Abu-Merhi S. Regulating intestinal function to reduce atherogenic lipoproteins. Clin Lipidol. 2013;8:481–490. doi: 10.2217/clp.13.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiam AR, Farese RV, Jr, Walther TC. The biophysics and cell biology of lipid droplets. Nat Rev Mol Cell Biol. 2013;14:775–786. doi: 10.1038/nrm3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demignot S, Beilstein F, Morel E. Triglyceride-rich lipoproteins and cytosolic lipid droplets in enterocytes: Key players in intestinal physiology and metabolic disorders. Biochimie. 2014;96:48–55. doi: 10.1016/j.biochi.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Lehner R, Lian J, Quiroga AD. Lumenal lipid metabolism: implications for lipoprotein assembly. Arterioscler Thromb Vasc Biol. 2012;32:1087–1093. doi: 10.1161/ATVBAHA.111.241497. [DOI] [PubMed] [Google Scholar]
- 16.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 17.Murphy DJ, Vance J. Mechanisms of lipid body formation. Trends Biochem Sci. 1999;24:109–115. doi: 10.1016/s0968-0004(98)01349-8. [DOI] [PubMed] [Google Scholar]
- 18.Ohsaki Y, Cheng J, Suzuki M, Shinohara Y, Fujita A, Fujimoto T. Biogenesis of cytoplasmic lipid droplets: From the lipid ester globule in the membrane to the visible structure. Biochim Biophys Acta. 2009;1791:399–407. doi: 10.1016/j.bbalip.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Sturley SL, Hussain MM. Lipid droplet formation on opposing sides of the endoplasmic reticulum. J Lipid Res. 2012;53:1800–1810. doi: 10.1194/jlr.R028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert JE, Parks EJ. Postprandial metabolism of meal triglyceride in humans. Biochim Biophys Acta. 2012;1821:721–726. doi: 10.1016/j.bbalip.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton RL, Wong JS, Cham CM, Nielsen LB, Young SG. Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J Lipid Res. 1998;39:1543–1557. [PubMed] [Google Scholar]
- 22.Raabe M, Véniant MM, Sullivan MA, Zlot CH, Björkegren J, Nielsen LB, Wong JS, Hamilton RL, Young SG. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue- specific knockout mice. J Clin Invest. 1999;103:1287–1298. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulinski A, Rustaeus S, Vance JE. Microsomal triacylglycerol transfer protein is required for lumenal accretion of triacylglycerol not associated with Apo B, as well as for Apo B lipidation. J Biol Chem. 2002;277:31516–31525. doi: 10.1074/jbc.M202015200. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Tran K, Yao Z. The activity of microsomal triglyceride transfer protein is essential for accumulation of triglyceride within microsomes in McA-RH7777 cells. A unified model for the assembly of very low density lipoproteins. J Biol Chem. 1999;274:27793–27800. doi: 10.1074/jbc.274.39.27793. [DOI] [PubMed] [Google Scholar]
- 25.Hussain MM, Rava P, Walsh M, Rana M, Iqbal J. Multiple functions of microsomal triglyceride transfer protein. Nutr Metab (Lond) 2012;9:14. doi: 10.1186/1743-7075-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apolipoprotein B-lipoprotein assembly. J Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 27.Hussain MM, Kancha RK, Zhou Z, Luchoomun J, Zu H, Bakillah A. Chylomicron assembly and catabolism: role of apolipoproteins and receptors. Biochim Biophys Acta. 1996;1300:151–170. doi: 10.1016/0005-2760(96)00041-0. [DOI] [PubMed] [Google Scholar]
- 28.Jiang ZG, Liu Y, Hussain MM, Atkinson D, McKnight CJ. Reconstituting initial events during the assembly of apolipoprotein B-containing lipoproteins in a cell-free system. J Mol Biol. 2008;383:1181–1194. doi: 10.1016/j.jmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hussain MM. A proposed model for the assembly of chylomicrons. Atherosclerosis. 2000;148:1–15. doi: 10.1016/s0021-9150(99)00397-4. [DOI] [PubMed] [Google Scholar]
- 30.Luchoomun J, Hussain MM. Assembly and secretion of chylomicrons by differentiated Caco-2 cells: Nascent triglycerides and preformed phospholipids are preferentially used for lipoprotein assembly. J Biol Chem. 1999;274:19565–19572. doi: 10.1074/jbc.274.28.19565. [DOI] [PubMed] [Google Scholar]
- 31.Kumar NS, Mansbach CM. II: Prechylomicron transport vesicle: isolation and partial characterization. Am J Physiol Gastrointest Liver Physiol. 1999;276:G378–G386. doi: 10.1152/ajpgi.1999.276.2.G378. [DOI] [PubMed] [Google Scholar]
- 32.Mansbach CM, Gorelick F. Development and Physiological Regulation of Intestinal Lipid Absorption. II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons. Am J Physiol Gastrointest Liver Physiol. 2007;293:G645–G650. doi: 10.1152/ajpgi.00299.2007. [DOI] [PubMed] [Google Scholar]
- 33.Black DD. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: cellular events in chylomicron assembly and secretion. Am J Physiol Gastrointest Liver Physiol. 2007;293:G519–G524. doi: 10.1152/ajpgi.00189.2007. [DOI] [PubMed] [Google Scholar]
- 34.Siddiqi SA, Mahan J, Siddiqi S, Gorelick FS, Mansbach CM. Vesicle-associated membrane protein 7 is expressed in intestinal ER. J Cell Sci. 2006;119:943–950. doi: 10.1242/jcs.02803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neeli I, Siddiqi SA, Siddiqi S, Mahan J, Lagakos WS, Binas B, Gheyi T, Storch J, Mansbach CM. Liver fatty acid-binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J Biol Chem. 2007;282:17974–17984. doi: 10.1074/jbc.M610765200. [DOI] [PubMed] [Google Scholar]
- 36.Siddiqi SA, Mansbach CM. PKC zeta-mediated phosphorylation controls budding of the pre- chylomicron transport vesicle. J Cell Sci. 2008;121:2327–2338. doi: 10.1242/jcs.022780. [DOI] [PubMed] [Google Scholar]
- 37.Siddiqi S, Saleem U, Abumrad NA, Davidson NO, Storch J, Siddiqi SA, Mansbach CM. A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J Lipid Res. 2010;51:1918–1928. doi: 10.1194/jlr.M005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iqbal J, Anwar K, Hussain MM. Multiple, independently regulated pathways of cholesterol transport across the intestinal epithelial cells. J Biol Chem. 2003;278:31610–31620. doi: 10.1074/jbc.M301177200. [DOI] [PubMed] [Google Scholar]
- 39.Williams KJ. Molecular processes that handle -- and mishandle -- dietary lipids. J Clin Invest. 2008;118:3247–3259. doi: 10.1172/JCI35206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iqbal J, Hussain MM. Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J Lipid Res. 2005;46:1491–1501. doi: 10.1194/jlr.M500023-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 42.Zha X, Gauthier A, Genest J, McPherson R. Secretory vesicular transport from the Golgi is altered during ATP-binding cassette protein A1 (ABCA1) mediated cholesterol efflux. J Biol Chem. 2003 doi: 10.1074/jbc.C300024200. [DOI] [PubMed] [Google Scholar]
- 43.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JA, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Temel RE, Lee RG, Kelley KL, Davis MA, Shah R, Sawyer JK, Wilson MD, Rudel LL. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J Lipid Res. 2005;46:2423–2431. doi: 10.1194/jlr.M500232-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Iqbal J, Parks JS, Hussain MM. Lipid Absorption Defects in Intestine-specific Microsomal Triglyceride Transfer Protein and ATP-Binding Cassette Transporter A1-deficient Mice. J Biol Chem. 2013;288:30432–30444. doi: 10.1074/jbc.M113.501247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao C, Lewis GF. Regulation of chylomicron production in humans. Biochim Biophys Acta. 2012;1821:736–746. doi: 10.1016/j.bbalip.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Veilleux A, Grenier E, Marceau P, Carpentier AC, Richard D, Levy E. Intestinal Lipid Handling: Evidence and Implication of Insulin Signaling Abnormalities in Human Obese Subjects. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.113.302993. [DOI] [PubMed] [Google Scholar]
- 48.Couture P, Tremblay AJ, Kelly I, Lemelin V, Droit A, Lamarche B. Key intestinal genes involved in lipoprotein metabolism are downregulated in dyslipidemic men with insulin resistance. J Lipid Res. 2014;55:128–137. doi: 10.1194/jlr.M040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrison EH, Hussain MM. Mechanisms involved in the intestinal digestion and absorption of dietary vitamin A. J Nutr. 2001;131:1405–1408. doi: 10.1093/jn/131.5.1405. [DOI] [PubMed] [Google Scholar]
- 50.Harrison EH. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim Biophys Acta. 2012;1821:70–77. doi: 10.1016/j.bbalip.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reboul E, Borel P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog Lipid Res. 2011;50:388–402. doi: 10.1016/j.plipres.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Anwar K, Kayden HJ, Hussain MM. Transport of vitamin E by differentiated Caco-2 cells. J Lipid Res. 2006;47:1261–1273. doi: 10.1194/jlr.M500523-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Nicod N, Parker RS. Vitamin E secretion by Caco-2 monolayers to APOA1, but not to HDL, is vitamer selective. J Nutr. 2013;143:1565–1572. doi: 10.3945/jn.113.176834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anwar K, Iqbal J, Hussain MM. Mechanisms involved in vitamin E transport by primary enterocytes and in vivo absorption. J Lipid Res. 2007;48:2028–2038. doi: 10.1194/jlr.M700207-JLR200. [DOI] [PubMed] [Google Scholar]