Abstract
Herpes simplex virus type 2 (HSV-2) resistance to antiviral drugs has been described primarily in immunocompromised patients. We report an apparently immunocompetent, human immunodeficiency virus–negative male patient who has experienced repeated HSV-2 genital outbreaks despite receiving antiviral prophylaxis with several different drugs. Several of the HSV-2 genital isolates from this patient have been confirmed as resistant to acyclovir and penciclovir. Antiviral resistance occurred in the setting of long-term prednisone treatment and intermittent acyclovir prophylaxis at suboptimal doses and persisted despite the cessation of oral steroid treatment. The patient’s genital herpes outbreaks were not controlled by high-dose prophylaxis with acyclovir, valacyclovir, and famciclovir. Cessation of antiviral prophylaxis resulted in reversion of this patient’s HSV-2 isolates to acyclovir and penciclovir sensitivity, although resistant virus reappeared when antiviral prophylaxis was resumed. Transmission of a sensitive HSV-2 strain from this patient to a female sex partner was observed. These observations confirm previous reports that resistance to acyclovir may develop during prophylactic therapy in an otherwise well, immunocompetent patient. These findings support the conclusion that both drug-sensitive and drug-resistant HSV-2 strains established latency in this patient and that both strains are capable of frequent reactivation.
Herpes simplex virus type 2 (HSV-2) has infected ~20% of the US population [1]. Of these 45 million infected persons, ~10% reported a history of genital herpes lesions. Some patients with genital herpes have frequent and/or severe recurrences that may have profound effects on their social functioning [2]. Genital herpes outbreaks may be treated for 3–5 days with oral antiviral drugs, including acyclovir (ACV), valacyclovir (Valtrex; GlaxoSmithKline), and famciclovir (Famvir; Novartis), but treatment only decreases the duration of symptoms by approximately one-quarter [3, 4]. Famciclovir and valacyclovir are orally bioavailable prodrugs that are metabolized to penciclovir (PCV) and ACV, respectively. Daily antiviral prophylaxis in patients with frequent or severe symptoms effectively prevents genital herpes outbreaks, decreasing the frequency of lesions by 80%–95% [5]. Disadvantages of daily prophylaxis for genital herpes include the expense and inconvenience of taking a medication once or twice daily.
Most ACV-resistant HSV-2 isolates lack expression or function of the thymidine kinase (tk) gene. ACV resistance may be due to a tk deletion, an alteration in tk, or a mutation in the viral DNA polymerase [6]. tk-deficient or -altered ACV-resistant viral isolates usually retain susceptibility to foscarnet and cidofovir, antivirals that do not require the activity of this enzyme for their activity. ACV-resistant HSV-2 in immunocompetent patients is rare, even in those who have received continuous antiviral prophylaxis for years [7, 8]. Despite the relatively widespread use of nucleoside antivirals in patients with genital herpes, to date, HSV-2 resistance has not emerged as a common concern for health care providers treating this condition [4].
Multiple previous reports have described clinically significant HSV resistance to ACV and PCV in immunocompromised patients, including those with AIDS, organ transplantation, and cancer [9–14]. In contrast to discrete genital herpes recurrences in immunocompetent persons, immunocompromised patients usually experience continuous, ongoing outbreaks of HSV-2 that do not clear until effective drug therapy is provided or the immunocompromised state resolves.
Kost et al. described a young homosexual man with frequently recurrent genital herpes due to a tk-altered HSV-2 strain [15]. Mouly et al. followed by describing an otherwise well, married, 64-year-old woman with a similar condition but without any risk factors for sexually transmitted diseases [16]. Finally, the patient described by Swetter et al. had a 15-month history of nonhealing vulvar ulcerations due to tk-deficient HSV-2, a distinctly different clinical appearance from that of the other 2 patients, who had clearing between genital herpes outbreaks [17].
We describe here an otherwise well, immunocompetent patient with recurrent genital herpes outbreaks caused by an HSV-2 strain that became highly resistant to ACV and PCV. Evidence for latency of both drug-sensitive and drug-resistant HSV-2 strains in this patient is presented.
MATERIALS AND METHODS
Specimens and viral cultures
Genital specimens were collected by swabbing the lesions with a Dacron swab moistened in MEM (Gibco) supplemented with penicillin, streptomycin, glutamine, and 2%–8% fetal calf serum (Hyclone). Isolation of HSV-2 was performed by placing the specimens on monolayers of mink lung cells and observing them for characteristic cytopathic effects [18].
Genotypic analysis
Viral DNA was isolated from Vero cells infected at high MOI with plaque-purified isolates from the index patient and his partner. The tk gene was amplified by polymerase chain reaction (PCR) in a DNA thermal cycler (Eppendorf), using primers flanking the gene that were designed to include either the EcoRI or HindIII restriction site (highlighted in bold): HSV2tkPCR1, 5′-CGGCAAGCTTCCGTGTTTGAACTAAACTCC-3′; and HSV2tkPCR2, 5′-CGAATTCTTGTAGAAGCGGGTATGGCTTC-3′. Amplification conditions included denaturation for 5 min at 98°C, 30 cycles of denaturation at 98°C for 45 s, annealing at 55°C for 45 s, and extension at 72°C for 2 min, followed by extension at 72°C for 10 min. PCR products were purified by electrophoretic separation on a 1% agarose gel; the fragment was excised and purified by use of a Qiagen spin column and then digested with EcoRI and HindIII for orientation-specific ligation into the plasmid vector pGEM-7Zf(+). Ligation reactions were transformed into competent DH5α cells (Stratagene). Plasmid DNA from tk subclones was obtained by a standard alkaline lysis miniprep procedure and purified using Qiagen miniprep spin columns. The purified DNA and primers were sent to Lone Star Laboratories (Houston, TX) for sequence analysis. Primers used for the sequence analysis were as follows: T72970, 5′-CCAGTGAATT GTAATACGACTCAC-3′; SP6147R, 5′-CCAAGCTATTTAGGTGACA CTATAG-3′; HSV2TK-47647R, 5′-GTAATGACCAGCGCCCAGATAAC-3′; and HSV2TK-47076, 5′-CAGGCAAAAATGTGGTACAAGTCC-3′. Viral DNA obtained from the partner was amplified using the forward primer 5′-GTTGAACTCCCGCACCTCT-3′ and the reverse primer 5′-GTTCTTTTATTGCCGTCATCG-3′. Amplification conditions were the same as those described above, but the PCR buffer was supplemented with 4% dimethyl sulfoxide. PCR products were sequenced directly with the amplification primers, as well as with 5′-TACCTCATGGGAAGCATGAC-3′ and 5′-GAAGGACACCCAGGACCAG-3′. Restriction profiles were obtained for viral genomic DNA isolated from the supernatant of tissue cultures. The virions were disrupted in Tris-EDTA supplemented with 1% SDS and 20 µL of 10 mg/mL proteinase K. DNA was purified by sequential extraction with phenol and chloroform and precipitated in ethanol. Restriction enzymes BamHI and HindIII were used to digest the DNA, and the fragments were separated on an agarose gel.
RESULTS
Case report
A 35-year-old man began to experience genital herpes outbreaks in 1992, on the shaft of the penis. These outbreaks were often preceded by prodromal pain at the site of the impending lesions and were usually accompanied by pain radiating into the buttocks. Episodic and then prophylactic ACV was prescribed during this period, with relatively good control of the outbreaks. In 1994, the patient developed persistent pruritis on his extremities. The pruritis was treated with oral prednisone, topical corticosteroids, and doxepin. In 1996, he developed biopsy-confirmed urticaria on his abdomen, back, and penis. The urticaria improved with prednisone treatment (30 mg daily) and recurred when the prednisone treatment was stopped. In 1997, a flare of urticaria was temporally related to the use of ACV. Daily doses of prednisone (2–20 mg) successfully controlled the chronic urticaria. Genital herpes outbreaks were suppressed during this period with valacyclovir treatment (500 mg once daily).
In March 1998, the patient was referred to the Infectious Diseases Clinic at the University of Utah for suspected ACV allergy and frequent genital herpes outbreaks. The patient was experiencing monthly genital herpes outbreaks with a prodrome of testicular and leg pain followed by visible pustules on the shaft of the penis. The use of ACV and valacyclovir was temporally associated with worsening urticaria, so the patient began taking famciclovir (250 mg once or twice daily) to suppress the herpes outbreaks. Nevertheless, at least 1 outbreak occurred during famciclovir prophylaxis. Prednisone treatment had been discontinued. The patient had no risk factors for HIV infection, and the results of several HIV ELISAs were negative, most recently in December 2000. Total lymphocyte, CD4 lymphocyte, and CD8 lymphocyte counts at that time were also normal. Results of other blood tests, including complete blood counts, hepatitis serologic tests, hepatitis C PCR, and chemistry panels were negative or normal. Noncompliance or drug-resistant genital herpes were suspected, and famciclovir (250 mg twice daily) was prescribed, with close follow-up arranged.
A few months after the initial referral, the patient experienced another apparent genital herpes outbreak during famciclovir prophylaxis. A small lesion on the left shaft of the penis was cultured but was negative. Noncompliance with antiviral prophylaxis was suspected, and twice-daily famciclovir was continued. Another visible outbreak occurred 2 months later, and, this time, HSV-2 was isolated in tissue culture. Antiviral susceptibility testing was not performed at that time, and famciclovir was continued. In November 1998, HSV-2 resistant to penciclovir and marginally sensitive to ACV was isolated (table 1). Famciclovir was stopped, and prophylaxis with high-dose valacyclovir (1000 mg twice daily) was initiated. The patient had several herpes outbreaks during the next few months, complaining that the episodes were coming approximately every 2 weeks.
Table 1.
Results of antiviral susceptibility testing on herpes simplex virus (HSV) type 2 isolates, 1998–2002.
| Patient, date | Herpes prophylaxis | Plaque reduction, EC50, µg/mL, | Testing laboratorya |
|||
|---|---|---|---|---|---|---|
| ACV | PCV | CDV | PFA | |||
| Index | ||||||
| Jun 1998 | Famciclovir, 250 mg twice daily | ND | ND | ND | ND | None |
| Nov 1998 | Famciclovir, 250 mg twice daily | 0.9 | 7.1 | ND | ND | Viromed |
| May 1999 | Valaciclovir, 1000 mg twice daily | 37.6 | >50 | ND | ND | Viromed |
| Nov 1999 | Valaciclovir, 1000 mg twice daily | >100 | >100 | 6.0 | 116 | UAB |
| Feb 2000 | Famciclovir, 250 mg twice daily | >100 | >100 | 9.8 | 103 | UAB |
| Mar 2000 | None | 0.4 | 2.0 | 3.0 | 90 | UAB |
| Apr 2000–1 | None | 0.2 | 2.0 | 2.2 | 74 | UAB |
| Apr 2000–2b | None | 0.5 | 3.2 | 7.4 | 110 | UAB |
| Aug 2000 | None | 0.3 | 3.0 | 8.7 | 127 | UAB |
| Sep 2000 | Valaciclovir, 1000 mg twice daily | 0.8 | 3.3 | 17.5 | 117 | UAB |
| Nov 2000–1 | Valaciclovir, 1000 mg twice daily | >100 | ND | ND | ND | UAB |
| Nov 2000–2b | None | 0.7 | ND | ND | ND | UAB |
| Partner | ||||||
| Oct 2001 | None | 0.5 | ND | ND | ND | UAB |
| Jun 2002 | None | 0.5 | ND | ND | ND | UAB |
NOTE. The isolates were cultured from outbreaks that occurred on the shaft of the index patient’s penis and on his partner’s vulva. ACV, acyclovir; CDV, cidofovir; EC50, 50% effective concentration; ND, not done; PCV, penciclovir; PFA, foscarnet.
HSV susceptibility testing was performed at Viromed Laboratories (Minneapolis, MN) or in the laboratory of E. Kern, University of Alabama at Birmingham (UAB).
Isolate from the second genital herpes outbreak during this month.
ACV- and penciclovir-resistant HSV-2 was isolated twice in 1999 and twice again in 2000. Relatively frequent outbreaks continued during this period while the patient was taking prophylactic antivirals. Daily antiviral prophylaxis was discontinued. Topical imiquimod was applied 3 times weekly at the site of the outbreaks, but this appeared to have no effect on the frequency of recurrences. Topical foscarnet was also prescribed in an attempt to limit the duration of the genital herpes outbreaks. However, the topical foscarnet did not have any noticeable effect on the duration, severity, or frequency of the outbreaks in this patient.
Several subsequent genital HSV-2 isolates showed a regained susceptibility to ACV, so high-dose valacyclovir prophylaxis was restarted. The outbreaks continued. Recurrent drug-resistant genital herpes was suspected, supported by the finding of an HSV-2 genital isolate that was, again, ACV resistant. Genital herpes episodes continue to occur every 1–2 months. Antiviral prophylaxis has been permanently discontinued. The patient remains otherwise well.
Transmission to a sex partner
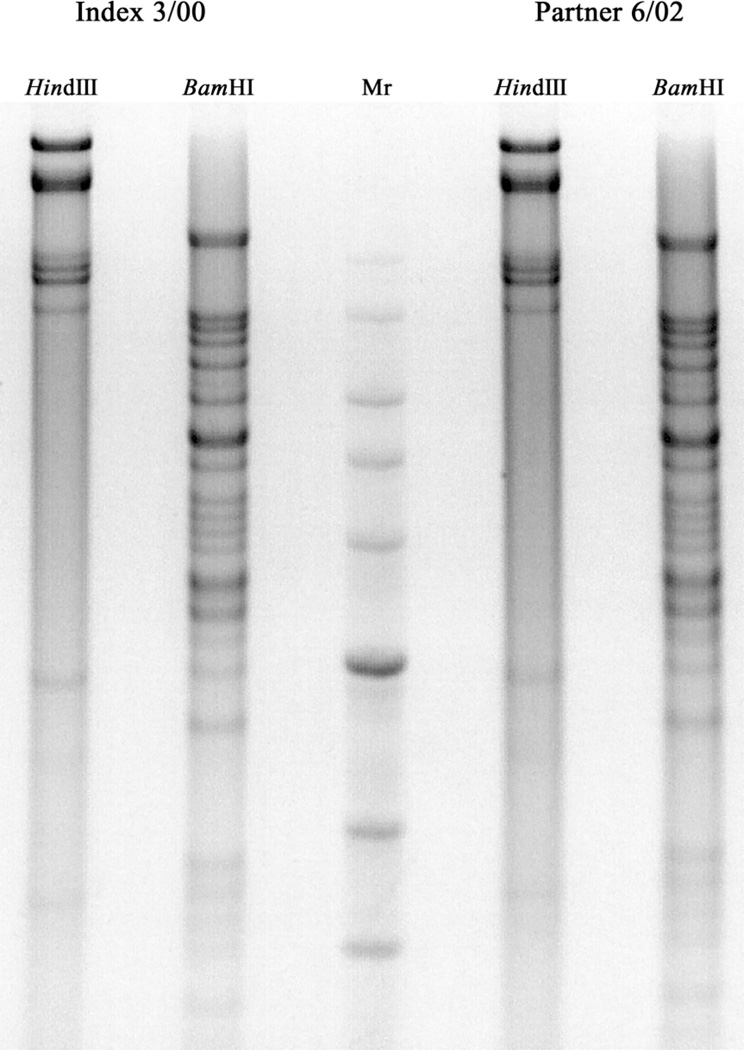
During the past 4 years, the index patient has had several sex partners. One of these already had genital herpes. To our knowledge, the others did not develop symptoms consistent with genital herpes, until 1 partner acquired primary genital herpes in late 2001. This apparent transmission was documented by seroconversion as assessed by gG-based HSV-2 serologic testing and by isolation of HSV-2 from her vulva during the primary infection. Genomic DNA from virus isolates “Index 3/00” and “Partner 6/02” was isolated, and restrictionsite polymorphisms were examined for these strains (figure 1). Restriction enzyme profiles for both BamHI and HindIII digests were indistinguishable between these strains, suggesting that they are closely related, and are consistent with the apparent transmission between these 2 individuals. This woman denies having had any sexual contacts other than the index patient described above. Her HSV-2 isolate was tested and found to be susceptible to all of the antivirals tested (table 1). She describes frequent genital herpes recurrences which, so far, are effectively treated with antiviral medications.
Figure 1.
Restriction enzyme profiles for the herpes simplex virus type 2 isolates Index 3/00 and Partner 6/02. Genomic DNA was isolated from virions and subjected to cleavage with the restriction endonucleases BamHI and HindIII. Restriction fragments were separated on an agarose gel and visualized with ethidium bromide. The image shown was captured on a VersaDoc and inverted with the software Quantity One (version 4.4.1; BioRad). Mr, migration rate.
Molecular analysis
To determine whether a mutation within the HSV-2 tk coding region was present in the 2 ACV-resistant isolates, the tk genes from several isolates were PCR amplified and subcloned into the plasmid vector pGEM-7Zf(+). Purified plasmid DNA was sent to Lone Star Laboratories for sequence analysis. As shown in table 2, both of the index patient’s ACV-resistant isolates contain a C nucleotide insertion at nt 556, resulting in a frameshift at aa 186. A truncated protein of 228 aa is predicted by such a frameshift. This insertion occurred in 1 of 2 homopolymer stretches within the tk coding sequence, previously identified as mutational hotspots within the tk gene [19]. Two ACV-sensitive isolates from the index patient and the single ACV-sensitive isolate from his partner had tk sequences that were identical to the wild-type reference strain except for the single-nucleotide polymorphisms (SNPs) G116A and A232G. Since these 2 SNPs are relatively common in human tk isolates, their presence supports but does not prove transmission of HSV-2 between the index patient and his partner.
Table 2.
Thymidine kinase (tk) mutations identified in herpes simplex virus type 2 (HSV-2) isolates from the index patient and his partner.
| Virus isolate | Acyclovir EC50, µg/mLa |
Polymorphismsb |
tk sequence (no. of amino acids) |
|
|---|---|---|---|---|
| Nucleotide | Amino acid | |||
| Index 2/00 | >100 | +C556, G116A, A232G | FS 186, Gly39Glu, Asn78Asp | Truncated (228) |
| Index 3/00 | 0.6 | G116A, A232G | Gly39Glu, Asn78Asp | Wild type (376) |
| Index 11/00-1 | >100 | +C556, G116A, A232G | FS 186, Gly39Glu, Asn78Asp | Truncated (228) |
| Index 11/00-2 | 0.6 | G116A, A232G | Gly39Glu, Asn78Asp | Wild type (376) |
| Partner 6/02 | 0.5 | G116A, A232G | Gly39Glu, Asn78Asp | Wild type (376) |
NOTE. EC50, 50% effective concentration.
Plaque purified HSV-2 isolates were retested to confirm the susceptibility of the isolate before proceeding with sequencing.
Nucleotide and amino acid changes are in comparison with strain HG52 (GenBank accession no. Z86099). A plus sign (+) indicates an insertion following the nucleotide, and “FS” indicates a frameshift following the amino acid.
DISCUSSION
We have described here an immunocompetent, HIV-negative patient who has experienced frequent ACV- and penciclovir-resistant HSV-2 genital outbreaks. This antiviral resistance occurred in the setting of long-term prednisone treatment and intermittent ACV prophylaxis at suboptimal doses. The drug-resistant and drug-sensitive HSV-2 outbreaks had similar frequencies and appearance. Cessation of antiviral prophylaxis resulted in reversion of this patient’s HSV-2 isolates to ACV sensitivity. Resuming antiviral prophylaxis caused immediate recurrence of the ACV-resistant HSV-2 phenotype. Transmission of a sensitive HSV-2 strain from this patient to a female partner occurred during a period when the index patient was not taking antiviral prophylaxis. These observations demonstrate that an HSV-2 strain that is highly resistant to ACV and penciclovir may develop during prophylactic therapy in an otherwise well, relatively immunocompetent patient.
Antiviral resistance developed during a period of prednisone use and, in retrospect, suboptimal suppressive doses of antivirals. The use of prednisone probably created a transient immunocompromised state that allowed the emergence of drug-resistant HSV-2. However, the drug-resistant HSV-2 phenotype persisted in this patient long after the prednisone treatment was stopped. Typical genital herpes outbreaks continued to occur despite daily prophylaxis with relatively high doses of valacyclovir and famciclovir. There is little doubt that the patient took his medications during this period, because the cessation of antiviral prophylaxis resulted in reversion of HSV-2 to its previous drug-sensitive phenotype. When antiviral prophylaxis was resumed, drug resistance reappeared rapidly, suggesting that the ACV-resistant HSV-2 strain had previously established latency in the sacral ganglia and was capable of frequent reactivation. These findings are similar to those described by Kost et al., in which recurrentACV-resistant HSV-2 infections were due to a tk-altered—not a tk-deleted—strain [15].
This insertion occurred in 1 of 2 homopolymer stretches within the tk coding sequence, previously identified as mutational hotspots within the tk gene [19, 20]. Isolate “89-353-1,” described by Sasadeusz et al. [19], appears to be identical to our index patient’s drug-resistant isolates, with a C insertion at nt 555/556 resulting in a truncated protein of 29 kDa, rather than the normal 40 kDa. The “89-353-1” isolate in the article by Kost et al. [15] came from a patient with AIDS, whereas those in the present study came from an immunocompetent person, albeit one exposed to steroids in the past. These isolates were all apparently capable of reactivating from latency to cause disease. Recently, 2 more ACV-resistant HSV-2 isolates selected in vitro were also found to have this identical mutation [21].
After several years of observation, our index patient did transmit HSV-2 to a female sexual contact. At the time of this transmission, the index patient had discontinued daily antiviral prophylaxis and, fortunately, an ACV-sensitive HSV-2 strain was transmitted. So far, we have not observed the emergence of ACV-resistant HSV-2 in this woman. Our plan was to be cautious and treat outbreaks of HSV-2 with standard 3–5 day courses of valacyclovir in this woman, avoiding daily prophylactic therapy, if possible. However, frequent recurrences eventually forced the institution of daily ACV prophylaxis, so far without the emergence of obvious antiviral resistance. This suggests that the partner was infected only with the wild-type, drug-sensitive strain.
There has been considerable discussion about whether to make ACV available over the counter, without a prescription, in the United States. If ACV were to become more readily available, many persons might treat or prevent outbreaks of their genital herpes with suboptimal doses of the drug. Fear of the emergence of drug-resistant HSV-2 in the immunocompetent population, in part, led the Food and Drug Administration to deny the request for over-the-counter status for ACV in 1998 [22]. The present article brings the total number of published cases of recurrent ACV-resistant HSV-2 genital infection in immunocompetent patients to 3—very few, considering the relatively widespread use of ACV and related antivirals. Concomitant use of steroids (prednisone in our index patient) was associated with the emergence of drug-resistant HSV-2 in the otherwise immunocompetent patient described here and in the patient described in the case report by Swetter et al. [17]. Steroid use is probably common enough in the general population to allow emergence of at least a few drug-resistant HSV-2 strains if ACV were to become available without a prescription. However, it is unlikely that drug-resistant HSV-2 strains would expand throughout the population, unless regular daily antiviral prophylaxis became much more widespread. These observations point to the need to control HSV-2 infections in the population through vaccination and other novel approaches.
Acknowledgments
We thank Mark B. McKeough and Brandt B. Jones, for technical assistance at the Utah site, and Emma Harden, for the performance of the antiviral assays at the University of Alabama at Birmingham.
Potential conflicts of interest: J.D.K. has a current research contract with Novartis and has served on the GlaxoSmithKline speakers’ bureau. S.L.S. is a consultant for and receives research support from both Novartis and Glaxo-SmithKline and is also on the GlaxoSmithKline speaker’s bureau.
Financial support: National Institute of Allergy and Infectious Diseases, National Institutes of Health (contract NO1-AI-85347 to the University of Alabama at Birmingham).
References
- 1.Fleming DT, McQuillan GM, Johnson RE, et al. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337:1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- 2.Cusini M, Ghislanzoni M. The importance of diagnosing genital herpes. J Antimicrob Chemother. 2001;47(Suppl T1):9–16. doi: 10.1093/jac/47.suppl_1.9. [DOI] [PubMed] [Google Scholar]
- 3.Reichman R, Badger G, Mertz G, et al. Treatment of recurrent genital herpes simplex infections with oral acyclovir. JAMA. 1984;251:2103–2107. [PubMed] [Google Scholar]
- 4.Marques AR, Straus SE. Herpes simplex type 2 infections—an update. Dis Mon. 2000;46:325–359. doi: 10.1016/s0011-5029(00)90012-9. [DOI] [PubMed] [Google Scholar]
- 5.Leung DT, Sacks SL. Current recommendations for the treatment of genital herpes. Drugs. 2000;60:1329–1352. doi: 10.2165/00003495-200060060-00007. [DOI] [PubMed] [Google Scholar]
- 6.Field HJ. Persistent herpes simplex virus infection and mechanisms of virus drug resistance. Eur J Clin Microbiol Infect Dis. 1989;8:671–680. doi: 10.1007/BF01963751. [DOI] [PubMed] [Google Scholar]
- 7.Villarreal EC. Current and potential therapies for the treatment of herpesvirus infections. Prog Drug Res. 2001;56:77–120. doi: 10.1007/978-3-0348-8319-1_2. [DOI] [PubMed] [Google Scholar]
- 8.Pottage JC, Jr, Kessler HA. Herpes simplex virus resistance to acyclovir: clinical relevance. Infect Agents Dis. 1995;4:115–124. [PubMed] [Google Scholar]
- 9.Gray JJ, Wreghitt TG, Baglin TP. Susceptibility to acyclovir of herpes simplex virus: emergence of resistance in patients with lymphoid and myeloid neoplasia. J Infect. 1989;19:31–40. doi: 10.1016/s0163-4453(89)94798-1. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Scieux C, Garrait V, et al. Resistant herpes simplex virus type 1 infection: an emerging concern after allogeneic stem cell transplantation. Clin Infect Dis. 2000;31:927–935. doi: 10.1086/314052. [DOI] [PubMed] [Google Scholar]
- 11.Darville JM, Ley BE, Roome AP, Foot AB. Acyclovir-resistant herpes simplex virus infections in a bone marrow transplant population. Bone Marrow Transplant. 1998;22:587–589. doi: 10.1038/sj.bmt.1701392. [DOI] [PubMed] [Google Scholar]
- 12.Safrin S. Treatment of acyclovir-resistant herpes simplex virus infections in patients with AIDS. J Acquir Immune Defic Syndr. 1992;5(Suppl 1):S29–S32. [PubMed] [Google Scholar]
- 13.Marrero M, Alvarez M, Millan JC, Mas Lago P, Soler M, Diaz M. Acyclovir resistant genital herpes virus infection in a patient with AIDS. Acta Virol. 1991;35:86–89. [PubMed] [Google Scholar]
- 14.Epstein JB, Scully C. Herpes simplex virus in immunocompromised patients: growing evidence of drug resistance. Oral Surg Oral Med Oral Pathol. 1991;72:47–50. doi: 10.1016/0030-4220(91)90188-i. [DOI] [PubMed] [Google Scholar]
- 15.Kost RG, Hill EL, Tigges M, Straus SE. Recurrent acyclovir-resistant genital herpes in an immunocompetent patient. N Engl J Med. 1993;329:1777–1782. doi: 10.1056/NEJM199312093292405. [DOI] [PubMed] [Google Scholar]
- 16.Mouly F, Baccard M, Scieux C, et al. Chronic recurrent acyclovir-resistant genital herpes in an immunocompetent patient [letter] Dermatology. 1995;190:177. doi: 10.1159/000246676. [DOI] [PubMed] [Google Scholar]
- 17.Swetter SM, Hill EL, Kern ER, et al. Chronic vulvar ulceration in an immunocompetent woman due to acyclovir-resistant, thymidine kinase-deficient herpes simplex virus. J Infect Dis. 1998;177:543–550. doi: 10.1086/514229. [DOI] [PubMed] [Google Scholar]
- 18.Salmon V, Stanberry L, Overall J. More rapid isolation of HSV in a continuous line of mink lung cells than in Vero or human fibroblast cells. Diagn Microbiol Infect Dis. 1984;2:317–324. doi: 10.1016/0732-8893(84)90063-4. [DOI] [PubMed] [Google Scholar]
- 19.Sasadeusz JJ, Tufaro F, Safrin S, et al. Homopolymer mutational hot spots mediate herpes simplex virus resistance to acyclovir. J Virol. 1997;71:3872–3878. doi: 10.1128/jvi.71.5.3872-3878.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaudreau A, Hill E, Balfour HH, Jr, Erice A, Boivin G. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex viruses from immunocompromised patients. J Infect Dis. 1998;178:297–303. doi: 10.1086/515626. [DOI] [PubMed] [Google Scholar]
- 21.Sarisky RT, Quail MR, Clark PE, et al. Characterization of herpes simplex viruses selected in culture for resistance to penciclovir or acyclovir. J Virol. 2001;75:1761–1769. doi: 10.1128/JVI.75.4.1761-1769.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sande MA, Armstrong D, Corey L, et al. Perspectives on switching oral acyclovir from prescription to over-the-counter status: report of a consensus panel. Clin Infect Dis. 1998;26:659–663. doi: 10.1086/514584. [DOI] [PubMed] [Google Scholar]