Abstract
Uterine decidualization, a key event for successful implantation, is critically controlled by stromal cell proliferation and differentiation. One hallmark event of decidualization is the acquisition of stromal cell polyploidy through terminal differentiation at the anti-mesometrial pole of the implantation site. Hoxa-10, a developmentally regulated homeobox transcription factor, is highly expressed in decidualizing stromal cells, and targeted deletion of Hoxa-10 in mice shows severe decidualization defects, primarily due to reduced stromal cell responsiveness to progesterone. However, the underlying molecular mechanism by which Hoxa-10 regulates this process remains largely unknown. Here, we show that Hoxa-10 deficiency confers diminished core cell cycle activity during stromal cell proliferation without disturbing polyploidy, suggesting that these events depend on local regulators that impact cell cycle machinery. To further address this question, we compared global gene expression profiles in uteri of wild-type and Hoxa-10−/− mice after inducing decidualization. Our studies show two major aspects of decidualization downstream of Hoxa-10. First, Hoxa-10 deficiency results in the aberrant region-specific expression of cyclin-dependent kinase-4 (cdk4) and -6 (cdk6), growth differentiation factor 10 (Gdf10), hepatocyte growth factor (Hgf) and Snail2. Second, Hoxa-10 deficiency compromises natural killer (NK) cell differentiation without altering trafficking of NK precursor cells during decidualization. Collectively, the results provide evidence that Hoxa-10 influences a host of genes and cell functions necessary for propagating normal decidual development during the post-implantation period.
Keywords: Mouse, Uterus, Decidualization, Hoxa-10, Microarray, uNK
Introduction
In mice, attachment of the blastocyst to the uterine luminal epithelium occurs on day 4 of pregnancy (day 1—vaginal plug) between 22:00 and 24:00 h. With the initiation of attachment, endometrial stromal cells surrounding implanting blastocysts undergo decidual transformation (decidualization). The progression of decidualization leads to unique morphological and cellular changes in the uterus (Dey et al., 2004). For example, between days 5 and 6 of pregnancy, stromal cells immediately surrounding the implanting blastocyst cease proliferating and undergo terminal differentiation into decidual cells, forming a zone called the primary decidual zone (PDZ). The PDZ is avascular and epithelioid in nature. However, stromal cells next to the PDZ continue to proliferate and differentiate into decidual cells forming the secondary decidual zone (SDZ). The SDZ is fully developed by day 7 (09:00h) and is characterized by terminally differentiated decidual polyploidy with acquisition of large mono- or bi-nucleated cells (Tan et al., 2002).
Cellular polyploidy is a unique process that requires transition of a mitotic cell cycle to an endoreduplication cycle (endocycle) in which cells undergo repeated rounds of DNA replication without successive cell division (cytokinesis) (Edgar and Orr-Weaver, 2001). Stromal cell polyploidy during decidualization is thought to limit the life span of decidual cells to accommodate the growing embryo (Sachs and Shelesnyak, 1955; Ansell et al., 1974). While this development is occurring at the anti-mesometrial pole, the mesometrial stromal cells also undergo proliferation and differentiation to give rise to smaller, less differentiated decidual cells that are critically important to establish the maternal–fetal interface through appropriate regulation of placental and trophoblast growth (Dey et al., 2004).
The implanting blastocyst stimulates the onset of decidualization in the uterus. This process, however, can also be initiated by experimental manipulation through intraluminal oil infusion in a receptive pseudopregnant uterus (Lim et al., 1997) presumably with similar morphological changes. The region-specific decidual development is associated with ultrastructural changes and differential gene expression (Gu and Gibori, 1995; O’Shea et al., 1983; Welsh and Enders, 1985), although the mechanism(s) involved are not yet fully understood.
Molecular and genetic approaches have identified numerous molecules, such as growth factors, cytokines and their receptors, homeotic genes and transcription factors important to implantation and post-implantation decidualization in mice and primates including humans (Paria et al., 2002; Dey et al., 2004). Among these signaling molecules, Hoxa-10, a member of the developmentally regulated AbdB subclass of Hox gene transcription factors, is critical to normal uterine function. For example, genetic studies in mice have shown that Hoxa-10 is essential for decidualization (Benson et al., 1996). In mice, Hoxa-10 is primarily expressed in the uterine stroma on day 4 of pregnancy (Daikoku et al., 2004). Following implantation on day 5, a dramatic upregulation of this gene occurs in stromal cells undergoing decidual transformation surrounding the implanting blastocyst. On days 6–8, the expression progressively expands to the decidualizing stroma on both mesometrial and anti-mesometrial poles of the implantation site (Satokata et al., 1995; Daikoku et al., 2004). In humans, Hoxa-10 expression in endometrial epithelial and stromal cells increases during the mid-secretory phase, which corresponds to the window of implantation (Taylor et al., 1998; Gui et al., 1999). It is to be noted that in humans stromal cell decidualization occurs during this phase prior to implantation. These observations suggest that Hoxa-10 is also important for implantation and decidualization in humans. Studies have also shown that progesterone (P4) is a primary regulator of Hoxa10 expression in mice and humans (Ma et al., 1998; Taylor et al., 1998; Lim et al., 1999; Daikoku et al., 2004).
Previous studies indicated that a small percentage (~40%) of Hoxa-10 null mice can initiate the implantation reaction (Benson et al., 1996), suggesting uterine that defects in these mice do not completely antagonize blastocyst attachment with the luminal epithelium. This is consistent with the finding of normal expression of several implantation related genes, such as leukemia inhibitory factor (LIF), heparin-binding EGF (HB-EGF), amphiregulin and cyclooxygenase-1 (COX-1) in the epithelium of Hoxa10−/− mice (Lim et al., 1999). Furthermore, while stromal cell proliferation is depressed in Hoxa-10 null uteri in response to P4 and estradiol-17β (E2), epithelial cell proliferation is normal in response to E2.
We have previously shown that the expression of cyclin D3, a G1 phase cell cyclin involved in cell proliferation, remains downregulated in Hoxa-10−/− uteri after application of a deciduogenic stimulus (Das et al., 1999). These results suggest that impaired stromal cell proliferation is a potential cause for the defective decidual response observed in Hoxa-10 null mice. Furthermore, Hoxa-10 as a transcriptional factor can mediate P4-dependent responses in the stroma (Lim et al., 1999). In this regard, a recent microarray study comparing P4 responsiveness between wild-type and Hoxa-10 null uteri found that two cell cycle inhibitory genes p15 and p17 were upregulated in Hoxa-10 null uteri (Yao et al., 2003). Our recent report also shows enhanced expression of the negative cell cycle regulatory cyclins cyclin G1 and G2 during implantation in Hoxa-10 null uteri (Yue et al., 2005). These results suggest that aberrant regulation of cell cycle activity in Hoxa-10−/− mice imposes inhibitory functions on stromal cell proliferation.
To determine downstream targets of Hoxa-10 signaling during decidualization, we examined global gene expression profiles in uteri of wild-type and Hoxa-10−/− mice following a decidualizing stimulus. Our results show numerous plausible gene targets during anti-mesometrial decidualization, organized into six functional categories, including growth regulation, tissue remodeling, immunomodulation, metabolism, membrane transport and signal transduction. Our analysis shows that Hoxa-10 plays an important role in establishing region-specific decidual development and differentiation of uterine NK cells at the implantation site.
Materials and methods
Animals and tissue preparation
Wild-type and Hoxa-10−/− mice on a 129/SvJ/C57BL6 background were housed in the animal care facility at Vanderbilt University Medical Center in accordance with the National Institutes of Health (NIH) and institutional guidelines for laboratory animals. Hoxa-10−/− mice were generated as previously described (Satokata et al., 1995) and originally provided by Richard L. Maas (Harvard Medical School). PCR analysis of tail genomic DNA was used to determine genotypes of mice. Adult females (8–10 weeks of age) were mated with fertile or vasectomized males of the same strain to induce pregnancy or pseudopregnancy (day 1—vaginal plug), respectively. Mice were sacrificed between 0830 and 0900 h on each day of pregnancy or pseudopregnancy. Implantation sites were identified by localized increased uterine vascular permeability at the site of blastocysts through intravenous (iv) injection of Chicago Blue B dye solution (1% in saline) (Das et al., 1994). To stimulate experimentally induced decidualization (deciduoma), sesame oil (25 μl) was infused intraluminally in one uterine horn on day 4 of pseudopregnancy, while the contralateral horn without infusion served as control. The decidual cell reaction was confirmed by recording uterine weight and histological examination of uterine sections on days 5 and 7 of pseudopregnancy. To perform microarray studies, deciduomal tissues were collected and separated of myometrium. Tissues were rapidly flash frozen and kept at −80°C for subsequent analysis or fixed in 10% formalin for paraffin blocks.
Isolation of RNAs
Decidual tissues were pooled, and total RNA was extracted in TRIzol reagent (Life Technologies, Gaithersburg, MD) according to the manufacturer’s recommendations. DNA-free RNA was obtained by treatment of total RNA (50 μg) with RNase-free DNase I (Sigma, St. Louis, MO) in the presence of placental RNase inhibitor (BRL, Gaithersburg, MD) for 30 min at 37°C. After phenol:chloroform (3:1) extraction, RNA was precipitated with 0.3 M sodium acetate and ethanol and the RNA pellet dissolved in diethyl pyrocarbonate-treated water.
Microarray hybridization and statistical analysis
Microarray hybridization and analysis were conducted according to Affymetrix’s recommended protocols with the help of Vanderbilt Micro-array Shared Resource Center, Vanderbilt University, Nashville, TN, USA. In brief, total RNA was used to prepare cDNAs, and biotin-labeled cRNAs were generated using RNA transcript labeling kit (Affymetrix, Inc.). Each preparation of cRNAs was used to hybridize GeneChip Mouse Genome 430 2.0 Array (Affymetrix, Inc.). We followed a similar procedure as previously described by us (Reese et al., 2001). In brief, triplicate hybridizations were performed using three independent RNA samples from each group. A single mean expression level for each gene along with a detection P value (indicating reliable detection) was derived using Affymetrix Microarray Suite version 5.0 software. The scanned image for each array was corrected for significant artifacts and bacterial gene contamination, background and noise with a P value below 0.05. Differences in fluorescence intensity were analyzed by multiple decision matrices to determine the presence or absence of gene expression and to derive an average difference score representing the relative levels of gene expression. Values for the mean and standard deviation of three replicate average difference scores were calculated for each gene. A Student’s t test was performed to identify genes differentially expressed between the groups (P < 0.05). Fold change in gene expression between groups was calculated from the mean average difference scores. The identification of differentially expressed genes for analyzing multiple samples in each group was carried out through Gene Traffic software based algorithmic comparison. Thus, paired comparisons of three replicate hybridizations resulted in nine possible outcomes for analysis. Transcripts with statistically significant difference (Student’s t test) that were also differentially expressed with a covariance (<30%) of the nine pair-wise comparisons (increase or decrease) were considered ≥1.0 for at least 2-fold change).
Reverse transcription-PCR
Reverse transcription (RT) and comparative PCR reactions have previously been described by us (Daikoku et al., 2005). The abundance of mRNAs for each gene expression was corrected against β-actin.
Hybridization probes
Mouse-specific cDNA clones (in pCRIITOPO vector) were generated by RT-PCR. The authenticity for each of these clones was confirmed by nucleotide sequencing. For in situ hybridization, sense and antisense 35S-labeled cRNA probes were generated. The probes had specific activities of 2 × 109 dpm/μg.
In situ hybridization
Frozen sections (10 μm) were hybridized with 35S-labeled cRNA probes as described previously (Das et al., 1994). Sections hybridized with sense probes served as negative controls and showed no positive signals.
Antibodies and other reagents
Anti-perforin rat monoclonal antibody was purchased from Abcam Inc.(Cambridge, MA). The affinity purified goat or rabbit polyclonal antibodies specific to granzyme A, CD45, cyclin D3, cyclin A, cyclin B, cyclin E, cdk1, cdk2, cdk4 and cdk6 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Rabbit polyclonal antibody to p21 was purchased from Oncogene Research Products (Cambridge, MA). A mouse monoclonal antibody for p27 was obtained from Zymed Laboratories Inc. (San Francisco, CA). Rat monoclonal anti-CD90.2 was purchased from BD Pharmingen (San Diego, CA). All of these antibodies are mouse-specific and show no cross-reactivity with related proteins. All secondary antibodies were purchased from Zymed laboratories Inc. (San Francisco, CA). Biotin-conjugated lectin from Dolichos biflorus agglutinin (DBA) was purchased from Sigma-Aldrich Co. (St. Louis, MO).
Immunohistochemical staining and dual immunofluorescence
Immunohistochemical staining and dual immunofluorescence were conducted as previously described by us (Tan et al., 2002, 2004). Immunohisto-chemical color reaction was developed through utilization of Zymed-Histostain-SP kit (Zymed Laboratories, San Francisco, CA). For dual immunofluorescence studies, FITC conjugated donkey anti-goat or TRITC conjugated donkey anti-rat secondary antibodies were directly visualized by fluorescence microscopy using a NIKON TS100 inverted microscope with a Q Imaging digital camera.
Western blot analysis
Western blotting was performed as previously described by us (Tan et al., 2002). In brief, tissue proteins (50 μg) were run on 10% SDS-PAGE gels under reducing conditions and transferred onto Immobilon membranes. Membranes were incubated with primary antibody containing 5% milk in TBST overnight at 4°C, followed by incubation with appropriate secondary antibody coupled to horseradish peroxidase (1:5000) in 5% milk for 1 h. Positive signals were detected by ECL kit (Amersham, Arlington Heights, IL).
Results
Aberrant cell-specific distribution of cyclin-dependent kinases-4 and -6 during anti-mesometrial decidualization in Hoxa-10 null mice
Hoxa-10 null uteri lack growth potential for cell proliferation and/or differentiation within the stromal compartment (Lim et al., 1999, Daikoku et al., 2004, 2005). Consistent with these observations, we previously showed that expression of cyclin D3, a G1-phase promoter of cell proliferation, is severely compromised in Hoxa-10 null uteri after application of a deciduogenic stimulus (Das et al., 1999). While increased stromal cell proliferation is considered an initiator of decidualization, no information is available regarding the regulation of cell cycle machinery that gives rise to polyploidy. We thus examined the regulation of cell cycle core components in the Hoxa-10 null uteri during decidualization in comparison to our previous information from wild-type uteri (Tan et al., 2002). Experimental decidualization was induced and analyzed on days 5 and 7 of pseudopregnancy in both wild-type (WT) and Hoxa-10−/− mice. The cell cycle phase-specific genes including cyclins A, B, D3 and E, cyclin-dependent kinases cdk1, cdk2, ckd4 and cdk6, and cyclin-dependent kinase inhibitors p21 and p27 were assessed in wild-type and Hoxa-10−/− uteri by Western blotting (Fig. 1A). We found that cyclin A, cdk1, cdk2 and p21 are downregulated in Hoxa-10−/− uteri on day 5 of pseudopregnancy, supporting proliferation defects. These results are consistent with our earlier report indicating cyclin D3 downregulation in Hoxa-10 null uteri (Das et al., 1999). However, no cell cycle regulators showed detectable changes in Hoxa-10 null uteri when compared to wild-type on day 7. Furthermore, analysis of cell-specific expression of these regulators on day 7 did not reveal any differences between wild-type and Hoxa-10−/− mice (data not shown). In contrast, the region-specific expression pattern of cdk4 in wild-type mice at the mesometrial pole and of cdk6 at the anti-mesometrial pole is completely lost in Hoxa-10 null deciduoma on day 7 (Fig. 1B). Immunohistochemical analysis of these proteins confirmed these patterns in Hoxa-10 null mice (Fig. 1C). It is also interesting to note that nuclear staining of cdk4 is detected in Hoxa-10 null mice as opposed to cytoplasmic staining in wild-type littermates. Overall, these results suggest that decidualization in Hoxa-10 null mice primarily depends on locally derived factors that impact core cell cycle machinery.
Fig. 1.
Analysis of core cell cycle regulators during decidualization between wild-type and Hoxa-10−/− mice. (A) Western blot analysis of various G1/S and G2/M phase-specific cell cycle regulatory molecules in deciduoma tissue extracts on d7 pseudopregnancy of wild-type mice. Immunoblotting with the primary antibodies was performed as described under Materials and methods. A constitutive protein actin was detected to analyze the levels of equal loading. (B) In situ hybridization of cdk4 and cdk6 during experimentally induced decidualization on d7 pseudopregnancy in Hoxa-10 mutants as compared to wild-type (WT) littermates. Hybridized sections were post-stained lightly with hematoxylin and eosin. Dark-field photomicrographs of representative uterine cross-sections are shown. M, mesometrial pole; AM, anti-mesometrial pole. Sections hybridized with corresponding sense probes did not show any positive signals (data not shown). Scale bars, 100 μm. Note: The region-specific expression of cdk4 or cdk6 in corresponding mesometrial or anti-mesometrial location as indicated by arrows in the wild-type mice is lost in Hoxa-10 null mice. (C) Immunohistochemical staining of cdk4 and cdk6 during decidualization at the site of embryo implantation on d7 in WT and Hoxa-10−/− mice. Red staining indicates the localization of immunoreactive proteins. No immunostaining was noted when similar sections were incubated with the primary antibody pre-neutralized with excess antigen (data not shown). M, mesometrial pole; AM, anti-mesometrial pole. Scale bars, 33.3 μm.
Gene expression profiling detects differentially regulated genes in Hoxa-10−/− mice during decidualization
Because Hox genes act locally to regulate effector genes during developmental processes (Gehring, 1987; Capecchi, 1997), we sought to identify Hoxa-10 downstream signaling gene networks to gain further insights into Hoxa-10-dependent regulation of decidualization. We performed microarray analysis utilizing a GeneChip (Affymetrix Inc.), which comprises 39,000 transcripts and variants, including over 34,000 well-characterized mouse-specific genes. We were specifically interested in anti-mesometrial decidualization including terminal differentiation of stromal cell to polyploidy, an event that is maximal on day 7 of pregnancy in mice (Tan et al., 2002). We collected three independent pools of artificial deciduoma from wild-type and Hoxa-10−/− mice on day 7 of pseudopregnancy (Fig. 2). Total RNA was isolated and subjected to gene expression profiling to identify differential gene expression between Hoxa-10−/− and littermate wild-type mice. We identified 130 genes that were either down- or upregulated (Supplementary Tables 1 and 2) by at least 2-fold change (mean log2 ratio > 1.0) in Hoxa-10−/− mutants when compared to wild-type mice. We also generated functional categories of differentially expressed genes to evaluate biological functions affected by Hoxa-10 deficiency during decidualization. This was achieved based on their described functions in the literature, and genes with multiple functions were classified to a single category. Our analysis revealed six functional categories of genes affected by Hoxa-10 deficiency: cell growth, tissue remodeling, immunomodulation, membrane transport, metabolism and signal transduction. Cell growth comprised the largest functional category (28/130), including genes involved in cell proliferation, differentiation and apoptosis. The next largest group of genes (22/130) consisted of those associated with tissue remodeling. The other groups were primarily represented by a range of 5–7% genes. All of these functional aspects are implicated in decidualization (Dey et al., 2004). It is also interesting to note that a largest category represented by total of ~29% genes is primarily unknown or ESTs.
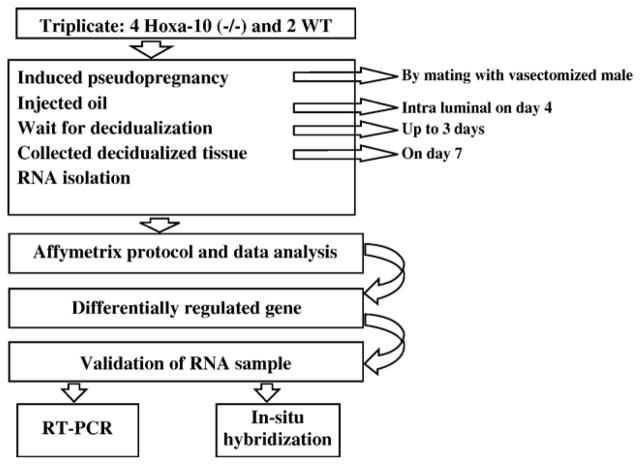
Fig. 2.
Experimental strategy for gene expression profiling and evaluation of differential gene expression in the Hoxa-10−/− mice during decidualization.
We arbitrarily chose 13 genes to confirm their up- or downregulation by RT-PCR. Three independent isolates of total RNA from wild-type and Hoxa-10−/− mice were analyzed by comparative RT-PCR at different PCR cycle numbers for linear amplification of each gene (Fig. 3A). The primers used for RT-PCR and the sizes of PCR products are indicated in Supplementary Table 3. The RT-PCR derived products were confirmed by cloning and sequencing. Band intensities were measured by densitometric analysis and corrected against β-actin (Fig. 3B). Consistent with our microarray data, expression of Snail2, Hgf, Timp3, Granzyme A, Granzyme C, Granzyme E, Granzyme G and Perforin was downregulated, whereas that of Igfbp2, MMP7, Gdf10, Tgfbi and UPA was upregulated in Hoxa-10 mutants when compared with wild-type littermates. These results suggest that our experimental strategy successfully identified differentially regulated genes during decidualization in Hoxa-10−/− mice.
Fig. 3.
RT-PCR analysis of gene expression in deciduoma RNA samples of Hoxa-10−/− and wild-type (WT) mice. (A) Comparative RT-PCR. Total RNA was extracted and subjected to RT-PCR at indicated PCR cycle numbers to achieve linear amplification for genes of interest. Amplified DNA bands were visualized by ethidium bromide staining. (B) Relative levels of gene expression. The band intensities shown in Fig. 2A were measured by densitometric analysis, and the relative levels of mRNAs for gene specific expression were determined after correction with that of β-actin.
Microarray-derived genes are aberrantly expressed during decidualization in Hoxa-10−/− mice
Because decidualization generally progresses with region-specific gene expression, we examined whether Hoxa-10 deficiency affects expression patterns of differentially regulated genes within the decidual bed. We used in situ hybridization to compare differential gene expression in deciduoma of wild-type and Hoxa-10−/− mice on day 7. We examined four downregulated genes (Hgf, Snail2, Granzyme A and Granzyme C) and two upregulated genes (Gdf10 and MMP7), since these genes have previously been implicated in cell proliferation, differentiation, immunomodulation and tissue remodeling (Galimi et al., 1994; Cunningham et al., 1995; Nagase and Okada, 1996; Takai et al., 1997; Nakayama et al., 1998; Dosiou and Giudice, 2005), which are key events of decidualization. In wild-type uteri, Hgf expression is localized to a population of cells at the mesometrial decidual bed, in contrast to Hoxa-10−/− uteri, with signals present in a few layers of subluminal stromal cells at the anti-mesometrial pole (Fig. 4A). With respect to Snail2, the expression is primarily detected at the mesometrial decidualizing stroma and in undifferentiated stoma below the muscle layer at the anti-mesometrial pole (Fig. 4A). In contrast, the expression of Snail2 in Hoxa-10−/− mice is distributed primarily in subluminal decidualizing stromal cells. With respect to Granzymes A and C, expression is detected in a subset of decidual cells at the mesometrial pole in wild-type uteri, but is undetectable in Hoxa-10−/− uteri (Fig. 4A).
Fig. 4.
In situ hybridization of (A) downregulated genes Hgf, Snail2, Granzyme A and Granzyme C and, (B) upregulated genes Gdf10 and MMP7 during experimentally induced decidualization on day 7 (d7) of pseudopregnancy in Hoxa-10 mutants as compared to wild-type (WT) littermates. Sections were hybridized with 35S-labeled sense or antisense riboprobes for 4 h at 45°C. RNase A resistant hybrids were detected after 5–7 days of autoradiography using Kodak NTB-2 liquid emulsion. Sections were post-stained lightly with hematoxylin and eosin. Dark-field photomicrographs of representative uterine cross-sections are shown. M, mesometrial pole; AM, anti-mesometrial pole. Sections hybridized with corresponding sense probes did not show any positive signals (data not shown). Scale bars, 100 μm.
Gdf10 and MMP7 expression is undetectable in wild-type decidual tissues but is distinctly present in subluminal decidualizing stromal cells in Hoxa-10−/− mice. Overall, these results suggest that these genes show aberrant cell-specific expression in Hoxa-10 mutant mice during progression of decidualization.
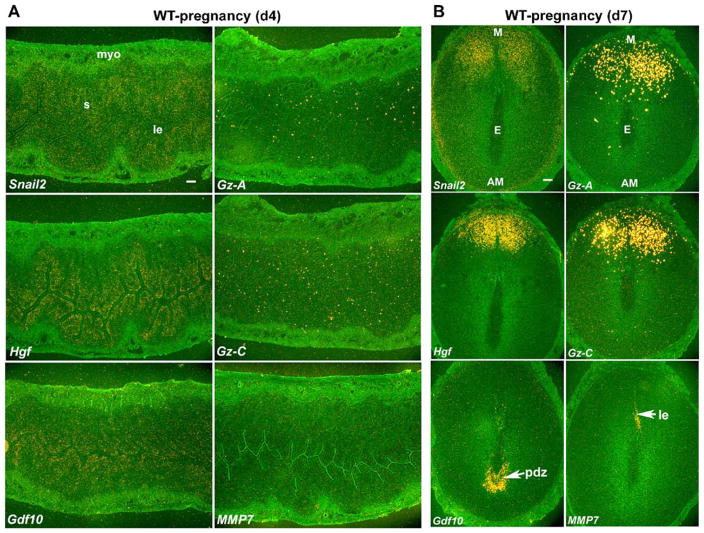
Micro-array derived genes are spatiotemporally regulated in periimplantation uteri of wild-type mice
To determine whether expression of the selected genes undergoes cell-specific regulation during early pregnancy, we analyzed uterine tissues on days 4 and 7 of pregnancy by in situ hybridization. On day 4 of pregnancy, the expression of Snail2, Hgf and Gdf10 is primarily localized in the stroma but with differing intensity (Fig. 5A). For example, Hgf and Gdf10 are primarily distributed in subluminal cells, as opposed to the more widespread localization of Snail2. The expression of Granzymes A (Gz-A) and C (Gz-C) is localized in a discrete population of cells within the endometrial stroma; MMP-7 expression is undetectable on this day (Fig. 5A).
Fig. 5.
In situ hybridization of Snail2, Hgf, Gdf10, Granzyme A (Gz-A) and Granzyme C (Gz-C) and MMP7 in uteri of wild-type mice during the periimplantation period. Hybridized sections were post-stained lightly with hematoxylin and eosin. Dark-field photomicrographs of representative longitudinal sections on day 4 (d4) and cross-sections on day 7 (d7) are shown. le, luminal epithelium; s, stroma; myo, myometrium; M, mesometrial pole; AM, anti-mesometrial pole; E, embryo; pdz, primary decidual zone. Sections hybridized with corresponding sense probes did not show any positive signals (data not shown). Scale bars, 100 μm.
With the progression of decidualization on day 7 (Fig. 5B), the pattern of expression is region-specific. For example, Snail2 is predominantly expressed at the mesometrial decidual bed together with submyometrial undifferentiated stromal accumulation at the anti-mesometrial pole. In contrast, Hgf is exclusively localized at the mesometrial decidualizing stroma. In the case of Gz-A and Gz-C, expression is limited to a subset of decidual cells at the mesometrial pole. Gdf10 expression is unique, present in a few layers of decidualizing stromal cells in the PDZ. Low levels of MMP-7 are detected primarily in the luminal epithelium. Analysis of expression for these uterine genes in the interimplantation regions on this day of pregnancy did not reveal any specific signals (data not shown). Collectively, the results show that expression of these genes during normal and artificially induced decidualization follows similar patterns with the exception of Gdf10 whose expression is influenced by embryonic signals.
Region-specific decidual expression of Gdf10 and Hgf is aberrant in Hoxa-10 null mice
Gdf10 and Hgf are uniquely expressed in the PDZ and mesometrial decidualizing stroma on day 7 of pregnancy in wild-type mice (Fig. 5B). We wanted to see whether Hoxa-10 deficiency alters their expression patterns. Our in situ hybridization analysis, indeed, shows that these genes are aberrantly expressed at the site of blastocysts (IS) on day 7 of pregnancy in Hoxa-10 null mice (Fig. 6). Furthermore, Gdf10 expression at the interimplantation region (IIS) had enhanced expression in the subluminal stromal cells in Hoxa-10−/− uteri as compared to wild-type uteri (Fig. 6). Overall, these results suggest that Hoxa-10 is necessary for region-specific decidual expression of genes at the site of embryo implantation.
Fig. 6.
In situ hybridization of Hgf and Gdf10 at the embryo implantation sites (IS) and interimplantation regions (IIS) on day 7 (d7) of pregnancy in Hoxa-10 mutants as compared to wild-type (WT) littermates. Hybridized sections were post-stained lightly with hematoxylin and eosin. Dark-field photomicrographs of representative uterine cross-sections are shown. M, mesometrial pole; AM, anti-mesometrial pole; E, embryo; pdz, primary decidual zone; le, luminal epithelium; s, stroma. Sections hybridized with corresponding sense probes did not show any positive signals (data not shown). Scale bars, 100 μm.
Uterine NK cell differentiation is specifically affected in Hoxa-10 null mice during decidualization
We next examined whether aberrant gene expression affected by Hoxa-10 deficiency is reflected in protein distribution patterns. Western blot analysis of perforin and Gz-A in deciduoma on day 7 shows that accumulation of both proteins in Hoxa-10 null mice is compromised (Fig. 7A). Because our microarray results showed suppression of immunomodulatory genes such as Granzymes A, C, E and G, and perforin and because these genes are implicated in uterine NK cell function (Parr et al., 1990; Zheng et al., 1991; Allen and Nilsen-Hamilton, 1998), we sought to determine the status of uterine NK cells during decidualization in the absence of Hoxa-10. Terminally differentiated uterine NK cells can be recognized by DBA-lectin reactivity in decidual cells (Paffaro et al., 2003). We therefore used double immunofluorescence to examine co-localization of these gene products with DBA. The results show that both Granzyme A and perforin are co-localized with DBA to a subset of cells at the mesometrial pole of deciduoma on day 7 in wild-type mice (Fig. 7B). In contrast, Hoxa-10 null mice exhibit dramatic suppression of this co-localized expression (Fig. 7B). Analysis of these markers at the implantation site (IS) on day 7 in wild-type and Hoxa-10 null mice showed similar results (data not shown). Overall, these results suggest that uterine NK cell differentiation is affected in Hoxa-10 null mice during decidualization.
Fig. 7.
Analysis of uterine NK cells during decidualization. (A) Western blot analysis of perforin and Granzyme A (Gz-A) proteins in separated deciduoma tissue extracts collected on d7 pseudopregnancy of wild-type (WT) and Hoxa-10−/− mice. Tissue proteins (50 μg) were fractionated by 10% SDS-PAGE gel. Immunoblotting was performed using primary antibodies specific to perforin, Granzyme A and actin. The primary antibodies and the protocol used for immunoblotting are described in Materials and methods. (B) Dual immunofluorescence staining for mutual localization of DBA/perforin/Gz-A in formalin-fixed paraffin-embedded deciduoma tissue sections of WT and Hoxa-10−/− mice on d7 pseudopregnancy. Sections were stained with TRITC (red) or FITC (green) tagged secondary antibodies as described in Materials and methods. Superimposed (merge) images showing yellow-colored cells represent expression of both. The analysis of distribution for DBA and perforin is exhibited in a similar co-localized manner (data not shown). M, mesometrial pole; le, luminal epithelium. Scale bar, 100 μm.
Previous studies showed that CD45 or CD-90 positive precursor NK cells undergo proliferation and differentiation in response to uterine decidualization (Croy et al., 1996; Ain et al., 2004). We sought to examine whether a decrease in differentiated uterine NK cells in Hoxa-10 null uteri is due to the lack of NK cell precursors in the endometrium during decidualization. Our analysis shows that CD45 (Fig. 8)- or CD90 (data not shown)-positive cells are abundant in the mesometrial pole of the deciduoma on day 7 of pseudopregnancy in wild-type and Hoxa-10 null mice, suggesting that precursor uterine NK cells are not the limiting factor for differentiation of NK cells in Hoxa-10 null mice. Co-localization of cyclin D3 and p21 with CD45 shows that precursor uterine NK cells express p21, not cyclin D3. However, no differences between wild-type and mutant mice were observed (data not shown), indicating a different mechanism for uterine NK cell differentiation.
Fig. 8.

Analysis of NK precursor cells in uterine decidual bed. Immunofluorescence staining of CD45 in formalin-fixed paraffin-embedded deciduoma tissue sections of WT and Hoxa-10−/− mice on d7 of pseudopregnancy. M, mesometrial pole. Scale bar, 100 μm.
Discussion
Previous studies have shown that Hox gene products are involved in pattern formation of segmented structures during development and perhaps have roles in regeneration and repair in adult biology (Kessel and Gruss, 1991; Simon and Tabin, 1993). In addition, Hoxa-10 deficiency has been shown to be critical for patterning of adult reproductive tracts (Benson et al., 1996). Consistent with this notion, the present study provides evidence that in reproduction, uterine development for decidualization is severely compromised in Hoxa-10 null mice. Distribution of the expression of several distinct molecular markers within the decidual bed is significantly affected in Hoxa-10 null uteri, suggesting a developmental anomaly for decidual patterning at the implantation site in these mice.
Uterine remodeling or transformation at the site of implantation is critical to pregnancy outcome. Uterine NK cells are believed to function to appropriately regulate and establish the maternal–fetal barrier at the implantation site (Croy et al., 1996; King, 2000; Liu and Young, 2001; Moffett-King, 2002; Dosiou and Giudice, 2005). In this regard, our present study provides novel evidence that Hoxa-10 deficiency causes dysregulation of uterine NK cell differentiation.
The dynamic nature of uterine stromal cell proliferation and differentiation during decidualization provides an attractive model to study cell growth and development. Our identification of a large number of gene candidates as downstream molecular targets of Hoxa-10 will generate more information concerning these processes.
We have previously shown that the D-type cyclin-dependent kinases cdk4 and cdk6 are differentially modulated at the implantation site with the onset of decidualization (Tan et al., 2002). Studies have suggested that cdk4 plays a role in proliferation and differentiation for the development of the mesometrial decidual bed, while cdk6 associates with stromal cell polyploidy, an event that occurs at the anti-mesometrial pole. The observed disturbances for regional distribution of expression for these two kinases in Hoxa-10 null uteri during decidualization (Fig. 1) suggest that Hoxa-10 is an important regulator of region-specific development of uterine decidua during implantation. Furthermore, these results imply that decidualization at these two poles is under the control of different growth regulatory factors.
Previous studies have shown that Gdf10, Hgf and Snail2 are primarily involved in cell proliferation and differentiation (Galimi et al., 1994; Cunningham et al., 1995; Takai et al., 1997; Nakayama et al., 1998). Our present observation of region-specific distribution of these genes within the decidual bed at the site of implantation in wild-type mice (Fig. 5B) suggests that these regulators possess distinct growth regulatory properties with the onset of decidualization. For example, Gdf10 may be involved in the developmental regulation of PDZ, while Hgf could participate in the generation of the mesometrial decidual bed. In contrast, Snail2 appears to control growth regulation of mesometrial decidual cells and anti-mesometrial undifferentiated stroma. It is again interesting to note that all these genes are specifically dysregulated with Hoxa-10 deficiency during decidualization (Figs. 4 and 6), implicating Hoxa-10 participates specifying regional decidualization at the site of implantation.
Previous studies have shown that Hgf regulates multiple processes including trophoblast cell proliferation and endome-trial invasion during early pregnancy (Patel et al., 2000; Dokras et al., 2001; Lala and Chakraborty, 2003), uterine epithelial cell morphogenesis (Sugawara et al., 1997; Spencer and Bazer, 2002), endothelial cell angiogenesis (Bussolino et al., 1992) and endometriosis (Yoshida et al., 2004). Genetic deletion of Hgf in mice reveals defects associated with the development of placenta, liver and muscle (Bladt et al., 1995; Schmidt et al., 1995; Uehara et al., 1995), resulting in death in utero. A role for Snail2 has not been proposed in uterine biology. Snail2 belongs to the Snail/Slug family of zinc finger transcriptional repressors. This family was first identified as regulators of cell migration during development (Nieto et al., 1994). Interestingly, Snail is implicated in enhancing transcription of genes important for maintaining cellular diploid status but suppressing transcription of genes involved in polyploidy (Ballester et al., 2001; Fuse et al., 1994; Nakayama et al., 1998). Signaling pathways including TGF-β, BMP, FGF and Wnt are implicated in the induction of Snail during developmental progression (Hemavathy et al., 2000). Interestingly, these same pathways are also implicated in decidualization (reviewed in Dey et al., 2004). Mice with genetic deletion of Snail2 are viable with growth retardation (Jiang et al., 1998) and female subfertility (Perez-Losada et al., 2002). In addition, previous studies suggest that Snail2 is involved in regulation of the cell survival signaling pathway (Inoue et al., 2002). Gdf10, a member of the transforming growth factor-β superfamily, primarily is involved in cellular differentiation processes (Cunningham et al., 1995). Although previous studies demonstrate that this gene is regulated in the mouse uterus during the cycle and early pregnancy, genetic deletion does not reveal detectable fertility defects (Zhao et al., 1999).
Many proteolytic enzymes participate in tissue remodeling through regulation of degradation of extracellular matrix (ECM) components at the site of implantation. Among them, matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) are thought to play central roles (Nagase and Okada, 1996). It has been suggested that a balance between TIMP-3 and MMP-9 regulates trophoblast invasion at the implantation site in mice (Das et al., 1997). Our gene array results show MMP-7 upregulation and TIMP-3 downregulation during decidualization in Hoxa-10 null mice (Fig. 3), implicating that Hoxa-10 may regulate these targets during trophoblast invasion. In addition, a recent report demonstrates molecular association of MMP-7 with HB-EGF precursor and subsequent regulation of ErbB4 mediated signaling for uterine tissue remodeling (Yu et al., 2002). In this regard, HB-EGF is considered important in implantation in mice (Das et al., 1994).
Previous studies have shown that Hoxa-10 deficiency alters immunological responses primarily through hyperproliferation of T lymphocytes in the mouse uterine stroma in response to progesterone (Yao et al., 2003). Uterine NK (uNK) cells are the most common lymphocytes found in the decidualizing uterus during the first half of pregnancy in rodents and humans (Croy et al., 1996; King, 2000; Liu and Young, 2001; Moffett-King, 2002; Dosiou and Giudice, 2005). Our present observation of Hoxa-10-dependent alteration of uNK cell function during decidualization is interesting. These results are clinically relevant, since dysregulated immune function is implicated in recurrent pregnancy loss in humans (Dosiou and Giudice, 2005).
After recruitment from the circulation, uNK cells rapidly proliferate and differentiate into a class of terminally differentiated cells, eventually acquiring cytoplasmic granules for various cytolytic mediators, including perforin, serine proteases like Granzymes, phosphatases, mucin-1 and IFN-γ. Successful differentiation of uNK cells is believed to play important roles in nurturing the embryo, normal placentation and uterine tissue remodeling. In the present study, we show that Granzymes and perforin, representing differentiated uNK cells, are down-regulated during decidualization in Hoxa-10 null mice. While precursor uNK cells are abundantly detected in Hoxa-10 null uteri, these cells lack differentiation potential. In this regard, Hgf or Snail2 could be interesting to examine this possibility. In conclusion, the present study adds novel information about Hoxa-10 downstream targets during decidualization and will help to better define how Hoxa-10 signaling regulates developmental aspects of implantation.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants (ES07814, HD37830, HD12304 and HD33994).
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ydbio.2005.11.016.
References
- Ain R, Trinh ML, Soares MJ. Interleukin-11 signaling is required for the differentiation of natural killer cells at the maternal–fetal interface. Dev Dyn. 2004;231:700–708. doi: 10.1002/dvdy.20183. [DOI] [PubMed] [Google Scholar]
- Allen MP, Nilsen-Hamilton M. Granzymes D, E, F, and G are regulated through pregnancy and by IL-2 and IL-15 in granulated metrial gland cells. J Immunol. 1998;161:2772–2779. [PubMed] [Google Scholar]
- Ansell JD, Barlow PW, McLaren A. Binucleate and polyploid cells in the decidua of the mouse. J Embryol Exp Morphol. 1974;31:223–227. [PubMed] [Google Scholar]
- Ballester A, Frampton J, Vilaboa N, Cales C. Heterologous expression of the transcriptional regulator escargot inhibits megakaryocytic endomitosis. J Biol Chem. 2001;276:43413–43418. doi: 10.1074/jbc.M106006200. [DOI] [PubMed] [Google Scholar]
- Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:1696–2687. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi MR. Hox genes and mammalian development. Cold Spring Harbor Symp Quant Biol. 1997;62:273–281. [PubMed] [Google Scholar]
- Croy BA, Luross JA, Guimond MJ, Hunt JS. Uterine natural killer cells: insights into lineage relationships and functions from studies of pregnancies in mutant and transgenic mice. Nat Immunol. 1996;15:22–33. [PubMed] [Google Scholar]
- Cunningham NS, Jenkins NA, Gilbert DJ, Copeland NG, Reddi AH, Lee SJ. Growth/differentiation factor-10: a new member of the transforming growth factor-beta superfamily related to bone morphogenetic protein-3. Growth Factors. 1995;12:99–109. doi: 10.3109/08977199509028956. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Song H, Guo Y, Riesewijk A, Mosselman S, Das SK, Dey SK. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol Endocrinol. 2004;18:1238–1250. doi: 10.1210/me.2003-0403. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Tranguch S, Friedman DB, Das SK, Smith DF, Dey SK. Proteomic analysis identifies immunophilin FK506 binding protein 4 (FKBP52) as a downstream target of Hoxa10 in the periimplantation mouse uterus. Mol Endocrinol. 2005;19:683–697. doi: 10.1210/me.2004-0332. [DOI] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- Das SK, Yano S, Wang J, Edwards DR, Nagase H, Dey SK. Expression of matrix metalloproteinases and tissue inhibitors of metallo-proteinases in the mouse uterus during the periimplantation period. Dev Genet. 1997;21:44–54. doi: 10.1002/(SICI)1520-6408(1997)21:1<44::AID-DVG5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Das SK, Lim H, Paria BC, Dey SK. Cyclin D3 in the mouse uterus is associated with the decidualization process during early pregnancy. J Mol Endocrinol. 1999;22:91–101. doi: 10.1677/jme.0.0220091. [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- Dokras A, Gardner LM, Seftor EA, Hendrix MJ. Regulation of human cytotrophoblast morphogenesis by hepatocyte growth factor/scatter factor. Biol Reprod. 2001;65:1278–1288. doi: 10.1095/biolreprod65.4.1278. [DOI] [PubMed] [Google Scholar]
- Dosiou C, Giudice LC. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev. 2005;26:44–62. doi: 10.1210/er.2003-0021. [DOI] [PubMed] [Google Scholar]
- Edgar BA, Orr-Weaver TL. Endoreplication cell cycles: more or less. Cell. 2001;105:297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- Fuse N, Hirose S, Hayashi S. Diploidy of Drosophila imaginal cells is maintained by a transcriptional repressor encoded by escargot. Genes Dev. 1994;8:2270–2281. doi: 10.1101/gad.8.19.2270. [DOI] [PubMed] [Google Scholar]
- Galimi F, Bagnara GP, Bonsi L, Cottone E, Follenzi A, Simeone A, Comoglio PM. Hepatocyte growth factor induces proliferation and differentiation of multipotent and erythroid hemopoietic progenitors. J Cell Biol. 1994;127:1743–1754. doi: 10.1083/jcb.127.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ. Homeoboxes in the study of development. Science. 1987;236:1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- Gu Y, Gibori G. Isolation, culture, and characterization of the two cell subpopulations forming the rat decidua: differential gene expression for activin, follistatin, and decidual prolactin-related protein. Endocrinology. 1995;136:2451–2458. doi: 10.1210/endo.136.6.7538462. [DOI] [PubMed] [Google Scholar]
- Gui Y, Zhang J, Yuan L, Lessey BA. Regulation of HOXA-10 and its expression in normal and abnormal endometrium. Mol Hum Reprod. 1999;5:866–873. doi: 10.1093/molehr/5.9.866. [DOI] [PubMed] [Google Scholar]
- Hemavathy K, Ashraf SI, Ip YT. Snail/slug family of repressors: slowly going into the fast lane of development and cancer. Gene. 2000;257:1–12. doi: 10.1016/s0378-1119(00)00371-1. [DOI] [PubMed] [Google Scholar]
- Inoue A, Seidel MG, Wu W, Kamizono S, Ferrando AA, Bronson RT, Iwasaki H, Akashi K, Morimoto A, Hitzler JK, Pestina TI, Jackson CW, Tanaka R, Chong MJ, McKinnon PJ, Inukai T, Grosveld GC, Look AT. Slug, a highly conserved zinc finger transcriptional repressor, protects hematopoietic progenitor cells from radiation-induced apoptosis in vivo. Cancer Cell. 2002;2:279–288. doi: 10.1016/s1535-6108(02)00155-1. [DOI] [PubMed] [Google Scholar]
- Jiang R, Lan Y, Norton CR, Sundberg JP, Gridley T. The Slug gene is not essential for mesoderm or neural crest development in mice. Dev Biol. 1998;198:277–285. [PubMed] [Google Scholar]
- Kessel M, Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- King A. Uterine leukocytes and decidualization. Hum Reprod. 2000;6:28–36. doi: 10.1093/humupd/6.1.28. [DOI] [PubMed] [Google Scholar]
- Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24:575–587. doi: 10.1016/s0143-4004(03)00063-8. [DOI] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Lim H, Ma L, Ma WG, Maas RL, Dey SK. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999;13:1005–1017. doi: 10.1210/mend.13.6.0284. [DOI] [PubMed] [Google Scholar]
- Liu CC, Young JD. Uterine natural killer cells in the pregnant uterus. Adv Immunol. 2001;79:297–329. doi: 10.1016/s0065-2776(01)79007-4. [DOI] [PubMed] [Google Scholar]
- Ma L, Benson GV, Lim H, Dey SK, Maas RL. Abdominal B (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progester-one and repression in mullerian duct by the synthetic estrogen diethylstilbestrol (DES) Dev Biol. 1998;197:141–154. doi: 10.1006/dbio.1998.8907. [DOI] [PubMed] [Google Scholar]
- Moffett-King A. Natural killer cells and pregnancy. Nat Rev, Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- Nagase H, Okada Y. Proteinases and matrix degradation. In: Kelley WN, Harris ED Jr, Ruddy S, Sledge CB, editors. The Textbook of Rheumatology. 5. WB Saunders; Philadelphia: 1996. pp. 323–341. [Google Scholar]
- Nakayama H, Scott IC, Cross JC. The transition to endoreduplication in trophoblast giant cells is regulated by the mSNA zinc finger transcription factor. Dev Biol. 1998;199:150–163. doi: 10.1006/dbio.1998.8914. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- O’Shea JD, Kleinfeld RG, Morrow HA. Ultrastructure of decidualization in the pseudopregnant rat. Am J Anat. 1983;166:271–298. doi: 10.1002/aja.1001660304. [DOI] [PubMed] [Google Scholar]
- Paffaro VA, Jr, Bizinotto MC, Joazeiro PP, Yamada AT. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta. 2003;24:479–488. doi: 10.1053/plac.2002.0919. [DOI] [PubMed] [Google Scholar]
- Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science. 2002;296:2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- Parr EL, Young LH, Parr MB, Young JD. Granulated metrial gland cells of pregnant mouse uterus are natural killer-like cells that contain perforin and serine esterases. J Immunol. 1990;145:2365–2372. [PubMed] [Google Scholar]
- Patel Y, Kim H, Rappolee DA. A role for hepatocyte growth factor during early postimplantation growth of the placental lineage in mice. Biol Reprod. 2000;62:904–912. doi: 10.1095/biolreprod62.4.904. [DOI] [PubMed] [Google Scholar]
- Perez-Losada J, Sanchez-Martin M, Rodriguez-Garcia A, Sanchez ML, Orfao A, Flores T, Sanchez-Garcia I. Zinc-finger transcription factor Slug contributes to the function of the stem cell factor c-kit signaling pathway. Blood. 2002;100:1274–1286. [PubMed] [Google Scholar]
- Reese J, Das SK, Paria BC, Lim H, Song H, Matsumoto H, Knudtson KL, DuBois RN, Dey SK. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J Biol Chem. 2001;276:44137–44145. doi: 10.1074/jbc.M107563200. [DOI] [PubMed] [Google Scholar]
- Sachs L, Shelesnyak MC. The development and suppression of polyploidy in the developing and suppressed deciduoma in the rat. J Endocrinol. 1955;12:146–151. doi: 10.1677/joe.0.0120146. [DOI] [PubMed] [Google Scholar]
- Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995;374:460–463. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Simon HG, Tabin CJ. Analysis of Hox-4.5 and Hox-3.6 expression during newt limb regeneration: differential regulation of paralogous Hox genes suggest different roles for members of different Hox clusters. Development. 1993;117:1397–1407. doi: 10.1242/dev.117.4.1397. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci. 2002;7:d1879–d1898. doi: 10.2741/spencer. [DOI] [PubMed] [Google Scholar]
- Sugawara J, Fukaya T, Murakami T, Yoshida H, Yajima A. Hepatocyte growth factor stimulated proliferation, migration, and lumen formation of human endometrial epithelial cells in vitro. Biol Reprod. 1997;57:936–942. doi: 10.1095/biolreprod57.4.936. [DOI] [PubMed] [Google Scholar]
- Takai K, Hara J, Matsumoto K, Hosoi G, Osugi Y, Tawa A, Okada S, Nakamura T. Hepatocyte growth factor is constitutively produced by human bone marrow stromal cells and indirectly promotes hematopoiesis. Blood. 1997;89:1560–1565. [PubMed] [Google Scholar]
- Tan J, Raja S, Davis MK, Tawfik O, Dey SK, Das SK. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech Dev. 2002;111:99–113. doi: 10.1016/s0925-4773(01)00614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol. 2004;265:181–195. doi: 10.1016/j.ydbio.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101:1379–1384. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- Welsh AO, Enders AC. Light and electron microscopic examination of the mature decidual cells of the rat with emphasis on the antimesometrial decidua and its degeneration. Am J Anat. 1985;172:1–29. doi: 10.1002/aja.1001720102. [DOI] [PubMed] [Google Scholar]
- Yao MW, Lim H, Schust DJ, Choe SE, Farago A, Ding Y, Michaud S, Church GM, Maas RL. Gene expression profiling reveals progesterone-mediated cell cycle and immunoregulatory roles of Hoxa-10 in the preimplantation uterus. Mol Endocrinol. 2003;17:610–627. doi: 10.1210/me.2002-0290. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Harada T, Mitsunari M, Iwabe T, Sakamoto Y, Tsukihara S, Iba Y, Horie S, Terakawa N. Hepatocyte growth factor/Met system promotes endometrial and endometriotic stromal cell invasion via autocrine and paracrine pathways. J Clin Endocrinol Metab. 2004;89:823–832. doi: 10.1210/jc.2003-030874. [DOI] [PubMed] [Google Scholar]
- Yu WH, Woessner JF, Jr, McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev. 2002;16:307–323. doi: 10.1101/gad.925702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Daikoku T, Hou X, Li M, Wang H, Nojima H, Dey SK, Das SK. Cyclin G1 and cyclin G2 are expressed in the periimplantation mouse uterus in a cell-specific and progesterone-dependent manner: evidence for aberrant regulation with Hoxa-10 deficiency. Endocrinology. 2005;146:2424–2433. doi: 10.1210/en.2004-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Lawler AM, Lee SJ. Characterization of GDF-10 expression patterns and null mice. Dev Biol. 1999;212:68–79. doi: 10.1006/dbio.1999.9326. [DOI] [PubMed] [Google Scholar]
- Zheng LM, Liu CC, Ojcius DM, Young JD. Expression of lymphocyte perforin in the mouse uterus during pregnancy. J Cell Sci. 1991;99:317–323. doi: 10.1242/jcs.99.2.317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.