| Patent Application Title: | Glycosidase Inhibitors | ||
| Patent Application Number: | WO 2014/159234 A1 | Publication date: | 2 October 2014 |
| Priority Application: | US 61/782,353 | Priority date: | 14 March 2013 |
| US 61/817,493 | 30 April 2013 | ||
| Inventors: | Yv, H.; Liu-Bujalski, L.; Johnson, T. L. | ||
| Assignee Company: | Merck Patent Gmbh; Frankfurter Strasse 250, 64293 Darmstadt (DE) | ||
| Disease Area: | Alzheimer’s disease | Biological Target: | Inhibition of the glycosidase O-GlcNAcase (OGA) |
| Summary: | The invention in this patent application relates to compounds represented generally by formula (I), which exhibit activities as glycosidase inhibitors that can inhibit O-GlcNAcase. These compounds may potentially be used for slowing the progression and/or the treatment of Alzheimer’s disease (AD) and possibly other diseases. | ||
| Many nuclear and cytoplasmic cellular proteins undergo several dynamic (reversible) modifications during their lifetime in which the monosaccharide 2-acetamido-2-deoxy-β-d-glucopyranoside (β-N-acetyl glucosamine) is attached and removed. The forward process is a glycosylation in which β-N-acetyl glucosamine is attached to specific serine and threonine residues on proteins via a post-translational O-glycosidic linkage to form O-linked N-acetylglucosamine (O-GlcNAc) proteins. This process is catalyzed by an enzyme called O-GlcNAc transferase (OGTase). The O-GlcNAc modification is abundant in the brain and was found on many cytoskeletal proteins, such as neurofilament proteins, synapsins, synapsin-specific clathrin assembly protein AP-3, and Ankyrin-G. The O-GlcNAc-modified proteins are responsible for the regulation of a wide range of vital cellular functions such as transcription, proteasomal degradation, and cellular signaling. The reverse process is a hydrolysis to cleave the β-N-acetyl glucosamine attachments and liberate the proteins. The cleavage of the O-GlcNAc is catalyzed by a glycosidase known as O-GlcNAcase or OGA, which is a member of family 84 of glycoside hydrolases. | |||
| The development of neurofibrillary tangles (NFTs) is one of the characteristics of neurodegenerative diseases such as AD, Downs’ syndrome, Pick’s disease, Niemann-Pick Type C disease, and amyotrophic lateral sclerosis (ALS). NFTs are aggregates of paired helical filaments (PHFs) made of an abnormal form of the cytoskeletal protein tau. In its normal function, tau stabilizes a key cellular network of microtubules that is essential for distributing proteins and nutrients within neurons. However, tau becomes hyperphosphorylated in AD patients to form PHFs and then aggregates to form NFTs. While the exact causes of hyperphosphorylation of tau are unknown, there is a clear correlation between the levels of NFTs and the severity of dementia in AD patients, which is indicative of a key role for tau dysfunction in AD. Considerable studies were directed toward understanding the causes, as well as identifying strategies to reduce tau hyperphosphorylation in order to halt or reverse the progression of AD. Some of these studies have shown a significant connection between the levels of O-GlcNAc modification and the phosphorylation of tau. A key observation was that O-GlcNAc modification occurs on many proteins at the same amino acid residues that can also be phosphorylated. Also, the hyperphosphorylated tau in human AD brains contains noticeably lower levels of O-GlcNAc than the levels found in healthy brains. It appears that there is a reciprocal relationship between the levels of O-GlcNAc modification and phosphorylation of tau; increased phosphorylation levels are accompanied by decreased O-GlcNAc levels and vice versa. This reciprocal relationship between O-GicNAc and phosphorylation was named the Yin-Yang hypothesis. The hypothesis was supported by the discovery that the enzyme OGTase forms a functional dynamic complex with phosphatases. This indicates that it is possible the same enzyme complex that removes O-phosphate would concomitantly attach O-GlcNAc. When PC-12 neuronal cells and brain tissue sections from mice were treated with a nonselective OGA inhibitor to increase the levels of O-GlcNAc on tau, the treatment resulted in decreased phosphorylation levels. It is thus believed that inhibition of the glycosidase OGA to maintain healthy elevated levels of O-GlcNAc in AD patients may also block the hyperphosphorylation of tau and its subsequent harmful effects including the formation of NFTs. Therefore, blocking of tau hyperphosphorylation and regulating its O-GlcNAc levels through the inhibition of OGA may be a promising and viable therapy to prevent the formation of NFTs and associated neurodegeneration to slow the progression of AD in patients. | |||
| The inhibition of OGA may also be beneficial for the treatment of other disorders. For example, studies have shown that decreased glucose availability due to impairment of its transport and metabolism in the brain leads to increased tau hyperphosphorylation. The inhibition of OGA decreases phosphorylation levels and that may compensate for age-related impairment of glucose metabolism within the brains of healthy individuals as well as patients suffering from AD or related neurodegenerative diseases. There is also evidence indicating that elevated levels of O-GlcNAc-modified proteins provide protection against pathogenic effects of stress in cardiac tissue, including stress caused by ischemia, hemorrhage, hypervolemic shock, and calcium paradox. Additional data indicates that the O-GlcNAc modification plays a role in other neurodegenerative diseases, including Parkinson’s disease and Huntington’s disease. | |||
| A major challenge in developing inhibitors of the glycosidase OGA is the large number of functionally related enzymes and the potential of their concomitant inhibition. Existing inhibitors of β-N-acetylglucosaminidases that can block OGA function are nonspecific and can also inhibit the lysosomal β-hexosaminidases. Therefore, there is a need for the discovery of low molecular weight compounds that can selectively inhibit OGA to prevent the cleavage of the O-GicNAc groups without inhibiting the lysosomal β-hexosaminidases. The compounds described in this patent application are introduced to address this need. | |||
| Important Compound Classes: | 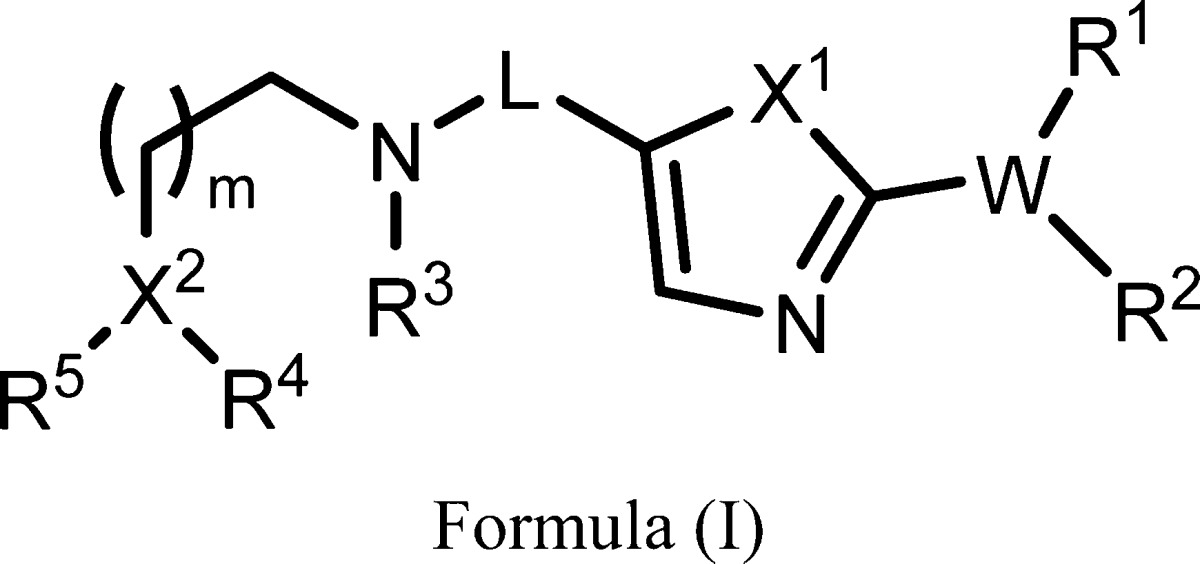 |
||
| Key Structures: | The inventors described the structures and syntheses of 65 examples representing formula (I) including the following four compounds: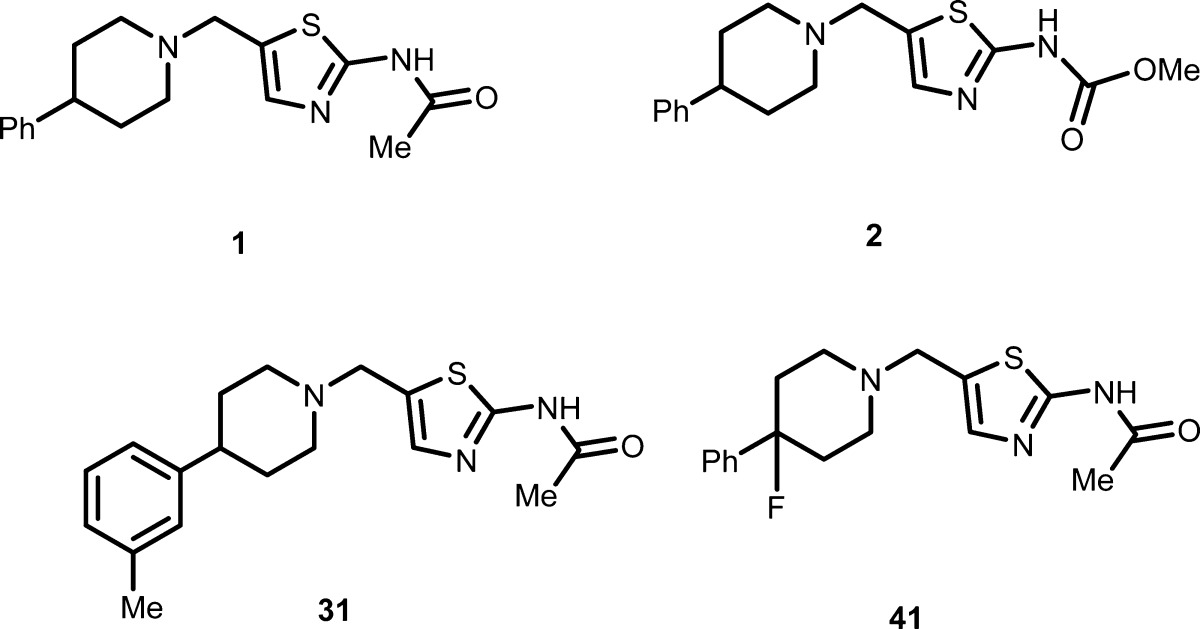
|
||
| Biological Assay: |
|
||
| Biological Data: | The inventors reported IC50 and EC50, ICC data from the above two assays, respectively, for most of the compounds of formula (I). The data obtained from the representative compounds 1, 2, 31, and 41 (structures above) are listed in the following table: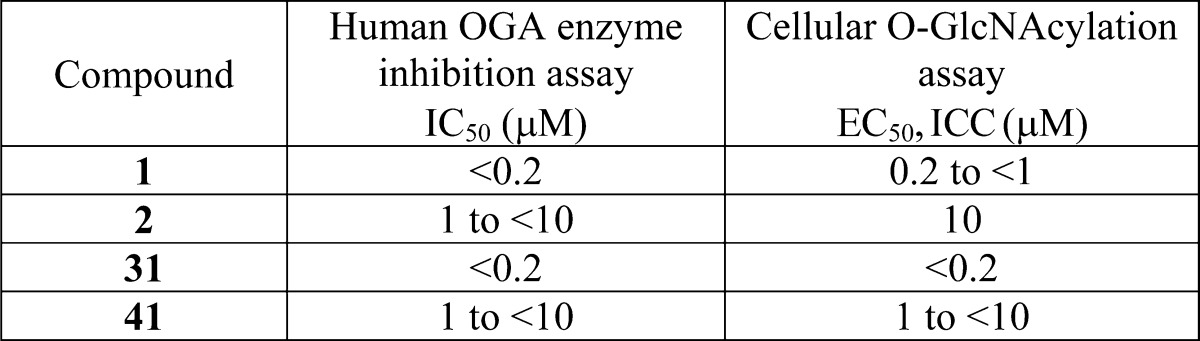
|
||
| Recent Review Articles: | Lefebvre T.Nat. Chem. Biol. 2012, 8 (4), 325–326. | ||
| Macauley M. S.; Vocadlo D. J.. Biochim. Biophys. Acta, Gen. Subj. 2010, 1800 (2), 107–121. | |||
| Yuzwa S. A.; Vocadlo D. J.. Curr. Alzheimer Res. 2009, 6 (5), 451–454. | |||
The authors declare no competing financial interest.


