Abstract
Drug resistance to Pseudomonas sp. has spread to such a level irrespective of the type of patients, that its pattern of distribution and antibiotic resistance needs to be studied in detail, especially in trauma patients and hence the study. A 6 year study was carried out among trauma patients to see the trend and type of resistance prevalent in the apex hospital for trauma care in India among nonduplicate isolates where multidrug-resistance (MDR), cross-resistance and pan-drug resistance in Pseudomonas sp. were analyzed. Of the total 2,269 isolates obtained, the species, which was maximally isolated was Pseudomonas aeruginosa (2,224, 98%). The highest level of resistance was seen in tetracycline (2,166, 95.5%, P < 0.001) and chloramphenicol (2,160, 95.2%, P < 0.001) and least in meropenem (1,739, 76.7%, P < 0.003). Of the total, 1,692 (74.6%) isolates were MDR in which P. aeruginosa (75%) were maximum. MDR Pseudomonas is slowing increasing since the beginning of the study period. Of 1,797 imipenem-resistant P. aeruginosa isolated during the study period, 1,763 (98%) showed resistance to ciprofloxacin or levofloxacin, suggesting that cross-resistance may have developed for imipenem due to prior use of fluoroquinolones. Antibiotic resistance in Pseudomonas sp. is fast becoming a problem in trauma patients, especially in those who requires prolong hospital stay, which calls for proper antimicrobial stewardship.
Keywords: Antibiotic resistance, Cross-resistance, Infections, Multi-drug resistance, Pseudomonas spp, Trauma
MICROBIOLOGY REPORT
Pseudomonas sp. is ubiquitously present worldwide of which Pseudomonas aeruginosa is a major nosocomial pathogen, which survives in moist environments and colonizes the respiratory tract of mechanically ventilated patients.[1] It causes severe infections such as pneumonia in critically ill and immunocompromised patients. Multidrug-resistant (MDR) P. aeruginosa is especially associated with increased mortality because no adequate therapeutic option exists.[2] MDR strains of P. aeruginosa were initially reported in patients with cystic fibrosis.[3,4] MDR in P. aeruginosa or Acinetobacter spp. has been variously defined.[4,5] Recently, it has been proposed that the term “extensive drug resistance” should be used to indicate resistance to all, but one or two classes of antimicrobial agents.[6]
Though many studies have been done on infections caused by Pseudomonas sp. and its drug resistance in different patients more so in patients with cystic fibrosis, but it is scarce among trauma patients. Hence, we have tried to study the distribution of different Pseudomonas spp., its antimicrobial resistance against commonly used antibiotics and the outcomes of such infections. Furthermore, we have tried to analyze, which type of trauma patients were mainly infected with multi drug resistant Pseudomonas.
This was a retrospective cross-sectional analysis of all the Pseudomonas isolates obtained from different samples of patients admitted in different wards of Jai Prakash Narayan Apex Trauma Center, New Delhi, India from January 2007 to December 2012. All the results were noted from the microbiology database and the resistance pattern observed and analyzed.
All the microbiology samples were scanned and the samples isolating Pseudomonas sp. were taken into consideration irrespective of their preoperative nor postoperative collection. All these samples were processed as per standard microbiological methods.[7] The bacterial isolates were identified to the species level by the VITEK 2® compact system (BioMérieux, Lyon, France). The antimicrobial susceptibility testing were done by the disc diffusion method, according to the Clinical and Laboratory Standard Institute (CLSI) guidelines[8,9] and the VITEK 2 system. Mueller — Hinton agar (HiMedia, Mumbai, India) was used uniformly for all the sensitivity testing and incubated aerobically at 37°C. ATCC control strains of P. aeruginosa (ATCC 27853) and Escherichia coli (ATCC 25922) were used as quality control. All isolates in this were nonduplicate.
The standardized custom sensitivity panel used in the VITEK 2 included 25 different antimicrobials for susceptibility testing of all Gram-negative isolates, but for this study only the susceptibilities of Pseudomonas spp. to common antipseudomonal agents were analyzed.
For this study, multi-drug resistance was defined as resistance of a Pseudomonas isolate to at least three of the following four drugs: Amikacin, imipenem, ceftazidime, ciprofloxacin and tobramycin. These antibiotics were chosen because they are representative of their antibiotic classes and their minimum inhibitory concentrations (MICs) were tested throughout the study period (2007 -2012). Antibiotics with intermediate susceptibility CLSI were considered resistant in the study analysis. Cross-resistance to formulary antibiotics were also evaluated for its efficacy in its empiric therapy. MDR P. aeruginosa was defined as P. aeruginosa resistant or intermediate to imipenem (MIC >8 mg/L), levofloxacin (MIC >8 mg/L) and amikacin (MIC >64 mg/L) according to CLSI criteria.[9] Furthermore, definitions of MDR Pseudomonas in other studies were noted too.[10]
Statistical analyses were performed using the SPSS software for Windows (SPSS Inc., Chicago, IL, USA, version 15.0).
A total of 2,269 nonduplicate Pseudomonas sp. were isolated. The mean age of the patients from whom they were isolated was 32 years ([1 -87 years], standard deviation of 15.1). Male patients (1,875, 82.6%) outnumbers female patients (394, 17.4%) from whom Pseudomonas spp. were isolated. In male patients, the mean age was 33 years with a range of 1 -87 years (± 14.22), whereas in female patients, the mean age was found to be 30 years with a range of 2 -83 years (± 18.38). Hence, the P value of the means of the age in male and female patients was 0.002 (95% confidence interval = 0.9481 -4.2187).
Of the total 2,269 isolates, the species which was maximally isolated was P. aeruginosa (2,224, 98%) followed by Pseudomonas putida (25, 1.1%), Pseudomonas stutzeri (8, 0.4%), Pseudomonas luteola (5, 0.2%), Pseudomonas flavescens (5, 0.2%), Pseudomonas alcaligenes (1) and Pseudomonas mendocena (1) in decreasing order of frequency. The most common sample from which P. aeruginosa was recovered was from urine samples (636, 29%), followed by tracheal aspirates (543, 24.4%), pus/wound swabs (442, 20%), blood (172, 8%), bronchalveolar lavage (171, 8%), cerebrospinal fluid (141), central vascular tips (43, 2%), drain fluids (27, 1.2%), tissues (12, 1%), pleural fluid (9, 0.4%), sputum (3,0.1%), bone (2, 0.1%) and others (11,1%). Among P. putida, maximum isolation was seen in pus samples (11, 44%), and followed by urine (5, 20%), tracheal aspirates (5, 20%) and equally by blood (2, 8%) and tissue (2, 8%). However, the highest rate of isolation was from blood samples (4, 50%) in P. stutzeri followed by equal isolation from urine (2, 25%) and pus/wound samples (2, 25%) each. Only a single isolate each of P. alcaligenes was obtained from urine sample and also P. mendocina from tissue. In P. luteola, maximum isolation was from urine (3, 60%) and equally from tracheal aspirate (1, 20%) and wound swab (1, 20%). Maximum isolation of P. flavescens was from blood (3, 60%) followed by bronchoalveolar lavage sample (2, 40%).
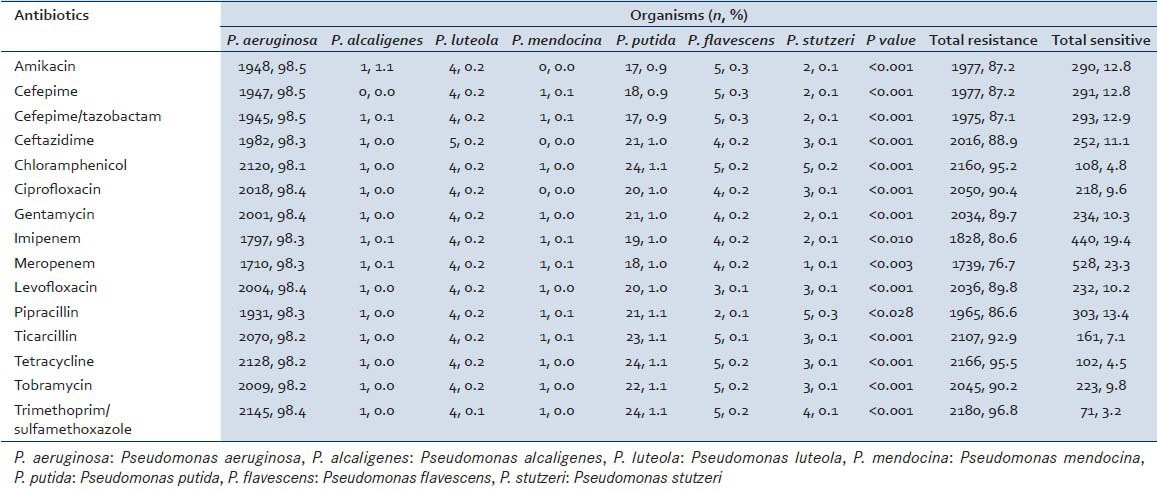
The sensitivity pattern of the different species of Pseudomonas isolated during this study period is given in Table 1. The highest level of resistance was seen in tetracycline (2,166, 95.5%, P < 0.001) and chloramphenicol (2,160, 95.2%, P < 0.001) and least in meropenem (1739, 76.7%, P < 0.003). P value of either <0.001 or <0.003 shows that the drug resistance is indeed significant and hence treatment with such antibiotics will not show much response.
Table 1.
Antimicrobial resistance pattern among the different Pseudomonas spp. to the various antibiotics used for its treatment
It was found that a total of 1,692 (74.6%) isolates were MDR. Also, a higher isolation rate of MDR was seen among the male patients (1,406, 75.0%) compared with the female patients (286, 72.6%) admitted for trauma care (χ2 = 0.987 and difference of freedom = 1).
Pseudomonas sp. was maximally isolated from the samples of patients admitted to the neurosurgery ward (781) followed by neurosurgery Intensive Care Unit (ICU) (568), surgery ICU (436), surgery ward (233), orthopedic ward (160), follow-up patients (70) and emergency department (16). Of the total isolates from different wards, maximum MDR Pseudomonas sp. were obtained from neurosurgery ward 605 (77.5%), followed by 453 (79.8%) MDR isolates from the neurosurgery ICU, 335(77.2%) from surgery ward, 91 (56.9%) from orthopedic ward, 40 (75.5%) from follow-up outpatient department and 6 (54.5%) from emergency. Its P < 0.001 which is highly significant (χ2 = 79.98, degree of freedom = 14).
When we analyzed the distribution of MDR Pseudomonas [Figures 1 and 2] among the different species obtained during the study period, P. aeruginosa accounted for the maximum MDR isolates with a total number of 1,667 (75%), followed in decreasing frequency by P. putida (14, 56%), P. luteola (4,80%), P. flavescens (4, 80%), P. stutzeri (2, 25%) and P. alcaligenes (1). However, no MDR was isolated among the P. mandocina. A P = 0.005 was seen (χ2 = 18.51, degree of freedom = 6) and hence not significant.
Figure 1.
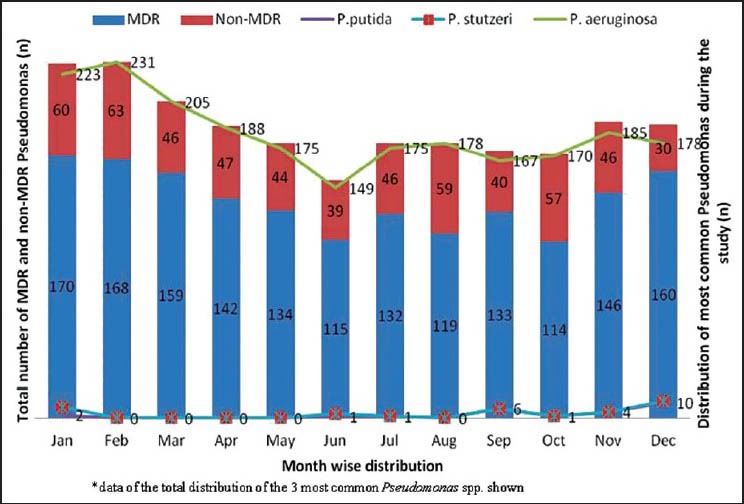
Monthly distribution of most commonly isolated Pseudomonas spp. along with multidrug-resistant (MDR) and non-MDR isolates
Figure 2.
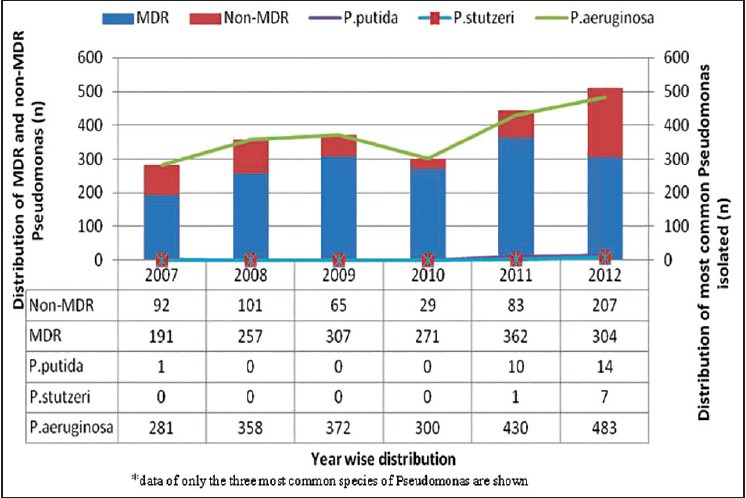
Year wise distribution of multidrug-resistant (MDR) and non-MDR Pseudomonas along with the three most commonly isolated species
Of the total 2,269 nonduplicate Pseudomonas spp., the pathogen was responsible for infections in 68% of the patients for whom their treatment had to be revised but later discharged healthy, 12% resulted in death and remaining 20% required no extra interventions in treatment protocol and the patients improved after removing the offending implant/devices or after minor debridement. Here, it was seen that more than 90% among the 20% Pseudomonas spp. were mostly colonizers as testing of their surrounding areas or instruments showed the same. So a simple cleaning was able to remove them.
Furthermore, it was observed that infections due to Pseudomonas spp. was more in the postoperative patients (1927, 84.9%) and those who are in the hospital for a long time hinting at the hospital acquired infections compared to that of the preoperative patients (342, 15%).
In this study, VITEK and CLSI antibiograms of 2,269 Pseudomonas spp. isolates from inpatients, outpatients of our trauma care facility, P. aeruginosa was categorized as the most important nosocomial pathogens among the MDR Gram-negative bacilli similar to a report from the Infectious Diseases Society of America.[11] In our study, even though the rate of isolation of P. aeruginosa was 98%, more than 68% of the patients improved after change of therapy and 12% of them had a fatal outcome.
In another retrospective case-control study in Turkey, it was found that the major risk factors for infection or colonization with multi-resistant P. aeruginosa were prolonged stay in the ICU, previous and lengthy imipenem usage, and mechanical ventilation.[12] Also, in our study, maximum isolates of Pseudomonas spp. were from the ICUs irrespective of the type of ICU. Our study observed an increase from 11.3% at the start of the study to 58% during the end of the study.
Prior fluoroquinolone use has been identified as a risk factor for the emergence of imipenem-resistant P. aeruginosa.[13,14] Out of 1,797 imipenem-resistant P. aeruginosa isolated during the study period, 1,763(98%) showed resistance to ciprofloxacin or levofloxacin, suggesting that cross-resistance may have developed for imipenem due to prior use of fluoroquinolones.
Pan-drug resistance from the MDR Pseudomonas isolated were those isolates resistant to all the antibiotics except colistin.[10] A total of 847 pan-drug resistant Pseudomonas were isolated and like the MDR isolates, a steady increase in pan-drug resistant Pseudomonas was seen over the study period in our set-up. We have also seen a high rate of isolation during the winter months compared with the summer months which may be influenced by the rate of patient admission pattern.
Though P. aeruginosa remained the predominant pathogen, other non-P. aeruginosa pathogens are also increasing as causative agents of nosocomial infections in trauma patients. However, our study found no mortality associated with the non-P. aeruginosa pathogens.
CONCLUSION
Pseudomonas aeruginosa is one of the most common causes of nosocomial infection in trauma patients, which is becoming highly drug resistant. Proper and timely use of antimicrobials is the need of the hour.
ACKNOWLEDGMENTS
The authors would like to thank All India Institute of Medical Sciences, New Delhi for supporting this study. Also, the authors extend their heartfelt gratitude to Mr. Pawan Kumar for his help in compiling the data and to Mrs. Kusum Chopra for her help in statistical analysis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Rossolini GM, Mantengoli E. Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosa. Clin Microbiol Infect. 2005;11(Suppl 4):17–32. doi: 10.1111/j.1469-0691.2005.01161.x. [DOI] [PubMed] [Google Scholar]
- 2.Giske CG, Monnet DL, Cars O, Carmeli Y. ReAct-Action on Antibiotic Resistance. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother. 2008;52:813–21. doi: 10.1128/AAC.01169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aris RM, Gilligan PH, Neuringer IP, Gott KK, Rea J, Yankaskas JR. The effects of panresistant bacteria in cystic fibrosis patients on lung transplant outcome. Am J Respir Crit Care Med. 1997;155:1699–704. doi: 10.1164/ajrccm.155.5.9154879. [DOI] [PubMed] [Google Scholar]
- 4.Park YS, Lee H, Chin BS, Han SH, Hong SG, Hong SK, et al. Acquisition of extensive drug-resistant Pseudomonas aeruginosa among hospitalized patients: Risk factors and resistance mechanisms to carbapenems. J Hosp Infect. 2011;79:54–8. doi: 10.1016/j.jhin.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Paterson DL. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006;43(Suppl 2):S43–8. doi: 10.1086/504476. [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Karageorgopoulos DE. Pandrug resistance (PDR), extensive drug resistance (XDR), and multidrug resistance (MDR) among Gram-negative bacilli: Need for international harmonization in terminology. Clin Infect Dis. 2008;46:1121–2. doi: 10.1086/528867. [DOI] [PubMed] [Google Scholar]
- 7.Collee JG, Miles RS, Watt B. Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology. 14th ed. New York: Churchill Livingstone; 1996. pp. 131–45. [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. Pennsylvania, USA: Clinical and Laboratory Standard Institute (CLSI) Document; 2010. Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement (June 2010 update). M100-S20. [Google Scholar]
- 9.Wayne, Pennsylvania, USA: Clinical and Laboratory Standard Institute (CLSI) Document; 2012. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. M100-S22. [Google Scholar]
- 10.Tsutsui A, Suzuki S, Yamane K, Matsui M, Konda T, Marui E, et al. Genotypes and infection sites in an outbreak of multidrug-resistant Pseudomonas aeruginosa. J Hosp Infect. 2011;78:317–22. doi: 10.1016/j.jhin.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, Bartlett JG, et al. Bad bugs need drugs: An update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–68. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 12.Gülay Z, Atay T, Amyes SG. Clonal spread of imipenem-resistant Pseudomonas aeruginosa in the intensive care unit of a Turkish hospital. J Chemother. 2001;13:546–54. doi: 10.1179/joc.2001.13.5.546. [DOI] [PubMed] [Google Scholar]
- 13.Lautenbach E, Weiner MG, Nachamkin I, Bilker WB, Sheridan A, Fishman NO. Imipenem resistance among Pseudomonas aeruginosa isolates: Risk factors for infection and impact of resistance on clinical and economic outcomes. Infect Control Hosp Epidemiol. 2006;27:893–900. doi: 10.1086/507274. [DOI] [PubMed] [Google Scholar]
- 14.Furtado GH, Bergamasco MD, Menezes FG, Marques D, Silva A, Perdiz LB, et al. Imipenem-resistant Pseudomonas aeruginosa infection at a medical-surgical intensive care unit: Risk factors and mortality. J Crit Care. 2009;24:625e9–14. doi: 10.1016/j.jcrc.2009.03.006. [DOI] [PubMed] [Google Scholar]


