Abstract
Soft tissue and wound infections due to Enterococcus spp. are increasing worldwide with current need to understand the epidemiology of the Enterococcal infections of wounds. Hence, we have looked into the distribution of Enterococcus spp. responsible for causing wound and soft tissue infections among trauma patients, its antibiotic resistance pattern and how it affects the length of hospital stay and mortality. A laboratory cum clinical-based study was performed over a period of 3 years at a level I trauma center in New Delhi, India. Patients with Enterococcal wound and soft tissue infections were identified using the hospital data base, their incidence of soft tissue/wound infections calculated, drug resistance pattern and their possible risk factors as well as outcomes analyzed. A total of 86 non-repetitive Enterococcus spp. was isolated of which E. faecium were maximally isolated 48 (56%). High level of resistance was seen to gentamicin HLAR in all the species of Enterococcus causing infections whereas a low level resistance to vancomycin and teicoplanin was observed among the isolates. Longer hospital stay, repeated surgical procedure, prior antibiotic therapy and ICU stay were observed to associate with increased morbidity (P < 0.05) and hence, more chances of infections with VRE among the trauma patients. The overall rate of wound and soft tissue infections with Enterococcus sp. was 8.6 per 1,000 admissions during the study period. Enterococcal wound infection is much prevalent in trauma care facilities especially in the ICUs. Here, a microbiologist can act as a sentinel, help in empirical therapeutic decisions and also in preventing such infections.
Keywords: Enterococcus sp, Infections, Soft tissue, Trauma, Vancomycin-resistant Enterococcus, Wounds
MICROBIOLOGY REPORT
Infections due to Gram-positive bacteria are on a rise, of which those due to Enterococcus spp. form a major part. Enterococci are reported to be the third leading cause of nosocomial infections in the world.[1] Enterococci, especially vancomycin-resistant Enterococcal (VRE) infections are becoming common and difficult to treat, appearing usually as long-lasting hospital outbreaks that present tremendous challenges for infection control.[2] The reason for the emerging Enterococcal infections is not fully understood, but an important contributory factor is probably the selection pressure from increasing consumption of cephalosporins.[3,4] This promotes emergence of Enterococci, which are inherently resistant to this group of antibiotics.[3,4] The major infections caused by Enterococci in general and VRE in particular include urinary tract infections (UTI), wound infections, intra-abdominal infections secondary to a perforated viscus or after surgery, cholecystitis, bacteremia, endocarditis, and rarely meningitis.[2] Wound and soft tissue infections due to Enterococcus spp. are also rising steadily. Risk factors for colonization and infection include previous antimicrobial therapy. However, data regarding the soft tissue and wound infections due to Enterococcus spp. and also its resistance pattern among trauma patients are scarce and hence the study was conducted with these lacunae in view.
The study was conducted with the following aims:
To determine the prevalence of various Enterococcal wound and soft tissue infections,
To look into the factors associated with mortality and length of hospital stay (LOS) with Enterococcal (VRE and VSE) wound infections,
To investigate the impact of vancomycin resistance and antibiotic therapy in Enterococcal soft tissue and wound infections among trauma patients, and
To observe its significance.
A cohort study was conducted from January 2011 to December 2013 at a level I trauma care center in New Delhi, India, serving a reference population of 16.3 to 17.8 million inhabitants during the study period. It has 176 functional beds with an average total admission per year of 5914 during the study period. The hospital bed occupancy rate was 83% with an average bed turnover rate per day of 24. Patients with Enterococcal wound and soft tissue infections were identified using the JPNATC, New Delhi Microbiology laboratory database. We have included all the wound swabs, pus samples and tissue sent for bacterial culture during the study period, irrespective of the diagnosis of the patients in this study. Tissue and pus culture results from the trauma patients during the 3-year period were observed regarding the changes in Enterococcus species distribution over time. The most frequent Enterococcal species were selected for analysis of changes in antibiotic sensitivity and species distribution over the years in the study period. For every patient, only one incident was included. An incident was defined as isolation of an Enterococcus sp. in one or more samples from the same operated site or wound. If patients underwent more than one operation within 6 months and the microbiology was the same, it was included as one incident.[4]
Possible risk factors like prolonged hospital stay, ICU admissions, previous multiple invasive surgical interventions, prior antibiotic therapy, repeated hospital admissions, presence of indwelling devices, methicillin-resistant Staphylococcus aureus co-infection etc were looked into.[5,6,7] For the purpose of this study, recent antimicrobial use was defined as receipt of any antimicrobial agent for more than 3 consecutive days in the 3 months before the date of culture detection; patients who received short courses of peri-operative prophylaxis were excluded by this criterion. Prior ICU stay of >1 week, surgical interventions including abdominal trauma surgeries were taken as criteria for the study. Also, possible outcomes like prolonging the length of stay (LOS), increased morbidity and mortality were analyzed.
Microorganisms recovered from these cultures were identified using the automated VITEK 2 compact systems (bioMérieux, Durham, USA) according to the manufacturer's protocol. Antibiotic sensitivity was done both by disc diffusion according to the CLSI guidelines,[8,9] EUCAST[10] and VITEK 2 in parallel. Throughout the study, Enterococcus faecalis ATCC 29212 (vancomycin sensitive), Enterococcus faecalis ATCC 51299 (vancomycin resistant) and Enterococcus faecium ATCC 700221 (vancomycin resistant) were used as controls.
Related information on patient demographics, antimicrobial use, repeated invasive surgical procedures, treatment of Enterococcal wound and soft tissue infections and outcomes of hospitalization was collected via a review of patient medical records from the electronic hospital database. Antibiotic sensitivity and its resistance pattern were noted and analyzed. The incidence of Enterococcus spp. was calculated as the number of patients with Enterococcus species in tissue or pus samples per 1,000 admissions for the period 2011-2013. Only one incident per patient was recorded. Descriptive statistics was used in most cases, but test for significance was done wherever feasible. A value of P < 0.05 was considered significant.
A total of 120 consecutive isolates were noted during this study period of which 86 non-repetitive isolates and hence patients were included. It was seen that male (72, 84%) predominated over female (14, 16%) patients with respect to the number of wound and tissue infections by Enterococcus sp. The clinical manifestations ranged from wound discharge (49%, 42/86), to swelling (36%, 31/86), redness (28%, 24/86), pain (62%, 53/86), abscess (30%, 26/86), fever (51%,44/86) and loss of function (24%, 21/86). Details regarding demographics, clinical characteristics and outcomes of patients with Enterococcal wound and soft tissue infections based on vancomycin susceptibility are shown in Table 1.
Table 1.
Baseline demographic data and co-morbidities of the 86 patients based on their vancomycin sensitivity
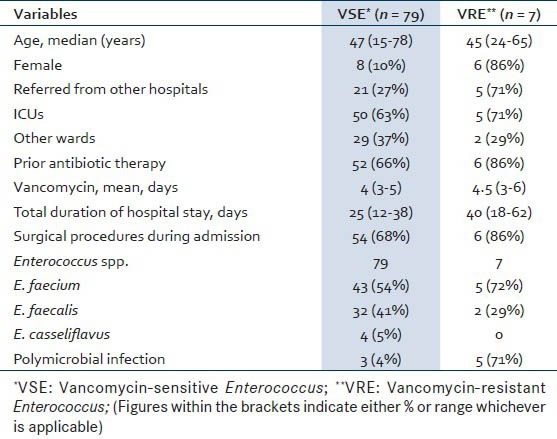
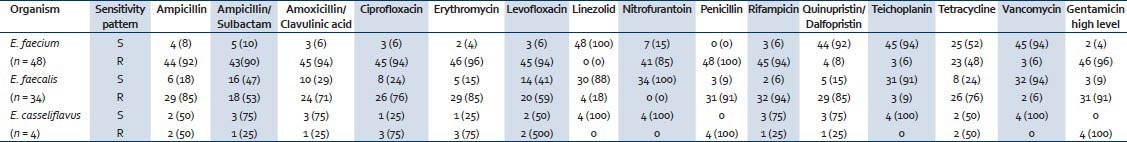
During the study period a total of 32,480 samples were received, of which 11,524 consists of pus and tissue samples for culture. From these, 86 non-repetitive Enterococcus spp. were isolated from different trauma patients. Of these, Enterococcus faecium (48/86, 56%) was the most common, followed by Enterococcus faecalis (34/86, 40%) and 4 (4%) of Enterococcus casseliflavus. The antimicrobial sensitivity pattern of different Enterococcus spp. is shown in Table 2. It was seen that a low level of vancomycin resistance was present in the Enterococcus sp. at our center. A total of 79 (92%) isolates were found to be vancomycin-sensitive Enterococcus (VSE) whereas the remaining 7 (8%) were found to be vancomycin-resistant Enterococcus (VRE). Our study also observed that the number of tissue and wound infections due to E. faecalis was high (24/86) in 2011, but was gradually overtaken by E. faecium during 2012 and 2013 (39/86). A total of 8 polymicrobial infections were observed among these patients, of which co-infection with Acinetobacter baumannii (5, 63%) was the highest followed by Klebsiella pneumoniae (2, 25%) and Staphylococcus aureus (1, 13%). All these polymicrobial infections were seen in the pus/wound samples along with E. faecium (7, 88%), except one case of co-infection of S. aureus with E. faecalis (1, 13%) in the tissue infection.
Table 2.
Distribution of various Enterococcus spp. and its antimicrobial sensitivity pattern
It was seen that longer hospital stay (LOS), repeated surgical procedure, prior antibiotic therapy and ICU stay were observed to associate with increased morbidity (P < 0.05) and hence, more chances of infections with VRE. Other factors like female gender or age did not have significant influence (P > 0.1) over the infection by Enterococcus sp. irrespective of their vancomycin sensitivity in our set up. This however did not influence the mortality of the admitted patients from whom Enterococcus sp. was isolated.
In our study, there was no significant change in the outcomes of the patient irrespective of whether they were infected with VSE or VRE (P > 0.1). No patient had a fatal outcome during the study period and all the patients were discharged healthy after treatment. This was seen in all the patients irrespective of whether they were admitted to ICUs or in other wards.
In the patients having wound and soft tissue infections due to VRE, 6 (86%) were treated with linezolid monotherapy, whereas 2 (29%) each with Quinupristin-Dalfopristin monotherapy or no antibiotics, respectively in the beginning. A combination of vancomycin with other antibiotics like teicoplanin, linezolid, ampicillin or benzylpenicillin was administered to 42 (53%) of the VSE patients either concurrently or in sequence later. However, combination therapy worked better in those patients with VRE or polymicrobial infection.
The overall incidence of wound and soft tissue infections with Enterococcus sp. was 8.6 per 1,000 admissions. However, the incidence of infection by E. faecalis was 3.4 per 1000 admissions and that due to E. faecium was 4.8 per 1,000 admissions.
We found that wound and soft tissue infections due to Enterococcus spp. irrespective of VSE or VRE were more in males. Prior ICU stay, multiple surgical interventions and longer hospital stay was associated with increased infection with VRE in our study. Similar findings have been seen in other studies.[11] Our study has seen a shift from a preponderance of E. faecalis to that of E. faecium in the later part of the study which also has been noted in other studies.[12,13] We found a low level of vancomycin resistance among the Enterococcus isolates, which is an encouraging finding. Also, Enterococcal wound or soft tissue infections were not a significant risk factor in our study though it influenced the morbidity and increased the LOS. This was also seen in other studies.[5,6,7] This might be explained by the prevalence of low level of VRE in our centre.
Our study found only 8 cases of polymicrobial infections with E. faecium and E. faecalis wound and soft tissue infections. Also, longer duration of hospital stay was seen in VRE patients compared to those of VSE, which might support the fact that longer LOS is an independent risk factor for VRE infections.[5,6] Prior ICU stay was more among VRE infected patients; however, other risk factors like co-infections or co-morbidities, cost of treatment/hospitalization could not be looked into detail due to the retrospective nature of the study.
In this study, a high level resistance to ampicillin was seen in both E. faecium (44, 92%) and E. faecalis (29, 85%) respectively. Similar high resistance was also seen in E. faecium (45, 94%) and E. faecalis (26, 76%) to ciprofloxacin, as reported in other studies.[2,3] A high level of resistance to gentamicin (HLAR) was seen in the Enterococcus spp. responsible for skin and soft tissue infections. Though the number of infections due to VSE among trauma patients has increased over the years in the study, there was no significant risk of mortality associated with it.
Our study has several strengths. It is a study of Enterococcal wound and soft tissue infections on trauma patients and no such large-scale study have been reported. Trauma patients generally lack underlying illness/co-morbidities and are of middle age. Thus, these infections are undoubtedly nosocomial. The incidence of wound/soft tissue infection was expressed per 1000 patient admissions instead of according to samples. The changing pattern of Enterococcus sp. was clearly noted in the study and may act as a baseline data for comparison in future. Also, the study tried to look into possible risk factors.
This study is not without limitations. First, it is the single-center study design, which makes extrapolation to other institutions difficult. Second, it is not a randomized study, with the resulting risk of bias due to confounding factors. As the majority of the patients had VSE wound/soft tissue infections during the study period (2011 to 2013), matching VRE and VSE patients for date of admission was not possible in the study period. Furthermore, we were unable to obtain the standardized data on costs of hospitalization due to Enterococcal wound and soft tissue infections.
CONCLUSION
Enterococcal wound and soft tissue infections are prevalent in trauma care facilities, especially in the ICUs. Appropriate infection control may be responsible for a low level of VRE.
ACKNOWLEDGEMENT
The authors would like to thank the team of Hospital Infection Control of JPNATC, All India Institute of Medical Sciences, New Delhi, for their efforts in conducting this study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. National Healthcare Safety Network Team. Participating National Healthcare Safety Network Facilities. NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention 2006-2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 2.Rubinstein E, Keynan Y. Vancomycin-resistant enterococci. Crit Care Clin. 2013;29:841–52. doi: 10.1016/j.ccc.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Leclercq R, Courvalin P. Enterococcus. In: White DG, Alekshun MN, McDermott PF, editors. Frontiers in antimicrobial resistance: A tribute to Stuart B. Levy. Washington, DC: ASM Press; 2005. [Google Scholar]
- 4.Siesing PC, Alva-Jørgensen JP, Brodersen J, Arpi M, Jensen PE. Rising incidence of Enterococcus species in microbiological specimens from orthopedic patients correlates to increased use of cefuroxime: A study concentrating on tissue samples. Acta Orthop. 2013;84:319–22. doi: 10.3109/17453674.2013.792028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shorman M, Al-Tawfiq JA. Risk factors associated with vancomycin-resistant enterococcus in intensive care unit settings in Saudi Arabia. Interdiscip Perspect Infect Dis 2013. 2013:369674. doi: 10.1155/2013/369674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byun SJ, Kang J. Risk factors and clinical outcomes for vancomycin-resistant enterococcus colonization on intensive care unit admission. J Korean Acad Nurs. 2013;43:287–95. doi: 10.4040/jkan.2013.43.2.287. [DOI] [PubMed] [Google Scholar]
- 7.Omotola AM, Li Y, Martin ET, Alshabani K, Yadav D, Sarkar M, et al. Risk factors for and epidemiology of community-onset vancomycin-resistant Enterococcus faecalis in southeast Michigan. Am J Infect Control. 2013;41:1244–8. doi: 10.1016/j.ajic.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Jorgensen JH, Hindler JA, Bernard K, Citron DM, Cockerill FR, Fritsche TR, et al. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2010. M45-A2: Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Approved Guideline. [Google Scholar]
- 9.Cockerill FR, Wikler MA, Alder J, Dudley MN, Eliopoulos GM, Ferraro MJ, et al. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI); 2012. M100-S22: Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. [Google Scholar]
- 10.Matuschek E, Brown DF, Kahlmeter G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect. 2014;20:O255–66. doi: 10.1111/1469-0691.12373. [DOI] [PubMed] [Google Scholar]
- 11.Barnaud G, Bingen E. Genotypic characterisation of endemic VanA Enterococcus faecium strains isolated in a paediatric hospital. J Med Microbiol. 2000;49:793–9. doi: 10.1099/0022-1317-49-9-793. [DOI] [PubMed] [Google Scholar]
- 12.Mundy LM, Sahm DF, Gilmore M. Relationships between enterococcal virulence and antimicrobial resistance. Clin Microbiol Rev. 2000;13:513–22. doi: 10.1128/cmr.13.4.513-522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sood S, Malhotra M, Das BK, Kapil A. Enterococcal infections & antimicrobial resistance. Indian J Med Res. 2008;128:111–21. [PubMed] [Google Scholar]


