Abstract
Objective
Long-acting basal insulin analogs have demonstrated positive effects on the balance between effective glycemic control and risk of hypoglycemia versus neutral protamine Hagedorn (NPH) insulin in randomized controlled trials. Evidence of severe hypoglycemic risk with insulin detemir, insulin glargine, or NPH insulin is presented from a nationwide retrospective database study.
Research design and methods
Data from hospital and secondary healthcare visits due to hypoglycemic coma from 75 682 insulin-naïve type 1 or 2 diabetes patients initiating therapy with NPH insulin, insulin glargine, or insulin detemir in Finland between 2000 and 2009 were analyzed. Incidence rates with 95% confidence intervals (CIs) were calculated using Poisson regression. Hazard ratios were estimated using Cox's regression with adjustments for relevant background variables.
Results
The adjusted risk of hospital/secondary healthcare visits due to the first severe hypoglycemic event was 21.7% (95% CI 9.6–32.1%, p < 0.001) lower for insulin detemir and 9.9% (95% CI 1.5–17.6%, p = 0.022) lower for insulin glargine versus NPH insulin. Risk of hypoglycemic coma recurrence was 36.3% (95% CI 8.9–55.5%, p = 0.014) lower for detemir and 9.5% but not significantly (95% CI −10.2 to 25.7%, p = 0.318) lower for glargine versus NPH insulin. Risk of all hypoglycemic coma events was 30.8% (95% CI 16.2–42.8%, p-value <0.001) lower for detemir and 15.6% (95% CI 5.1–25.0%, p-value 0.005) lower for glargine versus NPH. Insulin detemir had a significantly lower risk for first (13.1% lower [p = 0.034]), recurrent (29.6% lower [p = 0.021]), and all (17.9% lower [p = 0.016]) severe hypoglycemic events than insulin glargine.
Conclusions
There were considerable differences in risk of hospitalization or secondary healthcare visits due to hypoglycemic coma between basal insulin treatments in real-life clinical practice.
Keywords: hypoglycemia, basal insulin, register linkage study, pharmacoepidemiology
Severe hypoglycemia is a common acute complication in people with diabetes being treated with insulin.1 Although the prevalence of severe hypoglycemia in insulin-treated type 2 diabetes is generally considered to be lower than in type 1 diabetes, hypoglycemia occurs at clinically significant levels2,3 that increase in frequency with the duration of insulin therapy.1
Severe hypoglycemia has important implications for diabetes management, quality of life, and costs, both to the patient and the society. A recent survey of older adults in the USA showed that insulins were the second most common medication class associated with hospitalization due to adverse drug events, with hypoglycemia accounting for most of these events.4
Older patients with type 2 diabetes may be more vulnerable to hypoglycemia because of impaired counter-regulatory responses and awareness of autonomic symptoms.5 In type 2 patients with a history of major macrovascular or microvascular disease or at least one cardiovascular risk factor, severe hypoglycemia is strongly associated with adverse clinical outcomes, such as macrovascular events and death from a cardiovascular course.34 In addition, a history of hypoglycemic episodes severe enough to require hospitalization or emergency department visits is associated with an increase in the risk of dementia.6 Recurrent severe hypoglycemia can also increase the risk of long-term cognitive impairment in children with type 1 diabetes.7
The long-acting basal insulin analogs, insulin glargine and insulin detemir, which, owing to their improved pharmacokinetics, are able to more closely replicate endogenous 24-h basal insulin secretion, have demonstrated a positive effect on the balance between effective glycemic control and overall hypoglycemia risk compared with protaminated human insulin (neutral protamine Hagedorn [NPH] insulin)8–10; a trend toward lower risk of severe hypoglycemia has also been observed.10
The primary aim of this nationwide, retrospective, follow-up study was to evaluate the incidence of hospitalization and secondary healthcare visits due to severe hypoglycemia, hypoglycemic coma, in patients with diabetes, comparing the use of the three available long-acting insulins, NPH insulin, insulin glargine, and insulin detemir, in Finland during 2000–2009. The incidence of childhood type 1 diabetes is very high among this population,11 and prescription bias is minimized by the publicly funded healthcare system,12 which offers full (100%) reimbursement of insulin and oral antidiabetic medicine prescriptions for patients diagnosed with diabetes, and the lack of recommendations regarding insulin preference for specific patient groups.
RESEARCH DESIGN AND METHODS
Data sources
Data on insulin usage were obtained from the nationwide Finnish Prescription Register, which contains data on reimbursed drugs. Drugs prescribed by a doctor or dentist are partly reimbursed. Special refunds of medicine expenses are paid to patients who have a statement from their doctor attesting to their condition, such as diabetes and need of medication. The Finnish Registry for Reimbursed Medications identifies these patients. Prescription data in the register include the generic name of the drug, the Anatomical Therapeutic Chemical classification system (ATC) code,13 the defined daily dose, and the date of purchase of the reimbursed drug.
Information regarding hospitalization due to severe hypoglycemia (ICD-10 E10.00 for insulin-dependent diabetes with hypoglycemic coma or E11.00 for non-insulin-dependent diabetes with hypoglycemic coma), hereafter referred to as hypoglycemic coma, was obtained from the Finnish Hospital Care Register, which contains data concerning all hospitalization periods and outpatient secondary healthcare visits. All hospitals in Finland are included in this database. From the Finnish Hospital Care Register, we obtained the diagnosis (ICD-10 codes), start and end date of the period, and hospital district. Date and cause of death were obtained from the Finnish Causes of Death Register.14 Unique personal identification numbers are used in all these registers, enabling data to be identified for use in this study.15 Study database was constructed by linking these databases using unique personal identification numbers.
Study population
A total of 148 482 individuals made at least one insulin purchase (ATC A10A, ATC code of insulins and analogs) between 1 January 2000 and 31 December 2009 in Finland. Of these, 140 034 were entitled to special reimbursement for diabetes indicated by a statement from their doctor; 2003 patients who had reimbursement diagnosis related to diabetes types other than type 1 or 2 and 37 patients who had <1 day of follow-up time were excluded from the analysis. The resulting study population of 137 994 individuals was stratified into subgroups defined by pre-study and follow-up period: insulin use: naïve (no prior use of study insulins), non-naïve (use of study insulins during a 5-year period before the start of the follow-up study), other (no use of study insulins), and by type of diabetes (type1, type 2, or undefined). Definitions and a schematic representation of the study population are presented in Figure 1.
Figure 1.
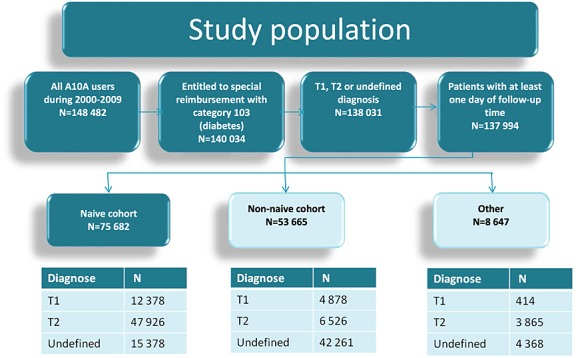
Schematic description of the study population with subgroup sizes
We report results on the naïve population (n = 75 682) to avoid the many types of biases inherent in observational pharmacoepidemiological studies.16 A new-user design identifies a population of patients with diabetes who have progressed to the stage of the disease no more controlled by other antidiabetic medications and therefore need to initiate basal insulin treatment.
Outcome, exposure, and background variables
The outcome measures of the study were incidence of the first severe hypoglycemic event with coma (codes E10.00 or E11.00 according to the Finnish version of ICD-10) during follow-up, recurrence of hypoglycemic coma, and incidence of all events, as obtained from hospitalizations and secondary care visits.
Current insulin treatment (NPH, insulin detemir, insulin glargine, or other) was used as a time-dependent exposure variable. Further details are given in the Statistical Methods Section.
The following variables evaluated from the baseline period at the start of follow-up were used in the analysis: type of diabetes (type 1, type 2, or undefined), age at the start of follow-up, gender, use of other insulins prior to the start of follow-up, history of hypoglycemia prior to the start of follow-up, calendar year at the start of follow-up, years from diagnosis of diabetes at the start of follow-up, and hospital district. Current use of other insulins, use of sulfonylureas prior to the start of follow-up, current use of sulfonylureas, and switch from one insulin to another during follow-up were used as time-dependent background variables. Other antidiabetic medications were not included as background variables because they are not associated with the risk of severe hypoglycemia.1,2
The start of follow-up was defined as the date of the first purchase of insulin detemir, glargine, or NPH between 1 January 2000 and 2009 December 31. In Finland, glargine became fully reimbursed in type 1 diabetes in 2003 and detemir in 2005, and both became fully reimbursed in type 2 diabetes in 2007. The time from 1995 until to the start of follow-up was considered as the baseline period. In the analysis of recurrence of hypoglycemic coma, the follow-up started at the end of the first severe hypoglycemic coma episode. The follow-up ended at death, time of the outcome event, or the end of the study period (31 December 2009) whichever came first. In the analysis of all hypoglycemic coma events, the follow-up ended at death or the end of the study period whichever came first.
Statistical methods
Crude incidence rates with 95% confidence intervals (CIs) were calculated on the basis of Poisson distribution. To adjust for background variables, a Cox's regression model17 was applied, which enabled the follow-up time of each patient to be divided into several periods, allowing adjustments for both baseline variables and time-dependent exposure and other time-dependent variables in the model.
The population was divided into three groups—naïve, non-naïve, and others—according to their use of insulin detemir, insulin glargine, or NPH insulin during (i) history (1995–1999); and (ii) follow-up (2000–2009) as follows:
An individual was considered naïve if (s)he had no history of use of detemir, glargine, or NPH and had at least one purchase of detemir, glargine, or NPH during the follow-up.
An individual was considered non-naïve if (s)he had history of use of detemir, glargine, or NPH and had at least one purchase of detemir, glargine, or NPH during the follow-up.
An individual was classified into others if (s)he had no history of use of detemir, glargine, or NPH and had no purchase of detemir, glargine, or NPH during the follow-up but had purchased some other insulin.
The study population was further divided into three groups, type 1 diabetes, type 2 diabetes, and undefined on the basis of the diagnoses in their reimbursement decisions for diabetes (special refund category 103).
Drug exposure periods started from the date of purchase and lasted until the effective length of a drug prescription. The effective length of a prescription was based on the number of defined daily doses contained in the prescription plus a 15% grace period in order to join consecutive drug exposure periods. The current use of detemir, glargine, or NPH at any given time during the follow-up was calculated from these drug exposure periods and used as a time-dependent exposure variable. It was possible that two consecutive drug exposure periods containing different insulins were overlapping. In such a case, we assumed that the patient switched to use another insulin at the time of purchase of the new prescription as it is unlikely for a patient to use two long-acting basal insulins at the same time. It was also possible that a patient did not use any of the insulins at some point, which was denoted by NO DGN (no detemir, glargine, or NPH use).
For every patient, a variable, years from diagnosis, was calculated as time difference from the minimum of the date of reimbursement decision, first date of purchase of sulfonylureas or insulins until the start of follow-up (as defined previously).
Several sensitivity analyses were performed to investigate the robustness of the findings. We carried out sensitivity analyses by modifying definition of the hypoglycemic coma event as an endpoint.
A subgroup analysis was performed in the populations of patients with type 1 and type 2 diabetes.
RESULTS
The mean follow-up period was 4.1 years, and NPH insulin was the most frequent first insulin for all diagnosis types (Table 1). In total, 9716 severe hypoglycemic events were identified, of which 5669 were first events during the follow-up period (Table 2). Crude incidence rate for the first hypoglycemic coma event (per 100 person-years) was lowest in patients with type 1 diabetes using insulin detemir and highest in patients with type 1 diabetes using insulin NPH. The adjusted risk of the first hypoglycemic coma event was 21.7% (95% CI 9.6–32.1%, p < 0.001) lower for insulin detemir and 9.9% (95% CI 1.5–17.6%, p = 0.022) lower for insulin glargine compared with NPH insulin (see Appendix 1 for a detailed description of risk of hypoglycemic coma events). Similarly, the adjusted risk for the recurrence of hypoglycemic coma was 36.3% (95% CI 8.9–55.5%, p = 0.014) lower for detemir (versus NPH) and 9.5% (95% CI −10.2 to 25.7%, p = 0.318) lower for glargine (versus NPH). Furthermore, the adjusted overall risk for hypoglycemic coma was 30.8% (95% CI 16.2–42.8%, p < 0.001) lower for detemir (versus NPH) and 15.6% (95% CI 5.1–25.0%, p = 0.005) lower for glargine (versus NPH). In comparison between detemir and glargine, detemir had a statistically significantly 13.1% (1.0–23.6%), 29.6% (5.1–47.8%), and 17.9% (3.6–30.1%) lower risk for first, recurrent, and overall hypoglycemic coma (p = 0.034, p = 0.021, and p = 0.016), respectively. Patients with type 2 diabetes had statistically significantly 15.5% (2.7–29.9%) and 41.7% (6.7–88.2%) higher risk for first and overall hypoglycemic coma (p = 0.016 and p = 0.016), respectively, when compared with type 1 diabetes. However, no statistically significant difference was observed for the risk of recurrent hypoglycemic coma between type 1 and type 2 diabetes. Sensitivity analyses for the first and recurrent severe hypoglycemic coma (Appendix 1) showed findings to be robust to the various alternative analysis strategies. The main findings, in absolute and relative risk terms, are presented for the naïve population graphically (Figure 2).
Table 1.
Basic characteristics of the naïve study population by reimbursement diagnosis (n and percentage)
| Type 1 diabetes | Type 2 diabetes | Undefined | Total | ||
|---|---|---|---|---|---|
| Mean age (years) | 26.56 | 63.33 | 68.06 | 58.27 | |
| Gender | Male/female (n) | 7658/4720 | 27 247/20 679 | 8077/7301 | 42 982/32 700 |
| Male/female (%) | 61.9/38.1 | 56.9/43.1 | 52.5/47.5 | 56.8/43.2 | |
| First insulin started | Detemir | 1909 | 4972 | 573 | 7454 |
| 15.4% | 10.4% | 3.7% | 9.8% | ||
| Glargine | 3359 | 12 504 | 2500 | 18 363 | |
| 27.1% | 26.1% | 16.3% | 24.3% | ||
| Neutral protamine Hagedorn | 7110 | 30 450 | 12 305 | 49 865 | |
| 57.4% | 63.5% | 80.0% | 65.9% | ||
| Years from diagnosis at the start of follow-up | 0–1 | 11 395 | 12 702 | 63 | 24 160 |
| 92.1% | 26.5% | 0.4% | 31.9% | ||
| 1–2 | 191 | 3721 | 61 | 3973 | |
| 1.5% | 7.8% | 0.4% | 5.2% | ||
| 2–5 | 313 | 12 699 | 607 | 13 619 | |
| 2.5% | 26.5% | 3.9% | 18.0% | ||
| 5–10 | 350 | 15 139 | 4285 | 19 774 | |
| 2.8% | 31.6% | 27.9% | 26.1% | ||
| 10+ | 129 | 3665 | 10 362 | 14 156 | |
| 1.0% | 7.6% | 67.4% | 18.7% | ||
| Total n | 12 378 | 47 926 | 15 378 | 75 682 |
Table 2.
Incidence of first, recurrent, and all hypoglycemic coma events for insulin detemir, insulin glargine, and NPH insulin, stratified by reimbursement diagnosis
| Diagnosis | Insulin | Events | Person-years | Rate with 95% confidence interval (per 100 person-years) | ||
|---|---|---|---|---|---|---|
| First hypoglycemic coma | ||||||
| Type 1 diabetes | Glargine | 272 | 18 850 | 1.443 | 1.281 | 1.625 |
| Detemir | 116 | 9520 | 1.218 | 1.016 | 1.462 | |
| NPH | 454 | 19 563 | 2.321 | 2.117 | 2.544 | |
| None/other | 100 | 6085 | 1.643 | 1.351 | 1.999 | |
| Type 2 diabetes | Glargine | 660 | 33 958 | 1.944 | 1.801 | 2.098 |
| Detemir | 135 | 10 120 | 1.334 | 1.127 | 1.579 | |
| NPH | 1937 | 96 164 | 2.014 | 1.927 | 2.106 | |
| None/other | 474 | 27 375 | 1.732 | 1.582 | 1.895 | |
| Undefined | Glargine | 268 | 12 943 | 2.071 | 1.837 | 2.334 |
| Detemir | 52 | 2772 | 1.876 | 1.430 | 2.462 | |
| NPH | 939 | 44 507 | 2.110 | 1.979 | 2.249 | |
| None/other | 262 | 11 533 | 2.272 | 2.013 | 2.564 | |
| Total | Glargine | 1200 | 65 751 | 1.825 | 1.725 | 1.931 |
| Detemir | 303 | 22 412 | 1.352 | 1.208 | 1.513 | |
| NPH | 3330 | 160 233 | 2.078 | 2.009 | 2.150 | |
| None/other | 836 | 44 993 | 1.858 | 1.736 | 1.988 | |
| Recurrent hypoglycemic coma events | ||||||
| Type 1 diabetes | Glargine | 305 | 1320 | 23.109 | 20.656 | 25.854 |
| Detemir | 117 | 806 | 14.514 | 12.108 | 17.397 | |
| NPH | 343 | 1084 | 31.651 | 28.473 | 35.184 | |
| None/other | 95 | 505 | 18.800 | 15.375 | 22.987 | |
| Type 2 diabetes | Glargine | 469 | 1967 | 23.840 | 21.777 | 26.099 |
| Detemir | 82 | 485 | 16.910 | 13.619 | 20.996 | |
| NPH | 1377 | 4557 | 30.215 | 28.660 | 31.854 | |
| None/other | 343 | 1853 | 18.506 | 16.648 | 20.572 | |
| Undefined | Glargine | 184 | 1013 | 18.156 | 15.713 | 20.978 |
| Detemir | 29 | 283 | 10.265 | 7.134 | 14.772 | |
| NPH | 526 | 2202 | 23.890 | 21.933 | 26.021 | |
| None/other | 177 | 964 | 18.368 | 15.852 | 21.283 | |
| Total | Glargine | 958 | 4301 | 22.276 | 20.909 | 23.733 |
| Detemir | 228 | 1574 | 14.489 | 12.726 | 16.498 | |
| NPH | 2246 | 7843 | 28.638 | 27.477 | 29.847 | |
| None/other | 615 | 3322 | 18.511 | 17.104 | 20.033 | |
| All hypoglycemic coma events | ||||||
| Type 1 diabetes | Glargine | 577 | 20 185 | 2.859 | 2.635 | 3.102 |
| Detemir | 233 | 10 333 | 2.255 | 1.983 | 2.564 | |
| NPH | 797 | 20 672 | 3.855 | 3.597 | 4.133 | |
| None/other | 195 | 6599 | 2.955 | 2.568 | 3.400 | |
| Type 2 diabetes | Glargine | 1129 | 35 951 | 3.140 | 2.962 | 3.329 |
| Detemir | 217 | 10 609 | 2.045 | 1.791 | 2.336 | |
| NPH | 3314 | 100 796 | 3.288 | 3.178 | 3.402 | |
| None/other | 817 | 29 285 | 2.790 | 2.605 | 2.988 | |
| Undefined | Glargine | 452 | 13 965 | 3.237 | 2.952 | 3.549 |
| Detemir | 81 | 3056 | 2.651 | 2.132 | 3.295 | |
| NPH | 1465 | 46 744 | 3.134 | 2.978 | 3.299 | |
| None/other | 439 | 12 523 | 3.506 | 3.193 | 3.849 | |
| Total | Glargine | 2158 | 70 101 | 3.078 | 2.951 | 3.211 |
| Detemir | 531 | 23 998 | 2.213 | 2.032 | 2.409 | |
| NPH | 5576 | 168 212 | 3.315 | 3.229 | 3.403 | |
| None/other | 1451 | 48 407 | 2.997 | 2.847 | 3.156 | |
NPH, neutral protamine Hagedorn.
Figure 2.
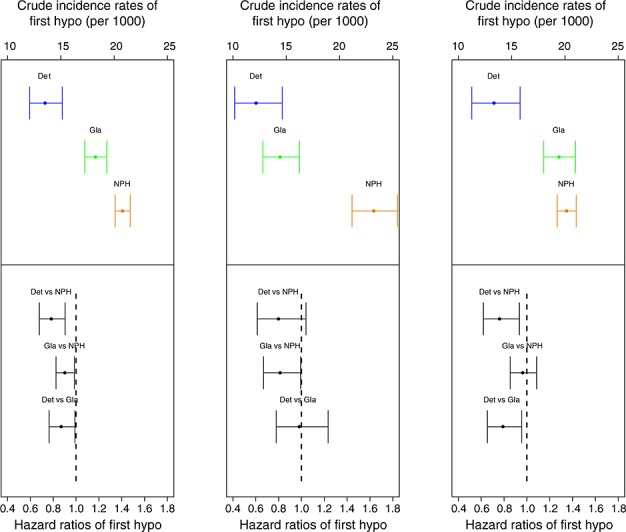
Crude incidence rates and adjusted hazard ratios of first hypoglycemic coma events among the naïve population by reimbursement diagnosis (left, all combined; middle, type 1 diabetes; and right, type 2 diabetes). Comparison of adjusted hazard ratios based on the Cox proportional hazards model with 95% CI
When background variables were considered, an increasing risk of first hypoglycemic coma event in older adults compared with the younger population was observed (Table 3). The risk of hypoglycemic coma was also higher in the youngest group of patients (0–9 years). Concomitant use of other insulin types was associated with increased risk of first hypoglycemic coma. The risk of first hypoglycemic coma was slightly lower in women (hazard ratio 0.943, 95% CI 0.894–0.996). History of severe hypoglycemia before the start of follow-up was associated with a greater than fourfold risk of first hypoglycemic coma event (hazard ratio 4.069, 95% CI 3.811–4.344) (Table 3).
Table 3.
Risk for first hypoglycemic coma estimated by the Cox proportional hazards model
| Hazard ratio with 95% confidence interval | p-value | ||||
|---|---|---|---|---|---|
| Gender | Male | (Reference) | |||
| Female | 0.943 | 0.894 | 0.996 | 0.036 | |
| Age group (years) | 0–9 | 1.378 | 1.113 | 1.706 | 0.003 |
| 10–19 | 1.097 | 0.878 | 1.370 | 0.417 | |
| 20–29 | (Reference) | ||||
| 30–39 | 1.223 | 0.978 | 1.529 | 0.077 | |
| 40–49 | 1.576 | 1.285 | 1.933 | <0.001 | |
| 50–59 | 1.467 | 1.200 | 1.792 | <0.001 | |
| 60–69 | 1.712 | 1.398 | 2.096 | <0.001 | |
| 70–79 | 1.960 | 1.596 | 2.406 | <0.001 | |
| 80+ | 2.183 | 1.760 | 2.708 | <0.001 | |
| Use of oral diabetes medication at the start of follow-up | No | (Reference) | |||
| Yes | 1.063 | 0.971 | 1.164 | 0.185 | |
| Current use of sulfonylureas | No | (Reference) | |||
| Yes | 0.961 | 0.898 | 1.027 | 0.240 | |
| History use of insulins at the start of follow-up | No | (Reference) | |||
| Yes | 1.150 | 1.044 | 1.268 | 0.005 | |
| Current use of insulins other than glargine, detemir, and NPH insulins | No | (Reference) | |||
| Yes | 1.318 | 1.233 | 1.409 | <0.001 | |
| History of hypoglycemia | No | (Reference) | |||
| Yes | 4.069 | 3.811 | 4.344 | <0.001 | |
| Switch of insulin | No | (Reference) | |||
| Yes | 1.109 | 1.000 | 1.229 | 0.049 | |
| Time since diagnosis (years) | <1 | (Reference) | |||
| 1–2 | 1.057 | 0.922 | 1.212 | 0.428 | |
| 2–5 | 0.929 | 0.835 | 1.034 | 0.176 | |
| 5–10 | 0.974 | 0.875 | 1.085 | 0.631 | |
| >10 | 1.117 | 0.980 | 1.273 | 0.097 | |
| Calendar year of the start of follow-up | ≤2005 | (Reference) | |||
| 2006 | 0.749 | 0.671 | 0.835 | <0.001 | |
| 2007 | 0.658 | 0.584 | 0.742 | <0.001 | |
| 2008 | 0.606 | 0.521 | 0.705 | <0.001 | |
| 2009 | 0.460 | 0.363 | 0.583 | <0.001 | |
Type of diabetes and current insulin were included in the model.
In subgroup analysis of patients with type 1 and type 2 patients, we found similar differences between the insulins as those reported for the overall population in the risk for the first, recurrent, and overall hypoglycemic coma events in the type 2 diabetes population and in the risk of first hypoglycemic coma event in the type 1 diabetes population (Appendices 2 and 3).
CONCLUSIONS
We compared the incidence of hypoglycemic coma between the long-acting insulins NPH, glargine, and detemir in a large, nationwide, unselected population of people with diabetes in a real-life clinical setting in Finland, where the cost of insulin medication is fully reimbursed. Considerable differences in the absolute risks and statistically significant differences in the adjusted relative risks of the first, recurrent, and overall hypoglycemic events between the use of insulin detemir, glargine, and NPH in the defined population were found. The observed risks for hypoglycemic coma were, in general, lowest with insulin detemir and highest with NPH. In subgroup analyses, these findings were consistent for the risk of first, recurrent, and overall hypoglycemic coma events in patients with type 2 diabetes and for the risk of first hypoglycemic coma in patients with type 1 diabetes.
In randomized controlled trials, both insulin glargine and insulin detemir have consistently shown a reduced incidence of overall and nocturnal hypoglycemia compared with NPH in type 1 and type 2 diabetes.9,18–23 Clinical trials generally report a very low incidence of severe hypoglycemia. Apart from the small number of events likely to occur in the limited timeframes and cohorts studied in randomized trials, there is also the issue that frequent and/or severe hypoglycemic events are a common exclusion criterion for patients' eligibility. Furthermore, incidence of severe hypoglycemia increases with longer duration of insulin therapy,1 thus studies focusing on insulin initiation will inevitably underestimate the long-term risk of severe hypoglycemia. Nevertheless, a recent Cochrane review found a trend similar to the findings reported here, with insulin glargine showing a 30% reduction and insulin detemir showing a 50% reduction in overall severe hypoglycemia risk compared with NPH in patients with type 2 diabetes.10
Compared with clinical trials, fewer hypoglycemic events were reported in routine clinical practice both with insulin glargine and insulin detemir when used as add-on to oral diabetic medications in type 2 diabetes.24 This may be due to several factors. Sulfonylureas were often discontinued when initiating insulin analogs, and the average daily dose of insulin was lower than that used in clinical trials. Observational studies carried out in real-life clinical practice have suggested that significant reductions in severe hypoglycemia can be achieved after switching insulin therapy to insulin detemir in patients with type 1 and type 2 diabetes.24–27 Non-interventional studies do not have tightly controlled populations or control groups, which limit the certainty with which clinical outcomes can be ascribed to treatment; and hypoglycemic data based on patient recall may be subject to recall bias. However, published results are consistent with the findings of this analysis.
One potential explanation for our finding of reduced hypoglycemic coma risk with insulin detemir or glargine is the decreased day-to-day variability of glucose-lowering action. A pharmacodynamic study in patients with type 1 diabetes injecting identical doses of the insulin in the same injection site once daily for 4 days showed that, compared with NPH, day-to-day variability of glucose-lowering action was 60% and 30% lower with detemir and glargine, respectively.28 In the randomized controlled clinical trials involving patients with type 1 diabetes, insulin detemir was associated with significantly less within-patient fasting blood glucose variability compared with NPH.20,23 We have previously reported an independent association between the variability of fasting plasma glucose and the risk of nocturnal hypoglycemia in type 1 and type 2 diabetes.29
The reduction in the risk of recurrent hypoglycemic coma was more prominent than the risk of first hypoglycemic coma event for insulin glargine and particularly for insulin detemir compared with NPH. This finding may have significant clinical relevance, as patients suffering from repeated severe hypoglycemic events may be the most vulnerable among patients with diabetes, and thus, there is a need for clinical measures to reduce this risk.
The increasing risk of hospitalization or secondary healthcare visits due to hypoglycemic coma in the older age group is another finding deserving attention. There is a possibility of bias in the data because of older patients with diabetes being less able to manage a severe hypoglycemic event at home than younger patients, but it is important to note that elderly patients may have a greater risk of associated morbidity, for example, cardiovascular events. In the ADVANCE study, severe hypoglycemia was strongly associated with increased risks of adverse clinical outcomes including macrovascular and microvascular events and death30; the subjects were patients with type 2 diabetes, >55 years of age. Severe hypoglycemia in the older age groups may also have longer standing consequences; according to a recent study, self-reported history of severe hypoglycemia was associated with age-related cognitive decline.31
Treatment of hypoglycemic coma can add considerably to healthcare costs. Although difficult to quantify, surveys attempting to assess the impact of providing acute treatment for hypoglycemic events, and subsequent follow-up care, have reported that, irrespective of the national healthcare system surveyed, cases where patients experience severe hypoglycemic events that require hospitalization outweigh all other diabetes-related costs.32,33 Furthermore, the registry-based data presented here may substantially underestimate the actual incidence of all hypoglycemic events. However, incidence of hypoglycemic coma is probably much less underestimated. A recent Finnish population-based survey reported that only 6% of all insulin-treated patients required intensive or emergency treatment for severe hypoglycemia.34 In this study, we had 5669 first events and in population of 75 682, which means that 7.5% of the study population had at least one hypoglycemic coma event during follow-up.
A number of sensitivity analyses were performed to address sources of potential bias and improve the strength of our findings, such as overlapping use of study insulins, different periods of commercial availability of the insulins, selection of the first insulin, and reclassification of no exposure periods; however, several factors must be kept in mind when evaluating results from any observational study on the basis of the record linkage. In this study, the use of prescribed drugs, especially oral antidiabetic medications, could not be verified with certainty and may be subject to misclassification. Although there were many variables included in this study, there was relatively limited information (age, sex, previous hospitalizations, and special refund data) about risk factors connected to hypoglycemia from the available registers. In particular, we had no information about glycemic control during follow-up, and insulins were prescribed empirically and not randomized. It is also unknown which caused the hypoglycemic event, insulin or an underlying disease, when many diabetes-related severity factors are not measured. It is therefore impossible to conclude definitively that there is a causal relationship between current insulin type and outcomes. However, the number of subjects and length of follow-up, plus the robustness of results after performing sensitivity analyses, gives support to the credibility of the results. The use of only naïve, new-insulin-user data reduces bias.16 Non-naïve (prevalent) insulin users are “survivors” of an earlier period of diabetes treatment, which can introduce substantial bias because it is known that the risk connected to the disease varies with time.
HbA1c documentation was not available for this study. The association of more intensive glycemic control with an increased risk of hypoglycemia is well established, and it would have been valuable to adjust the results for HbA1c levels. However, there is no evidence to suggest glycemic control is worse among patients using long-acting insulin analogs; in fact, average HbA1c in insulin-treated patients with type 1 as well as type 2 diabetes in Finland has slightly improved between the years 2000 and 2009.35 Thus, we do not believe the reduced risk of hypoglycemic coma during use of insulin detemir or glargine can be explained by higher average glucose levels.
In conclusion, the real-life data showed considerable differences in risk for hospitalization or secondary healthcare visit due to hypoglycemic coma between insulin detemir, insulin glargine, and NPH insulin in diabetic patients initiating basal insulin therapy during follow-up. Therefore, the risk of hospitalization due to hypoglycemic coma could potentially be modified by the selection of the long-acting insulin.
CONFLICT OF INTEREST
J. H. has had research agreements with Janssen-Cilag, Novartis, Orion Pharma, Abbott, Novo Nordisk Farma, Pfizer, Sanofi-Aventis, Astellas, and Takeda. S. M. and T. S. are employees of Novo Nordisk A/S, the manufacturer of insulin detemir. P. K. has had research agreements with Janssen-Cilag, Novartis, Orion Pharma, Abbott, Novo Nordisk Farma, Pfizer, Sanofi-Aventis, and Takeda. F. H. and P. E. have no financial disclosures to report.
KEY POINTS
The real-life data showed considerable differences in risk for hospitalization or secondary healthcare visits due to hypoglycemic coma between basal insulin treatments.
The risk of hospitalization due to hypoglycemic coma could potentially be modified by the selection of the long-acting insulin.
ETHICS STATEMENT
This is a register-based study with anonymous data and no patient contact. The study protocol was approved by the Ethical Review Board of the Hjelt Institute, University of Helsinki Medical Faculty.
Acknowledgments
This study was supported by Novo Nordisk Farma. The funders were not involved in the conduct of the study, collection, management, or analysis of the data. The research permission numbers to use the data were obtained from the Social Insurance Institute (Kela 14/522/2011), the National Institute for Health and Welfare (Dnro THL/408/5.05.00/2011), and the Statistics Finland (TK-53-367-11). The authors are grateful to Watermeadow Medical for their writing and editorial assistance, funded by Novo Nordisk A/S.
AUTHOR CONTRIBUTIONS
J. H. (principal investigator) was involved in the study planning, conduct, analysis and interpretation of the analyses, and preparation of the manuscript and took responsibility for the integrity of the data and the accuracy of the data analysis. F. H. (data analysis) was involved in the study planning, conduct, and analysis and interpretation of the analyses. P. E. (data analysis) was involved in the study planning, conduct, and analysis and interpretation of the analyses. S. M. (co-investigator) was involved in the study planning and interpretation of the analyses and preparation of the manuscript. T. S. (co-investigator) was involved in the study planning and interpretation of the analyses and preparation of the manuscript. P. K. (principal investigator) was involved in the study planning, conduct, and analysis and interpretation of the analyses and took responsibility for the integrity of the data and the accuracy of the data analysis.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web site:
Supplementary
Appendix 12.3. Results for the NAIVE population
Appendix 12.6. Results for the NON-NAIVE population
REFERENCES
- 1.UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 2.Garber AJ. Hypoglycaemia a therapeutic concern in type 2 diabetes. Lancet. 2012;379:2215–2216. doi: 10.1016/S0140-6736(12)60947-7. [DOI] [PubMed] [Google Scholar]
- 3.Donnelly LA, Morris AD, Frier BM, Ellis JD, Donnan PT, Durrant R, Band MM, Reekie G. Leese GP; DARTS/MEMO collaboration. Frequency and predictors of hypoglycaemia in type 1 and insulin-treated type 2 diabetes: a population-based study. Diabet Med. 2005;22:749–755. doi: 10.1111/j.1464-5491.2005.01501.x. [DOI] [PubMed] [Google Scholar]
- 4.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:200–212. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 5.Meneilly GS, Elahi D. Metabolic alterations in middle-aged and elderly lean patients with type 2 diabetes. Diabetes Care. 2005;28:1498–1499. doi: 10.2337/diacare.28.6.1498. [DOI] [PubMed] [Google Scholar]
- 6.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannonen R, Tupola S, Ahonen T, Riikonen R. Neurocognitive functioning in children with type-1 diabetes with and without episodes of severe hypoglycaemia. Dev Med Child Neurol. 2003;45(4):262–268. doi: 10.1017/s0012162203000501. [DOI] [PubMed] [Google Scholar]
- 8.Haak T, Tiengo A, Draeger E, Suntum M, Waldhäusl W. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2005;7:56–64. doi: 10.1111/j.1463-1326.2004.00373.x. [DOI] [PubMed] [Google Scholar]
- 9.Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 10.Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;2 doi: 10.1002/14651858.CD005613.pub3. CD005613. [DOI] [PubMed] [Google Scholar]
- 11.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371(9626):1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 12.Tynkkynen LK, Lehto J, Miettinen S. Framing the decision to contract out elderly care and primary health care services - perspectives of local level politicians and civil servants in Finland. BMC Health Serv Res. 2012;12:201. doi: 10.1186/1472-6963-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Collaborating Centre for Drug Statistics Methodology. Oslo, Norway: Norwegian Institute of Public Health, 2012; 2010. (Accessed July 10, at http://www.whocc.no/) [Google Scholar]
- 14.Lahti R, Penttilä A. The validity of death certificates: routine validation of death certification and its effects on mortality statistics. Forensic Sci Int. 2001;115:15–32. doi: 10.1016/s0379-0738(00)00300-5. [DOI] [PubMed] [Google Scholar]
- 15.Gissler M, Haukka J. Finnish health and social welfare registers in epidemiological research. Norsk Epidemiologi. 2004;14:113–120. [Google Scholar]
- 16.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 17.Andersen PK, Borgan O, Gill RD, Keiding N. Statistical models based on counting processes. New York: Springer-Verlag; 1993. [Google Scholar]
- 18.Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study group of insulin glargine in type 1 diabetes. Diabetes Care. 2000;23:639–643. doi: 10.2337/diacare.23.5.639. [DOI] [PubMed] [Google Scholar]
- 19.Home P, Bartley P, Russell-Jones D, et al. Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes a randomized clinical trial. Diabetes Care. 2004;27:1081–1087. doi: 10.2337/diacare.27.5.1081. [DOI] [PubMed] [Google Scholar]
- 20.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269–1274. doi: 10.2337/dc05-1365. [DOI] [PubMed] [Google Scholar]
- 21.Hermansen K, Fontaine P, Kukolja KK, Peterkova V, Leth G, Gall MA. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetes. Diabetologia. 2004;47:622–629. doi: 10.1007/s00125-004-1365-z. [DOI] [PubMed] [Google Scholar]
- 22.Kølendorf K, Ross GP, Pavlic-Renar I, et al. Insulin detemir lowers the risk of hypoglycaemia and provides more consistent plasma glucose levels compared with NPH insulin in type 1 diabetes. Diabet Med. 2006;23:729–735. doi: 10.1111/j.1464-5491.2006.01862.x. [DOI] [PubMed] [Google Scholar]
- 23.Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28:1569–1581. doi: 10.1016/j.clinthera.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Pollock RF, Erny-Albrecht KM, Kalsekar A, Bruhn D, Valentine WJ. Long-acting insulin analogs a review of “real-world” effectiveness in patients with type 2 diabetes. Curr Diabetes Rev. 2011;7:61–74. doi: 10.2174/157339911794273892. [DOI] [PubMed] [Google Scholar]
- 25.Home P, Naggar NE, Khamseh M, Gonzalez-Galvez G, Shen C, Chakkarwar P, Wenying Y. An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-western countries: the A1chieve study. Diabetes Res Clin Pract. 2011;94:352–363. doi: 10.1016/j.diabres.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Dornhorst A, Lüddeke H-J, Koenen C, et al. Transferring to insulin detemir from NPH insulin or insulin glargine in type 2 diabetes patients on basal-only therapy with oral antidiabetic drugs improves glycaemic control and reduces weight gain and risk of hypoglycaemia: 14-week follow-up data from PREDICTIVE. Diabetes Obes Metab. 2008;10:75–81. doi: 10.1111/j.1463-1326.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 27.Hermansen K, Dornhorst A, Sreenan S. Observational, open-label study of type 1 and type 2 diabetes patients switching from human insulin to insulin analogue basal-bolus regimens: insights from the PREDICTIVE study. Curr Med Res Opin. 2009;25:2601–2608. doi: 10.1185/03007990903262885. [DOI] [PubMed] [Google Scholar]
- 28.Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614–1620. doi: 10.2337/diabetes.53.6.1614. [DOI] [PubMed] [Google Scholar]
- 29.Niskanen L, Virkamäki A, Hansen JB, Saukkonen T. Fasting plasma glucose variability as a marker of nocturnal hypoglycemia in diabetes: evidence from the PREDICTIVE study. Diabetes Res Clin Pract. 2009;86:e15–e18. doi: 10.1016/j.diabres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 31.Aung PP, Strachan MWJ, Frier BM, Butcher I, Deary IJ, Price JF. Severe hypoglycaemia and late-life cognitive ability in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabet Med. 2012;29:328–336. doi: 10.1111/j.1464-5491.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- 32.Jönsson L, Bolinder B, Lundkvist J. Cost of hypoglycaemia in patients with type 2 diabetes in Sweden. Value Health. 2006;9:193–1938. doi: 10.1111/j.1524-4733.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 33.Hammer M, Lammert M, Mejías SM, Kern W, Frier BM. Costs of managing severe hypoglycaemia in three European countries. J Med Econ. 2009;12:281–290. doi: 10.3111/13696990903336597. [DOI] [PubMed] [Google Scholar]
- 34.Honkasalo MT, Elonheimo OM, Sane T. Severe hypoglycaemia in drug-treated diabetic patients needs attention a population-based study. Scand J Prim Health Care. 2011;29:165–1670. doi: 10.3109/02813432.2011.580090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valle T, Eriksson J, Peltonen M, Maria A, Koski S. Glycemic control in diabetic patients in Finland in 2009–2010. DEHKO report 2010:5. Finnish Diabetes Association, Tampere 2010 [in Finnish]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary
Appendix 12.3. Results for the NAIVE population
Appendix 12.6. Results for the NON-NAIVE population


