Abstract
Diabetes mellitus (DM) is the most prevailing disease with progressive incidence worldwide. Despite contemporary treatment type one DM and type two DM are frequently associated with long-term major microvascular and macrovascular complications. Currently restoration of failing β-cell function, regulation of metabolic processes with stem cell transplantation is discussed as complements to contemporary DM therapy regimens. The present review is considered paradigm of the regenerative care and the possibly effects of cell therapy in DM. Reprogramming stem cells, bone marrow-derived mononuclear cells; lineage-specified progenitor cells are considered for regenerative strategy in DM. Finally, perspective component of stem cell replacement in DM is discussed.
Keywords: Diabetes mellitus, Regenerative medicine, Stem cells, Cellular reprogramming, Transplantation
Core tip: Modern approaches to stem cell therapy are discussed a promising component of treatment program in diabetes mellitus. It is important to emphasize that the new technology that is associated with reprogramming of stem cells has a couple of disputes in accordance with the ethical considerations and practical issues. However, the extremely high cost of novel methods toward preventing immune rejection of graft tissue and the high risk of oncogenesis retain their value as major constraints to the implementation into routine clinical practice. The purpose of the review was to summarize and analyze data for existing knowledge and prospects for future researches in the field of regenerative therapy in patients with diabetes mellitus.
INTRODUCTION
Diabetes mellitus (DM) is the most common endocrine disease, which is considered one of the most important causes of morbidity and mortality worldwide[1]. Type I DM (T1DM) and type 2 DM (T2DM) have different origins, which significantly impact on the ability to achieve adequate glycemic control. T1DM is an autoimmune disease, which is based on absolute deficiency of insulin secretion due to inflammation, necrosis or apoptosis of β cells[2]. In opposite to T1DM, T2DM is defined as predominantly age-related metabolic disease associated with insulin resistance and forming β cell dysfunction that leads to glycemia and different types of metabolic disorders[3]. Although modern treatment of DM1 and DM2 are usually effective and may sufficiently improve clinical status in short-term perspective, it often associates with vascular complications in the long term period that is discussed as a main cause of ischemic lesions of tissues and target-organs damages. All these mediate manifestation of endothelial dysfunction, retinopathy, nephropathy and cardiomyopathy[4]. The molecular mechanisms that are turned up in resulting of ischemic tissue injury and restoration of tissue perfusion lead to onset and progression of the atherosclerotic damage[5]. As a consequence, atherothrombosis and the exaggerated ischemic tissue injury leading to cardiovascular remodeling mediate increased morbidity and mortality. Overall, DM increases age-related mortality and atherothrombotic related death in two-fold time[6]. It is needed to take into consideration that not all complications of DM appear to be resulting of ischemic causes. As known there are several none-vascular factors associated with an increased risk of manifestation of DM complications, such as not adequate control for hyperglycemia, drug-induced and none-drug-induced hypoglycemia, as well as age-related metabolic comorbidity. It is well known, all they may contribute malignant evolution of DM and negatively relate with poor prognosis and tendency to low effectiveness of therapies. Currently guidelines for diabetic patient treatment focus an opinion of physicians on molecular targets that affects insulin secretion, glucose regulator peptides, hormone regulators, enzymes and transporters. However, it is predisposed that treatment approaches would also mediate improving of hypoglycemia associated with suppression of advanced glycation end products accumulation, decreasing of reactive oxygen species overproduction, improving dyslipidemia and endothelial dysfunction, prevention of atherosclerosis, modification of coexisting cardiovascular risk factors and achieving of adequate control for metabolic comorbidities[7].
Therefore, taking into consideration of particularities of pathogenesis of DM, there are several alternative approaches toward improving of efficacy of contemporary therapy. They are directed to reparation and restoration of β-cell function, improving of metabolic processes by specific way, such as stem cell transplantation[8]. Indeed, therapeutic potency of pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced PCSs in diabetes cure is very promised[9,10]. According novel investigations, several ESCs and induced PSCs lines have to be great differential capacities for DM patients. As expected, they are able to translate into all cell types that have a high ability to differentiate into insulin-secreting β cells with low risk of rejection[10]. However, the data on regenerative DM care obtained several investigators are controversial[11]. Currently we have profound discrepancies in this field between results obtained in animal studies and clinical investigations. On the one hand, unexpected inconsistencies might be related with several strategies of recruitment and maturation of stem cells and using of different types of stem cells. On the other hand, DM patient populations are not uniform that negatively associates with results of stem cells transplantation[12,13]. The purpose of the review was to summarize and analyse data for knowledge and prospects for future researches in the field of regenerative therapy in DM patients.
PARADIGM OF THE REGENERATIVE CARE
The main paradigm of regenerative care bases on new knowledge in DM pathogenesis and several molecular repair mechanisms[14]. Conceived to halt or reverse disease progression, stem cell therapies are applied essentially as adjuvants to standard of care with the goal of furthering an otherwise limited self-renewal capacity of the disease[15].
EFFECTS OF CELL THERAPY
The possibly effects of regenerative therapy might have a many faces and they affect different sides of pathophysiological mechanisms of DM evolution (Figure 1).
Figure 1.
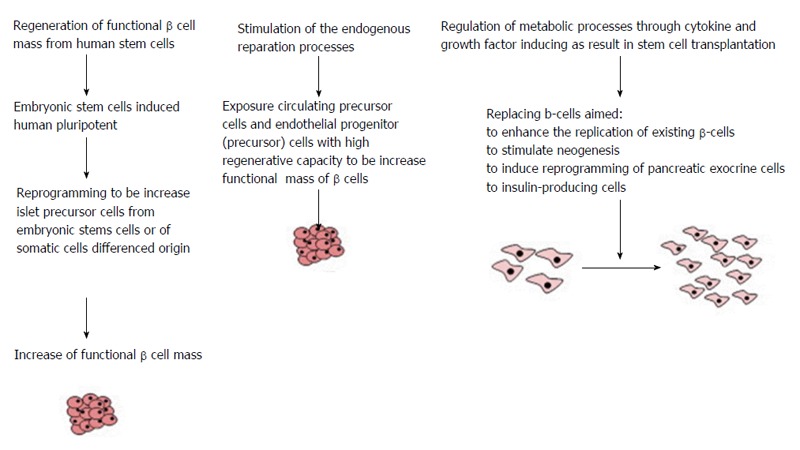
The possible approaches of cell therapy in diabetes patients.
The possible approaches for care are: (1) Regeneration of β cell mass and restoring of functional properties of β cell with human stem cells; (2) Stimulation of the endogenous repair mechanisms; and (3) Modulation of metabolic processes in stem cells transplanted through use of appropriate cytokines and growth factors that might be induced direction for further differentiation of stem cells.
However, the innate intimae molecular mechanisms leaded to realize the favorable effects of stem cell transplantation are different (Figure 2).
Figure 2.
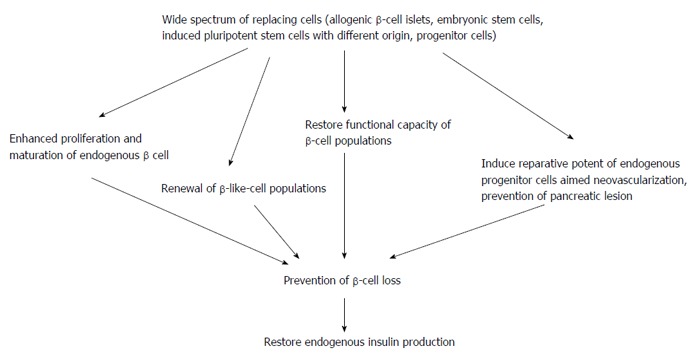
The potent molecular mechanisms that lead to realize an effect of cell therapy in diabetes.
Regeneration of β cell mass and restoring of functional properties of β cell with human stem cells
The progressive loss of functional pancreatic β cells and insufficient insulin secretion by β cells due to endogenous stimuli are suitable for all forms of DM[8]. As a variant of achieving of increased desired pancreatic β cell mass is allogenic pancreatic islet transplantation. This method is currently considered a most efficient approach for DM treatment in routine clinical practice[16]. However, there are many distinguished strategies to be restoring desired β cell mass from stem cell pools. One of it is strategies is directed to increasing of islet precursor cells from embryonic stem cells under influence of relevant transcription factors (Pdx1, Ngn3, Isl-1, etc.), as well as with the use of several extracellular factors. Once a high enough proportion of islet precursors have been obtained there is a need for cell-lineage selection in order to purify the desired cell pools[17]. More detail cellular mechanisms for stem cell reprogramming aimed regeneration of pancreatic β-cell mass are described in excellent review represented by Pandian et al[18]. It has emphasis that there is transplantation of exogenous pancreas/islets or artificial islets, enhanced proliferation and maturation of endogenous β cells, prevention of β-cell loss, or fortified renewal of β-like-cell populations from stem cell pools and non-β-cell sources[19,20]. Results of recently performed investigations have been revealed that there are serious limitations regarding efficacy and safety of various types of cell replacement therapies aimed restoration of functional β-cell sources[21]. However, when several strategies were compared each other the restoring of functional β cell mass from human stem cells for cure in T1DM appears to be most promising approach[22]. One of explanation of this phenomenon was use of specific methods and techniques for generating of stem cells from different source[23].
As known there are at least two practically important sources for human pluripotent stem cells: (1) Deriving of ESCs from blastocysts that were created in vitro; and (2) Induced PSCs generated from different cell lineages of somatic cells using reprogram methods[17-19].
As we can see, ESC deriving is an attractive area of scrutinizes. Now there are at least two clinical trials that were recently finished and the results obtained have let to approve the performing technique for further clinical practice. However, the closely discussion with various specialists are required to be understand whether will the results have serious clinical value or not[24]. Overall, it is not exactly known whether will different cell lineages of embryonic or adult stem cells have high potency to differentiation into β-like cells or not. Moreover, we cannot say that only isolated restoring of the original insulin secretory activity of the transferred cells is expected. It is needed to take also into consideration that immunomodulatory effect of cells transferred affected other tissue cells may be possible and that this phenomenon may lead to autoimmune destruction of previously transplanted cells and other tissue cells[21].
Because human induced PSCs appear to be highly similar to human ESCs, novel technology based on reprogramming of various originated PSCs is discussed as one of the most promising technique[25]. Now it is known that PSCs may be successfully derived from various human somatic cells, such as dermal fibroblasts and keratinocytes[26]. Therefore, autologous pancreatic islets may be differentiated from induced PSCs that derived from DM subjects using integrating retroviral vectors that integrate into the host genome and after then it may replace to donor[27-29]. Importantly, that use embryonic cells in this case is not required. Based on the results of the contemporary investigations, it is possibility emphases that induced PSCs that have been derived from DM subjects with helping of various trans-differentiation techniques are not similar on their biological safety[27-30]. There are needing for continuously investigations of more representative technologies that may let us sufficiently improve of biological hazardless around strategy based on induced PSC transfer. However, before clinical implementation of induced PSCs transplantation there is required to perform fundamental investigations related the specificity, efficiency, kinetics, and biological safety of novel methods of cell reprogramming. Despite results of controlled studies in this field are limited, novel approaches regarding improve and change the induced PSC process promise to be more successful than previous[31]. Currently there are some transcription factors (molecular factors, vectors, various small molecules) that might be useful for improving functionality of induced PSCs before replacement. All these may increase an attractive of trans-differentiation technique to derive one somatic cell type to another patient-specific cell through step associated with induced PSCs obtained[29]. Results of the recently studies have been found that using transcription factors for trans-differentiation of induced PSC into patient-specific cells may open a new era of regenerative medicine. The use of different types of somatic cells with trans-differentiation technology is consider an important approach for improving plastic of induced PSC reprogramming and as serious extend of possibilities for increasing efficacy and biological safety of regenerative medicine[29,31]. Finally, irrespective several limitation of clinically-based evidences of implementation of trans-differentiation on routine clinical practice, it is required to accumulate efforts toward summarize of knowledge about novel method of induced PSC transcription.
The contemporary investigations regarding clinical using of insulin-producing surrogate cells derived from ESCs have been revealed controversial results. This would be related with uniformness in transcription factors use and in the sufficiently differentiation affected techniques of ESC deriving. However, there is no consensus on common standard protocols regarding clinical approaches mentioned above[32,33]. Despite the contemporary statements are required improvement, they present requirement about uniform technology regarding differentiation methods of deriving pancreatic progenitor cells from pluripotent cells[25]. Therefore, another source of deriving of autologic insulin-producing β-cells is tested. Indeed, human bone marrow mesenchymal stem cells (hBM-MSCs) might be considered a source for restoring functionally capacity of β-cell and also probably islet-like clusters that leads to β-cell mass increasing[20]. It is expected that microenvironmental of hBM-MSCs may improve trans- differentiation this type of cell into insulin-produced β-cells. There are data that platelet-rich plasma might be useful for increasing of differentiation capacity of the hBM-MSCs[34]. Moreover, it has been postulated that hBM-MSCs probably would be considered more optimal candidates for further clinical implementation when compared with induced PSC, while this predisposition is required strong and continuous investigations.
Stimulation of the endogenous reparation processes
There are evidences that circulating precursor cells and endothelial progenitor (also known as precursor) cells (EPC) are reduced in DM with advanced complications such as critical limb ischemia, peripheral neuropathy and neuropathic diabetic foot. It is expected that EPC labeled CD34+KDR+ and CD31+CD133+ could have not only a sufficient prognostic value, but and therapeutic significance in DM patients with neuropathic and ischemic lesions[35]. The expected effect of EPC associates with stimulation of the endogenous repair process in the field of the endothelium that may lead to improving of clinical evolution of DM. It is needed to emphases the signaling pathways that lets EPC to differentiate into functional β-cells and mature endothelial cells are still poorly understood and their clinically potency is being be currently unresolved[36].
The strategy of regulation of metabolic processes with stem cells
Some alternative approaches for replacing β-cells include follow principal ways toward to enhance the replication of β-cells, stimulation of neogenesis of the tissues affected DM-related injury, and reprogramming of autologic pancreatic exocrine cells to patient-specific insulin-producing cells. The contemporary approaches based on various type stem-cell deriving might also be useful for effective modulation of the immune system response in T1DM patients. It is also possible the problems of obesity and insulin resistance appearance in T2DM could resolve with immune system response modulation through patient-specific insulin-producing cells transfer[19]. It is predisposed that such approaches may lead to increased efficacy regeneration of pancreatic β-cell mass and functional activity of restoring β-cells[17,18]. Another potential factor could be mediated the effects of stem cells are cytokine and growth factor, but their clinically importance in DM patients is not still understood.
RESULTS OF PRE-CLINICAL STUDIES OF STEM CELL-BASED THERAPY
Early experience in the treatment of diabetes employs stem cells in their native state, as well as unfractionated or enriched in progenitor subpopulation cells, but next generation of cell delivery such as reprogramming stem cells, bone marrow-derived mononuclear cells; lineage-specified progenitor cells are considered more perspective (Table 1).
Table 1.
Summary preclinical data among stem cell transplantation in diabetic animals
| Type of cell replaced | Positive effect expected | Negative effect expected |
| Embryonic stem cells | Direct effect: Differentiation into functional insulin-producing cells Indirect effect: Improving of the fasting blood glucose due to restore the function of islet β cells Decreasing of blood lipid levels Increasing of serum C-peptide level Prevention of free-radical induced oxidative stress injury of beta-cells Improving of pancreatic microcirculation | Ethical problems Rejection High frequency of autoimmune-mediated destruction of the β cells and other autoimmune reactions High immunogenency Malignancy Potential tumor mediated effect |
| Pluripotent stem cells | Direct and indirect effects: See mentioned above | High frequency of rejection High immunogenency Low frequency of autoimmune-mediated destruction of the β cells and other autoimmune reactions Potential tumor mediated effect |
| Bone marrow derived mesenchymal stem cells | Direct and indirect effects: See mentioned above | Low frequency of autoimmune-mediated destruction of the β cells Moderate immunogenency Potential tumor mediated effect Low frequency of rejection |
| Adipose-derived stem cells | Direct and indirect effects: See mentioned above | Extremely low incidences in comparison with bone marrow derived mesenchymal stem cells of rejection, potential tumor mediated effect and autoimmune-mediated destruction of the β cells |
Reprogramming stem cells
A new era in reprogramming of stem cells is related with techniques of therapeutic cloning. Recently it has been reported to have a high potency in DM treatment[37]. Now there are essential requirements of a material designed as stem cells differenced origin recruited for further reprogramming process[38]. These include ESCs and multipotent adult stem/progenitor cells derived from a wide range of tissues (pancreas, intestine, liver, bone marrow, brain, etc.)[39]. There are various evidence for using of recombinant proteins or pharmacologic drugs to induce and mediate the reprogramming process[40,41]. The strategic approaches include follow important direction affected development of generating methods and technologies that associates with non-integrating, non-viral, and non-genetic techniques toward induced PSCs deriving[41]. There are some basic conditions for pluripotency determination that have been identified in vitro, and aimed at specific types of somatic cells[42]. The high quality review presented by Hindley et al[43] that is devoted current understanding of possible interrelationship between the core cell cycle machinery and the maintenance of pluripotency in ESCs and induced PSCs. However, there are advantages of therapeutic cloning affected the potential of cells originated from non-β-cell and related with avoiding of the autoimmune response after transplantation[44]. Despite there is a high similarity of different types of ESCs, effectiveness of reprogramming methods is low and successful result of stem cell culturing appears in 0.01%-0.1% cases[26]. These facts are considered a cause for design of stem cell bank in short-term perspective[45]. Although tremendous clinical effects of stem cell transfer are related with induced PSC transplantation, majority experts have been believed that differentiation of self-renew autologic somatic cells into specific patient-related cells are more desirable approach then ESCs and induced PSC transplantation[46]. However, fully pluripotency is remained available capacity for various lines of human induced PSC[37]. Little known whether these advances for new treatment care in DM patients will preserve[47,48]. Currently new lines of PSC might be powerful for mediation of the molecular mechanism regulation affected the reprogramming process of stem cells different origin[49].
Bone marrow derived mesenchymal stem cells transplantation
Although there is significant progress in the development of safety in turn of clinical implementation of the first derivation of ESCs and induced PSCs, transgene-free induced PSC methods of reprogramming technology have to be attractive as the best technique for culturing of pluripotent stem cells[50]. Cell therapy based on mesenchymal stem cell (MSC) transplantation is considered an effective in the treatment of DM with higher level of safety and tolerability when compared with ESCs. Bone marrow mesenchymal stem cells (BMSCs) have individual particularities that appear to be self-renewing capacity. Therefore, BMSCs represent multipotent activity and may migrate to appropriate pathological sites for realizing their therapeutic potency. The successful BMSC transplantation was presented in animal model of T2DM and it was associated with significantly improving of the fasting glucose and decreased atherogenic circulating lipids in blood. Other biological markers of cardiovascular and metabolic risk were modulated also after transfer of BMSCs. Indeed, circulating C-peptide levels were significantly increased in resulting of BMSCs transplantation[51]. El-Tantawy et al[52] reported that autologous BMSCs appear a significantly potency to prevention of tissue alterations in animals with DM. This effect was probably associated with attenuation of the alloxan-induced oxidative stress. Authors have believed that BMSCs demonstrate rigorous ability for differentiation into functional insulin-producing β-cells and that therapeutic effect of BMSCs may allow achieving an adequate control for hyperglycemia, improve hyperlipidemia, and suppress oxidative stress. All these mentioned above may be helpful in the global strategy toward prevention of DM-related complications.
Tang et al[53] investigated the effect of transplantation of autologous BMSCs in streptozotocin-induced DM pigs. The results obtained in the animal model have been showed that transplantation of autologous BMSCs may help to reverse a streptozotocin-induced DM. Moreover, after transplantation the autologous BMSCs leaded to restoring of blood glucose levels, improving of glucose tolerance test and pancreatic microcirculation, increasing of circulating insulin and C-peptide, as well as the number of islets was significantly increased. Obviously these data suggested that autologous BMSCs implantation might be useful as alternative strategy of DM. Overall, majority investigators have been concluded that the transplantation of BMSCs aimed alternative treatment of DM added to conventional strategy is safe and effective[52,53].
Limitation of the cell therapy in DM
There is wide spectrum of serious limitations for transplantation of the stem cell. The main obstacles affected success of the strategy in T1DM is autoimmune-mediated destruction of the transplanted β-cells and pancreatic islets[54]. One of the possible causes leaded to low efficacy of stem cell transplantation is cellular damage during the isolation process and donor shortages[55]. All these stimulate efforts for creating of novel techniques for increase transplantation efficacy by co-culturing single primary islet cells with adipose-derived stem cells (ADSCs). Now it has suggested that ADSCs may have a sufficient potency to islet cell protection from damage during culturing. Despite this expectation, no significant evidences that the ADSC use improve survival of islet cells and their functionality prior to transplantation procedure. In this context many investigators point that culturing technique is crucial for efficacy of xenotransplantation procedure. Indeed, in vivo experiments with involving xenotransplantation of microfiber-encapsulated spheroids into a mouse model of DM have found that co-culture-transplanted mice lead to higher glucose metabolism modulation when compared with mono-culture-transplanted mice. The novel method for culturing islet spheroids were tested by Jun et al[55]. Investigators concluded that new technique is potentially over helmed the traditional technologies in turn of cell shortages. Moreover, islet spheroids culturing may probably consider a biological artificial pancreas. Currently, both cell source, ESC and induced PSC, allow achieving a high levels of insulin-produced β-cell differentiation, but due to ethical issues and the potential malignancy risk after transplantation clinical use of these approaches are limited Next alternative strategy to be overcome the such seriously obstacles mentioned above is attempts to use pancreatic epithelial cells that may also represent capacities for differentiation into patient-specific insulin-produced β-cells. However, there are major reasons for limitation in clinical implementation of pancreatic epithelial cells due to their high immunogenency. Finally, induced PSCs, ADSCs, and BMSCs are currently discussed the great promise for regenerative medicine in DM field.
EXPECTANCIES OF STEM CELL-BASED THERAPY IN DIABETIC PATIENT POPULATIONS
The expectations that cell therapy may appear new strategy approach for restoring of β-cell mass and their functionality is based on the results of recent investigations. They have been indicated that full glycemic control may be achieve after replacement of autological β-cells and induced PSCs[56]. The pre-clinical studies in support of regenerative paradigms in DM have been tested in different clinical settings with using of various stem cell culturing[57]. It is traditional techniques for human ESCs culturing are incompatible with the generation of genetically diverse, patient- or disease-specific stem cells[58]. The basic data among stem cell-based therapy in diabetic patient population are presented in Table 2. However, the overall efficiency of the conversional nuclear transfer is very low and the safety issue remains a major concern for induced PSCs implementation in various DM patient populations[59]. Overall, the results of the recent studies are controversial due to lack uniformity of design and protocols related techniques of the cell isolation and delivery methods[33]. Moreover, accordingly opinion Soejitno et al[26], the implementation of the stem cell in the routine clinical setting is limited due to risk of malignancy, autoimmune response and rejection of the transplanted cells. Indeed, the allogeneic immune rejection of human ESC-derived cells is considered the main cause of efficacy limitation in recipients[23]. This important problem might be attenuate by implementation of the novel technology affected nuclear reprogramming of induced PSCs in DM patients. However, despite many significant advances novel technological approaches recent clinical studies did not shown superiority new treatment when compared with traditionally methods based on induced PSCs therapy[23]. Finally it is required novel clinical investigations with greater statistical power to be resolving of the situation around efficacy of various methods of the cell therapy in DM[60].
Table 2.
The basic data among current and completed stem cell-based investigations in diabetic patient population
| Title of the study/ClinicalTrials.gov identifier | Phase | n | Gender | Age group | Cell type | Interventions | Results |
| Tissue distribution of F18-FDG labelled autologous bone marrow derived stem cells in patients with type 2 DM (NCT01694173) | Phase 2/3 | 28 | Both gender | Adult/ senior | Stem cell harvest | Splenic artery transplantation vs placebo | No data, current study |
| Efficacy of autologous bone marrow derived stem cell transplantation in patients with type 2 diabetes mellitus (NCT00644241) | Phase 2 | 10 | Both gender | Adult/ senior | Stem cell harvest | Angiographic transplantation of stem cells | No data, current study |
| A pilot study on transplantation therapy using autologous bone marrow mononuclear cells and umbilical cord mesenchymal stem cells in patients with type 1 diabetes mellitus (NCT01143168) | Phase 1 | 24 | Both gender | Adult | Autologous bone marrow mononuclear cells and umbilical cord mesenchymal stem cells | Angiographic transplantation of stem cells | No data, current study |
| A open labeled and self controlled, safety/efficacy assessed pilot study on transplantation therapy using bone marrow mesenchymal stem cells for insulin resistance of type 2 diabetes mellitus (NCT01142050) | Phase 1 | 24 | Both gender | Adult | Mesenchymal stem cells | Angiographic transplantation of stem cells | No data, current study |
| Autologous hematopoietic stem cell transplantation in type 1 diabetes mellitus (NCT01121029) | Phase 1/2 | 15 | Both gender | 2-35 yr | Autologous hematopoietic stem cell | Transplantation | Beta cell function was increased in all but 1 patient and induced prolonged insulin independence in the majority of the patients |
| Autologous bone marrow mononuclear cell infusion with hyperbaric oxygen therapy in type 2 diabetes mellitus (NCT00767260) | Phase 1/2 | 82 | Both gender | 45-65 yr | Autologous bone marrow mononuclear cell | Autologous bone marrow mononuclear cell Infusion vs standard medical therapy | No data, current study |
| Phase 1 and 2 study of the use of human adipose derived mesenchymal stem cells as regenerative therapy in diabetic patients with critical limb ischemia (NCT01257776) | Phase 1/2 | 36 | Both gender | 18-85 yr | Autologous adipose derived mesenchymal stem cells | Intra-arterial administration through a selective cannulation of target common femoral artery vs no intervention | No data, current study |
| Efficacy of autologous bone marrow derived stem cell transplantation in patients with type 2 diabetes mellitus (NCT01065298) | Phase 1/2 | 30 | Both gender | 30-75 yr | Autologous Bone marrow derived stem cell | Injection into superior pancreatico duodenal artery vs standard combined medical therapy | No data, current study |
| Study on induced wound healing through application of expanded autologous bone marrow stem cells in diabetic patients with ischemia-induced chronic tissue ulcers affecting the lower limbs (NCT01065337) | Phase 2 | 30 | Both gender | 18-80 yr | Bone marrow stem cells | Intraarterial administration vs standard of care wound treatment according guideline of the American Diabetes Association | No data, completed study |
| Phase 2 study of autologous stem cell and hyperbaric oxygen therapy in type 2 diabetes mellitus (NCT01786707) | Phase 1/2 | 2 | Both gender | 45-65 yr | Autologous stem cells | Autologous stem cells and hyperbaric oxygen therapy vs No Intervention | No data, completed study |
| Reversal of type 1 diabetes in children by stem cell educator therapy (NCT01996228) | Phase 1/2 | 20 | Both gender | 6-14 yr | Human Cord Blood-derived multipotent stem cells | Apharesis and stem cell educator therapy | No data, current study |
| Phase 2 study of stem cell educator therapy in type 1 diabetes (NCT01350219) | Phase 2 | 100 | Both gender | 14-60 yr | Human cord blood-derived multipotent stem cells | Apharesis and stem cell educator therapy | No data, current study |
| A trial of high dose immunosuppression and autologous hematopoietic stem cell support vs intensive insulin therapy in adults with early onset type 1 diabetes mellitus (NCT01285934) | Phase 1/2 | 30 | Both gender | 16-35 yr | Autologous hematopoietic stem cell | Autologous hematopoietic stem cell transplantation vs intensive insulin therapy | No data, current study |
| Stem cell educator therapy in type 2 diabetes (NCT01415726) | Phase 1/2 | 25 | Both gender | 14-65 yr | Human cord blood-derived multipotent stem cells | Stem cell educator used for the isolation and purification of cord blood stem cells. No comparator | No data, current study |
| Safety and efficacy study of umbilical cord/placenta-derived mesenchymal stem cells to treat type 2 diabetes (NCT01413035) | Phase 2 | 30 | Both gender | 18-80 yr | Human umbilical cord/placenta-derived mesenchymal stem cells | Human umbilical cord/placenta-derived mesenchymal stem cells iv infusion + oral hypoglycemic drugs, insulins or their combination vs oral hypoglycemic drugs, insulins or their combination | No data, current study |
| Open study to evaluate the safety and efficacy of autologous mesenchymal stem cells in treatment of recently diagnosed patients with type 1 diabetes mellitus (NCT01068951) | Phase 2 | 20 | Both gender | 18-40 yr | Autologous mesenchymal stem cells | Autologous transplantation of the patients own mesenchymal stem cells (approximately 2 × 106 cells/kg body weight) intravenously. | No data, completed study |
| Umbilical cord mesenchymal stem cells and liraglutide in diabetes mellitus (NCT01954147) | Phase 1/2 | 100 | Both gender | 35-65 yr | Umbilical cord mesenchymal stem cell | Umbilical cord mesenchymal stem cell infusion combined with liraglutide vs liraglutide | No data, current study |
| Umbilical mesenchymal stem cells and mononuclear cells infusion in type 1 diabetes mellitus: a randomized controlled open-label study (NCT01374854) | Phase 1/2 | 44 | Both gender | 18-40 yr | UC-MSCs | 1 × 106/kg UC-MSCs is infused through pancreatic artery along with mononuclear cells by interventional therapy and another same dose of UC-MSCs is administered one week post-intervention | No data, current study |
| Autologous transplantation of mesenchymal stem cells for treatment of patients with onset of type 1 diabetes (NCT01157403) | Phase 2 | 80 | Both gender | 10-40 yr | Autologous bone marrow mesenchymal stem cells | Autologous transplantation of bone marrow mesenchymal stem cells (approximately 2.5 × 106 cells/kg body weight) intravenously | No data, current study |
| A phase II, multicenter, randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of prochymal® (ex vivo cultured adult human mesenchymal stem cells) for the treatment of recently diagnosed T1DM (NCT00690066) | Phase 2 | 60 | Both gender | 12-35 yr | Ex vivo cultured adult human mesenchymal stem cells | Intravenous infusion of ex vivo cultured adult human mesenchymal stem cells | No data, current study |
| A randomized, controlled, parallel design, safety and efficacy study of granulocyte colony stimulating factor mobilized autologous peripheral blood mononuclear cell therapy in subjects with diabetic limb ischemia (NCT00922389) | Phase 1/2 | 36 | Both gender | 18-65 yr | Peripheral blood derived mononuclear cells | Implanting stem cells derived from peripheral blood after G-CSF mobilization | No data, current study |
| Phase 1/2 study: treatment of patients with diabetic foot complications with allogeneic bone marrow derived mesenchymal stromal cells (NCT01686139) | Phase 1/2 | 10 | Both gender | 18-81 yr | Cultured Bone Marrow Mesenchymal Stromal Cells (BM-MSCs) from allogeneic donors or autologous BM-MSCs | Multiple injections of ABMD-MSC cells (10-20 × 106 cells) | No data, current study |
| Autologous hematopoietic stem cell transplantation for the treatment of limb ischemia and diabetic neuropathy in patients with diabetes mellitus type 2: a randomized controlled trial (NCT00730561) | Phase 1/2 | 20 | Both gender | 18-74 yr | Hematopoietic stem cell (totipotential, hematopoietic or endothelial lineages) | Intramuscular application of CD34+ hematopoietic stem cells (with a minimum of 2 million CD34+ cells/kg) into the gastrocnemius muscles after stimulation with subcutaneous filgrastim 600 micrograms/kilogram a day for 4 d | No data, completed study |
FUTURE PERSPECTIVES OF REGENERATIVE THERAPY
The ability to interconvert terminally differentiated cells could serve as a powerful tool for cell-based treatment of DM. Using wide spectrum of reprogramming factors investigators could activate de novo conversion of intestinal epithelial cells into insulin-produced β-like cells[61]. Authors concluded that the intestine is an accessible and abundant source of functional insulin-producing cells. This fact is intriguing and may have a serious clinically significant value.
The other way is the transplantation several types of stem cells derived from adult cells of pancreas, bone morrow, liver, and cells various originated is under consideration[57]. The lack of transplantable pancreatic islets is a serious problem that affects the treatment of patients with T1DM. The new strategy of regenerative medicine suggests that these obstacles are potentially to be overcame and that the aim of this approach is transformation of any somatic cells into insulin-produced patient-specific β-cells[57]. Contemporary biological and analytical techniques help us to predict the transcription factors that are needed for β-cell regeneration and restoring of the β-cell mass[62]. The transcription factors mediate β-cell renewing with diverse culturing methods[63]. In this context novel cellular strategies toward reprogramming may have better clinical prospects[64,65]. In has been expected that small molecules might be successful to be inducing pancreatic β-cell modification. Recently, a synthetic DNA-based small molecule triggered targeted transcriptional activation of pancreas-related genes to suggest the possibility of achieving desired cellular phenotype in a precise model[66]. Besides providing new β-cells, cell therapy also has to address the question on how to protect the transplanted cells from destruction by the immune system via either allo- or autoimmunity[66,67].
In conclusion, stem cell replacement as a perspective component of therapy for DM has received much attention. Importantly, novel technologies for reprogramming of stem cells, such as somatic cell nuclear transfer, meet several ethical and practical concerns. Other significant obstacles remain high cost, methods to prevent immune rejection of grafted tissues, and suppression of the risks of tumorigenesis. For overcoming these obstacles probably more scientific discussions around ethical principles, methods of culturing of stem cells, routine clinical procedures and protocol evaluation, as well as more clinical investigations in this field are required.
Footnotes
P- Reviewer: Chapel A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Scully T. Diabetes in numbers. Nature. 2012;485:S2–S3. doi: 10.1038/485s2a. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft FM, Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell. 2012;148:1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34:2436–2443. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali MA, Dayan CM. The importance of residual endogenous beta-cell preservation in type 1 diabetes. Br J Diabetes Vasc Dis. 2009;9:6. [Google Scholar]
- 5.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322–1335. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nwaneri C, Cooper H, Bowen-Jones D. Mortality in type 2 diabetes mellitus: magnitude of the evidence from a systematic review and meta-analysis. Brit J Diabetes and Vascular Disease. 2013;13:192–207. [Google Scholar]
- 7.Howangyin KY, Silvestre JS. Diabetes mellitus and ischemic diseases: molecular mechanisms of vascular repair dysfunction. Arterioscler Thromb Vasc Biol. 2014;34:1126–1135. doi: 10.1161/ATVBAHA.114.303090. [DOI] [PubMed] [Google Scholar]
- 8.Holditch SJ, Terzic A, Ikeda Y. Concise review: pluripotent stem cell-based regenerative applications for failing β-cell function. Stem Cells Transl Med. 2014;3:653–661. doi: 10.5966/sctm.2013-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liew CG. Generation of insulin-producing cells from pluripotent stem cells: from the selection of cell sources to the optimization of protocols. Rev Diabet Stud. 2010;7:82–92. doi: 10.1900/RDS.2010.7.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdelalim EM, Bonnefond A, Bennaceur-Griscelli A, Froguel P. Pluripotent stem cells as a potential tool for disease modelling and cell therapy in diabetes. Stem Cell Rev. 2014;10:327–337. doi: 10.1007/s12015-014-9503-6. [DOI] [PubMed] [Google Scholar]
- 11.Chidgey AP, Layton D, Trounson A, Boyd RL. Tolerance strategies for stem-cell-based therapies. Nature. 2008;453:330–337. doi: 10.1038/nature07041. [DOI] [PubMed] [Google Scholar]
- 12.Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:139–148. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]
- 13.Anastasia L, Pelissero G, Venerando B, Tettamanti G. Cell reprogramming: expectations and challenges for chemistry in stem cell biology and regenerative medicine. Cell Death Differ. 2010;17:1230–1237. doi: 10.1038/cdd.2010.14. [DOI] [PubMed] [Google Scholar]
- 14.Terzic A, Behfar A. Regenerative heart failure therapy headed for optimization. Eur Heart J. 2014;35:1231–1234. doi: 10.1093/eurheartj/ehu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi SD, Smith PD, Choong PF. Nuclear reprogramming and induced pluripotent stem cells: a review for surgeons. ANZ J Surg. 2014;84:E1–11. doi: 10.1111/ans.12419. [DOI] [PubMed] [Google Scholar]
- 16.Nostro MC, Keller G. Generation of beta cells from human pluripotent stem cells: Potential for regenerative medicine. Semin Cell Dev Biol. 2012;23:701–710. doi: 10.1016/j.semcdb.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soria B. In-vitro differentiation of pancreatic beta-cells. Differentiation. 2001;68:205–219. doi: 10.1046/j.1432-0436.2001.680408.x. [DOI] [PubMed] [Google Scholar]
- 18.Pandian GN, Taniguchi J, Sugiyama H. Cellular reprogramming for pancreatic β-cell regeneration: clinical potential of small molecule control. Clin Transl Med. 2014;3:6. doi: 10.1186/2001-1326-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weir GC, Cavelti-Weder C, Bonner-Weir S. Stem cell approaches for diabetes: towards beta cell replacement. Genome Med. 2011;3:61. doi: 10.1186/gm277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafarian A, Taghikhani M, Abroun S, Pourpak Z, Allahverdi A, Soleimani M. Generation of high-yield insulin producing cells from human bone marrow mesenchymal stem cells. Mol Biol Rep. 2014;41:4783–4794. doi: 10.1007/s11033-014-3349-5. [DOI] [PubMed] [Google Scholar]
- 21.Calafiore R, Montanucci P, Basta G. Stem cells for pancreatic β-cell replacement in diabetes mellitus: actual perspectives. Curr Opin Organ Transplant. 2014;19:162–168. doi: 10.1097/MOT.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 22.Lindahl M, Danilova T, Palm E, Lindholm P, Võikar V, Hakonen E, Ustinov J, Andressoo JO, Harvey BK, Otonkoski T, et al. MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell Rep. 2014;7:366–375. doi: 10.1016/j.celrep.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu X. The immunogenicity of cells derived from induced pluripotent stem cells. Cell Mol Immunol. 2014;11:14–16. doi: 10.1038/cmi.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philonenko ES, Shutova MV, Chestkov IV, Lagarkova MA, Kiselev SL. Current progress and potential practical application for human pluripotent stem cells. Int Rev Cell Mol Biol. 2011;292:153–196. doi: 10.1016/B978-0-12-386033-0.00004-9. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Nur O, Russ HA, Efrat S, Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Soejitno A, Prayudi PK. The prospect of induced pluripotent stem cells for diabetes mellitus treatment. Ther Adv Endocrinol Metab. 2011;2:197–210. doi: 10.1177/2042018811420198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudva YC, Ohmine S, Greder LV, Dutton JR, Armstrong A, De Lamo JG, Khan YK, Thatava T, Hasegawa M, Fusaki N, et al. Transgene-free disease-specific induced pluripotent stem cells from patients with type 1 and type 2 diabetes. Stem Cells Transl Med. 2012;1:451–461. doi: 10.5966/sctm.2011-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiland S, Salekdeh GH, Krijgsveld J. Defining pluripotent stem cells through quantitative proteomic analysis. Expert Rev Proteomics. 2011;8:29–42. doi: 10.1586/epr.10.100. [DOI] [PubMed] [Google Scholar]
- 29.Ma T, Xie M, Laurent T, Ding S. Progress in the reprogramming of somatic cells. Circ Res. 2013;112:562–574. doi: 10.1161/CIRCRESAHA.111.249235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sommer AG, Rozelle SS, Sullivan S, Mills JA, Park SM, Smith BW, Iyer AM, French DL, Kotton DN, Gadue P, et al. Generation of human induced pluripotent stem cells from peripheral blood using the STEMCCA lentiviral vector. J Vis Exp. 2012;(68):e4327. doi: 10.3791/4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai Q, Desprat R, Klein B, Lemaître JM, De Vos J. Embryonic stem cells or induced pluripotent stem cells? A DNA integrity perspective. Curr Gene Ther. 2013;13:93–98. doi: 10.2174/1566523211313020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naujok O, Burns C, Jones PM, Lenzen S. Insulin-producing surrogate β-cells from embryonic stem cells: are we there yet? Mol Ther. 2011;19:1759–1768. doi: 10.1038/mt.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naujok O, Lenzen S. [Pluripotent stem cells for cell replacement therapy of diabetes] Dtsch Med Wochenschr. 2012;137:1062–1066. doi: 10.1055/s-0032-1304936. [DOI] [PubMed] [Google Scholar]
- 34.Lian Z, Yin X, Li H, Jia L, He X, Yan Y, Liu N, Wan K, Li X, Lin S. Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma in streptozotocin-induced diabetic rats. Ann Dermatol. 2014;26:1–10. doi: 10.5021/ad.2014.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambataro M, Seganfreddo E, Canal F, Furlan A, Del Pup L, Niero M, Paccagnella A, Gherlinzoni F, Dei Tos AP. Prognostic significance of circulating and endothelial progenitor cell markers in type 2 diabetic foot. Int J Vasc Med. 2014;2014:589412. doi: 10.1155/2014/589412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayhew CN, Wells JM. Converting human pluripotent stem cells into beta-cells: recent advances and future challenges. Curr Opin Organ Transplant. 2010;15:54–60. doi: 10.1097/MOT.0b013e3283337e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang L, Kou Z, Zhang Y, Gao S. Induced pluripotent stem cells (iPSCs)--a new era of reprogramming. J Genet Genomics. 2010;37:415–421. doi: 10.1016/S1673-8527(09)60060-6. [DOI] [PubMed] [Google Scholar]
- 38.Zhou H, Ding S. Evolution of induced pluripotent stem cell technology. Curr Opin Hematol. 2010;17:276–280. doi: 10.1097/MOH.0b013e328339f2ee. [DOI] [PubMed] [Google Scholar]
- 39.Nsair A, MacLellan WR. Induced pluripotent stem cells for regenerative cardiovascular therapies and biomedical discovery. Adv Drug Deliv Rev. 2011;63:324–330. doi: 10.1016/j.addr.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burns CJ, Persaud SJ, Jones PM. Diabetes mellitus: a potential target for stem cell therapy. Curr Stem Cell Res Ther. 2006;1:255–266. doi: 10.2174/157488806776956832. [DOI] [PubMed] [Google Scholar]
- 41.Tancos Z, Nemes C, Polgar Z, Gocza E, Daniel N, Stout TA, Maraghechi P, Pirity MK, Osteil P, Tapponnier Y, et al. Generation of rabbit pluripotent stem cell lines. Theriogenology. 2012;78:1774–1786. doi: 10.1016/j.theriogenology.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Lu X, Zhao T. Clinical therapy using iPSCs: hopes and challenges. Genomics Proteomics Bioinformatics. 2013;11:294–298. doi: 10.1016/j.gpb.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hindley C, Philpott A. The cell cycle and pluripotency. Biochem J. 2013;451:135–143. doi: 10.1042/BJ20121627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teng S, Liu C, Krettek C, Jagodzinski M. The application of induced pluripotent stem cells for bone regeneration: current progress and prospects. Tissue Eng Part B Rev. 2014;20:328–339. doi: 10.1089/ten.TEB.2013.0301. [DOI] [PubMed] [Google Scholar]
- 45.Taylor CJ, Bolton EM, Bradley JA. Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc Lond B Biol Sci. 2011;366:2312–2322. doi: 10.1098/rstb.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeo JC, Ng HH. Transcriptomic analysis of pluripotent stem cells: insights into health and disease. Genome Med. 2011;3:68. doi: 10.1186/gm284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu X, Xu Y. Challenges to the clinical application of pluripotent stem cells: towards genomic and functional stability. Genome Med. 2012;4:55. doi: 10.1186/gm354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Z, Han Y, Cao X. Induced pluripotent stem cell (iPSCs) and their application in immunotherapy. Cell Mol Immunol. 2014;11:17–24. doi: 10.1038/cmi.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kao CF, Chuang CY, Chen CH, Kuo HC. Human pluripotent stem cells: current status and future perspectives. Chin J Physiol. 2008;51:214–225. [PubMed] [Google Scholar]
- 50.Jung Y, Bauer G, Nolta JA. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells. 2012;30:42–47. doi: 10.1002/stem.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan XH, Song QQ, Dai JJ, Yao X, Wang JX, Pang RQ, He J, Li ZA, Sun XM, Ruan GP. Transplantation of bone marrow mesenchymal stem cells for the treatment of type 2 diabetes in a macaque model. Cells Tissues Organs. 2013;198:414–427. doi: 10.1159/000358383. [DOI] [PubMed] [Google Scholar]
- 52.El-Tantawy WH, Haleem EN. Therapeutic effects of stem cell on hyperglycemia, hyperlipidemia, and oxidative stress in alloxan-treated rats. Mol Cell Biochem. 2014;391:193–200. doi: 10.1007/s11010-014-2002-x. [DOI] [PubMed] [Google Scholar]
- 53.Tang K, Xiao X, Liu D, Shen Y, Chen Y, Wang Y, Li B, Yu F, Ma D, Yan J, et al. Autografting of bone marrow mesenchymal stem cells alleviates streptozotocin-induced diabetes in miniature pigs: Real-time tracing with MRI in vivo. Int J Mol Med. 2014;33:1469–1476. doi: 10.3892/ijmm.2014.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao F, Ma L, Zhao M, Huang G, Mirenda V, Dorling A, Lechler R, Lombardi G. Ex vivo expanded human regulatory T cells delay islet allograft rejection via inhibiting islet-derived monocyte chemoattractant protein-1 production in CD34+ stem cells-reconstituted NOD-scid IL2rγnull mice. PLoS One. 2014;9:e90387. doi: 10.1371/journal.pone.0090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jun Y, Kang AR, Lee JS, Park SJ, Lee DY, Moon SH, Lee SH. Microchip-based engineering of super-pancreatic islets supported by adipose-derived stem cells. Biomaterials. 2014;35:4815–4826. doi: 10.1016/j.biomaterials.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 56.Schroeder IS. Potential of pluripotent stem cells for diabetes therapy. Curr Diab Rep. 2012;12:490–498. doi: 10.1007/s11892-012-0292-5. [DOI] [PubMed] [Google Scholar]
- 57.Kojima N. In vitro reconstitution of pancreatic islets. Organogenesis. 2014;10:225–230. doi: 10.4161/org.28351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng W. Exploiting pluripotency for therapeutic gain. Panminerva Med. 2010;52:167–173. [PubMed] [Google Scholar]
- 59.Hao J, Zhu W, Sheng C, Yu Y, Zhou Q. Human parthenogenetic embryonic stem cells: one potential resource for cell therapy. Sci China C Life Sci. 2009;52:599–602. doi: 10.1007/s11427-009-0096-2. [DOI] [PubMed] [Google Scholar]
- 60.Li M, Chen M, Han W, Fu X. How far are induced pluripotent stem cells from the clinic? Ageing Res Rev. 2010;9:257–264. doi: 10.1016/j.arr.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Chen YJ, Finkbeiner SR, Weinblatt D, Emmett MJ, Tameire F, Yousefi M, Yang C, Maehr R, Zhou Q, Shemer R, et al. De novo formation of insulin-producing “neo-β cell islets” from intestinal crypts. Cell Rep. 2014;6:1046–1058. doi: 10.1016/j.celrep.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jang J, Yoo JE, Lee JA, Lee DR, Kim JY, Huh YJ, Kim DS, Park CY, Hwang DY, Kim HS, et al. Disease-specific induced pluripotent stem cells: a platform for human disease modeling and drug discovery. Exp Mol Med. 2012;44:202–213. doi: 10.3858/emm.2012.44.3.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boland MJ, Hazen JL, Nazor KL, Rodriguez AR, Martin G, Kupriyanov S, Baldwin KK. Generation of mice derived from induced pluripotent stem cells. J Vis Exp. 2012;(69):e4003. doi: 10.3791/4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sohn YD, Han JW, Yoon YS. Generation of induced pluripotent stem cells from somatic cells. Prog Mol Biol Transl Sci. 2012;111:1–26. doi: 10.1016/B978-0-12-398459-3.00001-0. [DOI] [PubMed] [Google Scholar]
- 65.Zou C, Chou BK, Dowey SN, Tsang K, Huang X, Liu CF, Smith C, Yen J, Mali P, Zhang YA, et al. Efficient derivation and genetic modifications of human pluripotent stem cells on engineered human feeder cell lines. Stem Cells Dev. 2012;21:2298–311. doi: 10.1089/scd.2011.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lysy PA, Weir GC, Bonner-Weir S. Concise review: pancreas regeneration: recent advances and perspectives. Stem Cells Transl Med. 2012;1:150–159. doi: 10.5966/sctm.2011-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim C. Disease modeling and cell based therapy with iPSC: future therapeutic option with fast and safe application. Blood Res. 2014;49:7–14. doi: 10.5045/br.2014.49.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]


