Abstract
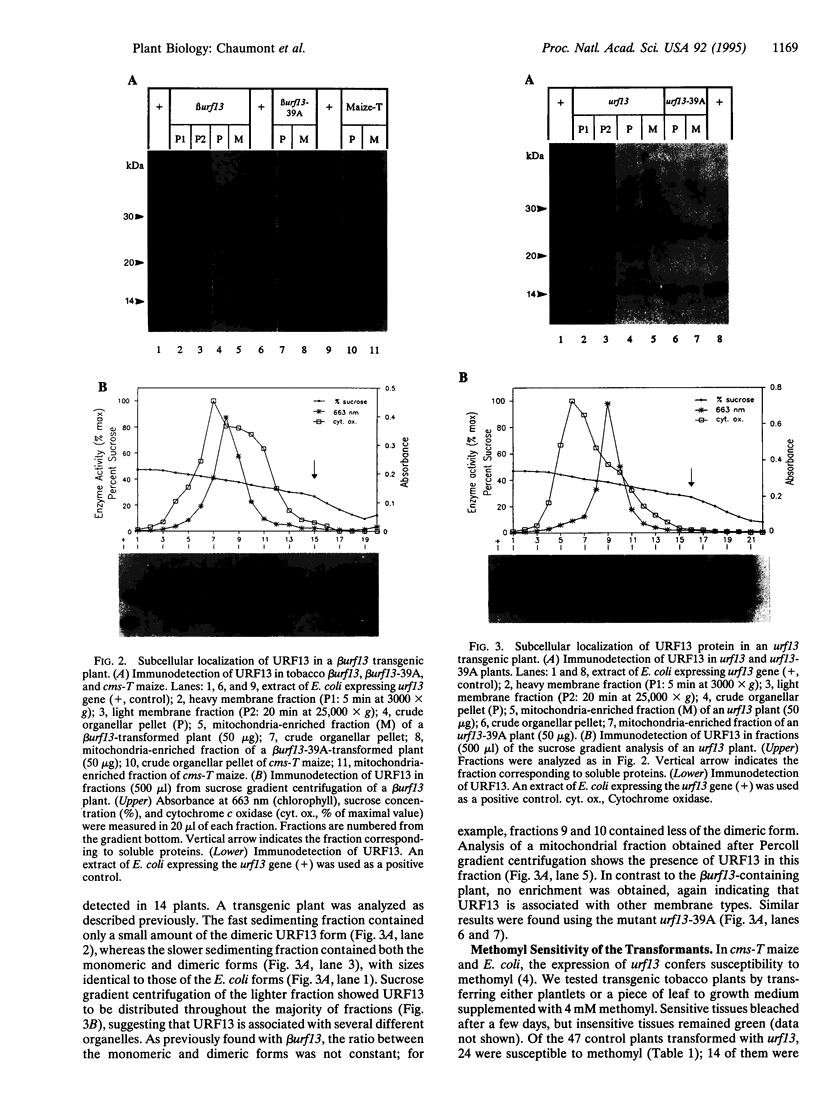
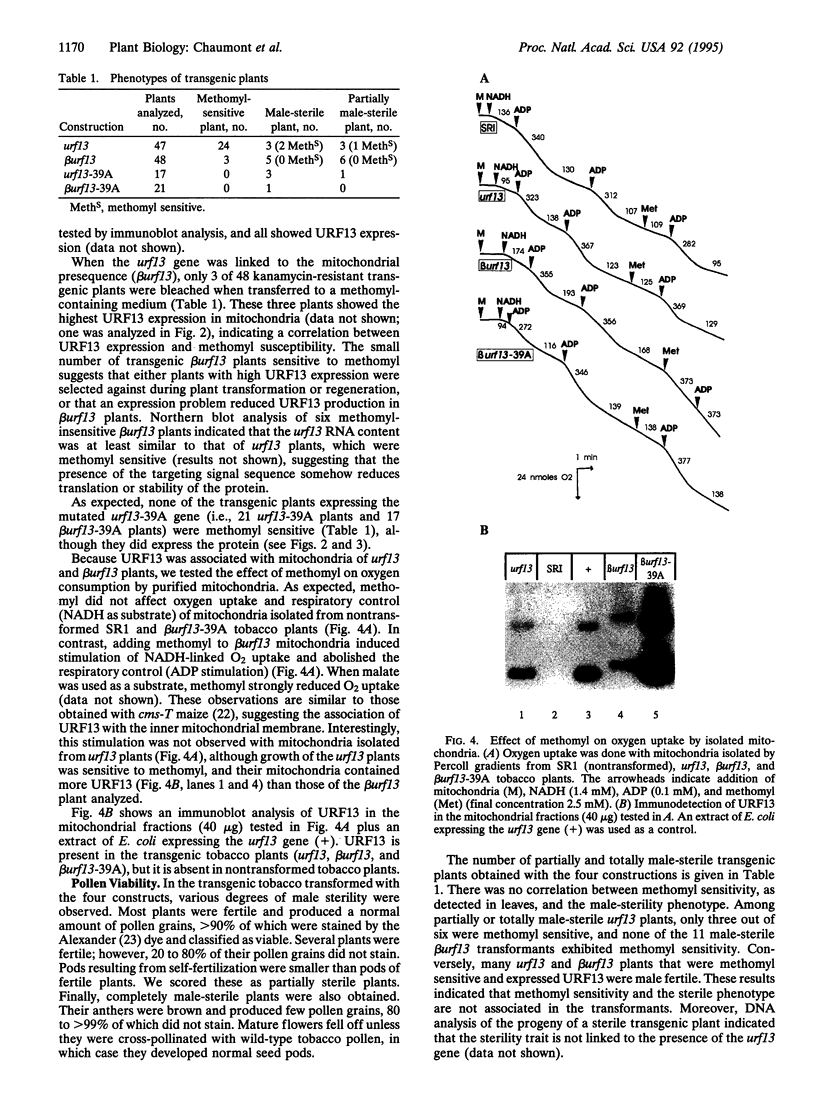
The URF13 protein, which is encoded by the maize mitochondrial T-urf13 gene, is thought to be responsible for pathotoxin and methomyl sensitivity and male sterility. We have investigated whether T-urf13 confers toxin sensitivity and male sterility when expressed in another plant species. The coding sequence of T-urf13 was fused to a mitochondrial targeting presequence, placed under the control of the cauliflower mosaic virus 35S promoter, and introduced into tobacco by Agrobacterium tumefaciens-mediated transformation. Plants expressing high levels of URF13 were methomyl sensitive. Subcellular analysis indicated that URF13 is mainly associated with the mitochondria. Adding methomyl to isolated mitochondria stimulated NADH-linked respiration and uncoupled oxidative phosphorylation, indicating that URF13 was imported into the mitochondria, and conferred toxin sensitivity. Most control plants, which expressed the T-urf13c construct lacking the mitochondrial presequence, were methomyl sensitive and contained URF13 in a membrane fraction. Subcellular fractionation by sucrose gradient centrifugation showed that URF13 sedimented at several positions, suggesting the protein is associated with various organelles, including mitochondria. No methomyl effect was observed in isolated mitochondria, however, indicating that URF13 was not imported and did not confer toxin sensitivity to the mitochondria. Thus, URF13 confers toxin sensitivity to transgenic tobacco with or without import into the mitochondria. There was no correlation between the expression of URF13 and male sterility, suggesting either that URF13 does not cause male sterility in transgenic tobacco or that URF13 is not expressed in sufficient amounts in the appropriate anther cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. P. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969 May;44(3):117–122. doi: 10.3109/10520296909063335. [DOI] [PubMed] [Google Scholar]
- Anstis S. M., Rogers B. J. Illusory continuous motion from oscillating positive-negative patterns: implications for motion perception. Perception. 1986;15(5):627–640. doi: 10.1068/p150627. [DOI] [PubMed] [Google Scholar]
- Braun C. J., Siedow J. N., Levings C. S., 3rd Fungal toxins bind to the URF13 protein in maize mitochondria and Escherichia coli. Plant Cell. 1990 Feb;2(2):153–161. doi: 10.1105/tpc.2.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C. J., Siedow J. N., Williams M. E., Levings C. S., 3rd Mutations in the maize mitochondrial T-urf13 gene eliminate sensitivity to a fungal pathotoxin. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4435–4439. doi: 10.1073/pnas.86.12.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F., Silva Filho M. de C., Thomas D., Leterme S., Boutry M. Truncated presequences of mitochondrial F1-ATPase beta subunit from Nicotiana plumbaginifolia transport CAT and GUS proteins into mitochondria of transgenic tobacco. Plant Mol Biol. 1994 Feb;24(4):631–641. doi: 10.1007/BF00023559. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., 3rd, Timothy D. H. Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell. 1986 Feb 14;44(3):439–449. doi: 10.1016/0092-8674(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Siedow J. N., Timothy D. H., Levings C. S., 3rd A 13-kilodalton maize mitochondrial protein in E. coli confers sensitivity to Bipolaris maydis toxin. Science. 1988 Jan 15;239(4837):293–295. doi: 10.1126/science.3276005. [DOI] [PubMed] [Google Scholar]
- Fauron C. M., Havlik M., Brettell R. I. The mitochondrial genome organization of a maize fertile cmsT revertant line is generated through recombination between two sets of repeats. Genetics. 1990 Feb;124(2):423–428. doi: 10.1093/genetics/124.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. G., Oliver R. J., Leaver C. J. Variation in mitochondrial translation products associated with male-sterile cytoplasms in maize. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3841–3845. doi: 10.1073/pnas.75.8.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glab N., Wise R. P., Pring D. R., Jacq C., Slonimski P. Expression in Saccharomyces cerevisiae of a gene associated with cytoplasmic male sterility from maize: respiratory dysfunction and uncoupling of yeast mitochondria. Mol Gen Genet. 1990 Aug;223(1):24–32. doi: 10.1007/BF00315793. [DOI] [PubMed] [Google Scholar]
- Guerrero F. D., Crossland L., Smutzer G. S., Hamilton D. A., Mascarenhas J. P. Promoter sequences from a maize pollen-specific gene direct tissue-specific transcription in tobacco. Mol Gen Genet. 1990 Nov;224(2):161–168. doi: 10.1007/BF00271548. [DOI] [PubMed] [Google Scholar]
- Huang J., Lee S. H., Lin C., Medici R., Hack E., Myers A. M. Expression in yeast of the T-urf13 protein from Texas male-sterile maize mitochondria confers sensitivity to methomyl and to Texas-cytoplasm-specific fungal toxins. EMBO J. 1990 Feb;9(2):339–347. doi: 10.1002/j.1460-2075.1990.tb08116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe D. E., Cox J. K., Malone C. P. Mitochondrial heredity: a determinant in the toxic response of maize to the insecticide methomyl. Science. 1978 Sep 29;201(4362):1227–1229. doi: 10.1126/science.201.4362.1227. [DOI] [PubMed] [Google Scholar]
- Korth K. L., Kaspi C. I., Siedow J. N., Levings C. S., 3rd URF13, a maize mitochondrial pore-forming protein, is oligomeric and has a mixed orientation in Escherichia coli plasma membranes. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10865–10869. doi: 10.1073/pnas.88.23.10865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth K. L., Levings C. S., 3rd Baculovirus expression of the maize mitochondrial protein URF13 confers insecticidal activity in cell cultures and larvae. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3388–3392. doi: 10.1073/pnas.90.8.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings C. S., 3rd, Siedow J. N. Molecular basis of disease susceptibility in the Texas cytoplasm of maize. Plant Mol Biol. 1992 May;19(1):135–147. doi: 10.1007/BF00015611. [DOI] [PubMed] [Google Scholar]
- Levings C. S., 3rd The Texas cytoplasm of maize: cytoplasmic male sterility and disease susceptibility. Science. 1990 Nov 16;250(4983):942–947. doi: 10.1126/science.250.4983.942. [DOI] [PubMed] [Google Scholar]
- Rottmann W. H., Brears T., Hodge T. P., Lonsdale D. M. A mitochondrial gene is lost via homologous recombination during reversion of CMS T maize to fertility. EMBO J. 1987 Jun;6(6):1541–1546. doi: 10.1002/j.1460-2075.1987.tb02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. P., Pring D. R., Gengenbach B. G. Mutation to male fertility and toxin insensitivity in Texas (T)-cytoplasm maize is associated with a frameshift in a mitochondrial open reading frame. Proc Natl Acad Sci U S A. 1987 May;84(9):2858–2862. doi: 10.1073/pnas.84.9.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Allmen J. M., Rottmann W. H., Gengenbach B. G., Harvey A. J., Lonsdale D. M. Transfer of methomyl and HmT-toxin sensitivity from T-cytoplasm maize to tobacco. Mol Gen Genet. 1991 Oct;229(3):405–412. doi: 10.1007/BF00267463. [DOI] [PubMed] [Google Scholar]