Abstract
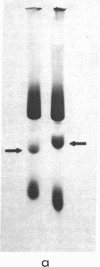
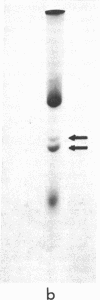
An early, spontaneous amber mutation in the lac i-gene allows translational reinitiation, which results in a mutant lac repressor. Comparison of the amino-terminal sequence of this mutant repressor with the partial amino-acid sequence of the wild-type lac repressor shows that reinitiation occurs at the first internal AUG codon, and results in a mutant protein lacking 42 residues at the amino-terminal end. This protein binds the inducer isopropyl-β-D-thiogalactoside with normal affinity, and is capable of maintaining a tetrameric structure; however, it does not repress in vivo. These data suggest that the amino-terminal portion of the wild-type lac repressor is necessary either for direct binding to the lac operator or for the correct conformation for binding to DNA.
Keywords: protein biosynthesis, antibody, gel electrophoresis, amino-acid sequence, E. coli
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capecchi M. R. Initiation of E. coli proteins. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1517–1524. doi: 10.1073/pnas.55.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh H. P., Söll D., Khorana H. G. Studies on polynucleotides. LXVII. Initiation of protein synthesis in vitro as studied by using ribopolynucleotides with repeating nucleotide sequences as messengers. J Mol Biol. 1967 Apr 28;25(2):275–298. doi: 10.1016/0022-2836(67)90142-8. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. Isolation of the lac repressor. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1891–1898. doi: 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicker T., Zipser D. A mutation which creates a new site for the re-initiation of polypeptide synthesis in the z gene of the lac operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):305–314. doi: 10.1016/0022-2836(68)90388-4. [DOI] [PubMed] [Google Scholar]
- Kumar S., Szybalski W. Orientation of transcription of the lac operon and its repressor gene in Escherichia coli. J Mol Biol. 1969 Feb 28;40(1):145–151. doi: 10.1016/0022-2836(69)90303-9. [DOI] [PubMed] [Google Scholar]
- Lengyel P., Söll D. Mechanism of protein biosynthesis. Bacteriol Rev. 1969 Jun;33(2):264–301. doi: 10.1128/br.33.2.264-301.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels C. A., Zipser D. Mapping of polypeptide reinitiation sites within the beta-galactosidase structural gene. J Mol Biol. 1969 May 14;41(3):341–347. doi: 10.1016/0022-2836(69)90280-0. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Beckwith J., Muller-Hill B. Direction of transcription of a regulatory gene in E. coli. Nature. 1968 Dec 28;220(5174):1287–1290. doi: 10.1038/2201287a0. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B., Crapo L., Gilbert W. Mutants that make more lac repressor. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1259–1264. doi: 10.1073/pnas.59.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A. Effect of nonsense mutations on translation of the lactose operon of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1966;31:181–187. doi: 10.1101/sqb.1966.031.01.026. [DOI] [PubMed] [Google Scholar]
- Newton A. Re-initiation of polypeptide synthesis and polarity in the lac operon of Escherichia coli. J Mol Biol. 1969 May 14;41(3):329–339. doi: 10.1016/0022-2836(69)90279-4. [DOI] [PubMed] [Google Scholar]
- Platt T., Miller J. H., Weber K. In vivo degradation of mutant lac repressor. Nature. 1970 Dec 19;228(5277):1154–1156. doi: 10.1038/2281154a0. [DOI] [PubMed] [Google Scholar]
- Sarabhai A., Brenner S. A mutant which reinitiates the polypeptide chain after chain termination. J Mol Biol. 1967 Jul 14;27(1):145–162. doi: 10.1016/0022-2836(67)90357-9. [DOI] [PubMed] [Google Scholar]
- Stewart J. W., Sherman F., Shipman N. A., Jackson M. Identification and mutational relocation of the AUG codon initiating translation of iso-1-cytochrome c in yeast. J Biol Chem. 1971 Dec 25;246(24):7429–7445. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zipser D., Zabell S., Rothman J., Grodzicker T., Wenk M. Fine structure of the gradient of polarity in the z gene of the lac operon of Escherichia coli. J Mol Biol. 1970 Apr 14;49(1):251–254. doi: 10.1016/0022-2836(70)90392-x. [DOI] [PubMed] [Google Scholar]