Abstract
The reaction of horse-heart cytochrome c with hydrated electrons has been studied by the pulseradiolysis technique. In neutral solution, the ferriheme group was reduced in a bimolecular reaction that takes place at a rate equal to that of the decay of the e-aq, and approaches the diffusion-controlled limit. This reduction is assigned mainly to a direct reaction, proceeding via the exposed edge of the porphyrin projecting into the cytochrome c crevice.
The reaction absorption spectrum observed 20 μsec after an electron pulse was very similar, yet blue-shifted relative to, the difference spectrum between the reduced and oxidized forms of cytochrome c. However, this shift vanishes in a slow monomolecular reaction, which seems to reflect the conformational relaxation of the protein to the final equilibrium state of its reduced form. In alkaline solutions, the transition of cytochrome c molecules into an irreducible conformation causes a proportionate decrease in the amount of ferricytochrome c reduced in the direct reaction. The rate of conformational transition of the protein into the reactive form is now the limiting step for a substantial part of the reduction that takes place via this slow monomolecular reaction.
Pseudomonas cytochrome c 551 which, in contrast to horse-heart cytochrome c, is a negatively charged protein at neutral pH reacts with e-aq at a rate lower than does the horse-heart protein. The reduction of the heme group follows that of the e-aq decay with a small, yet significant, delay.
Keywords: horse heart cytochrome c, pulse radiolysis, Pseudomonas cytochrome c, protein relaxation, difference spectra
Full text
PDF
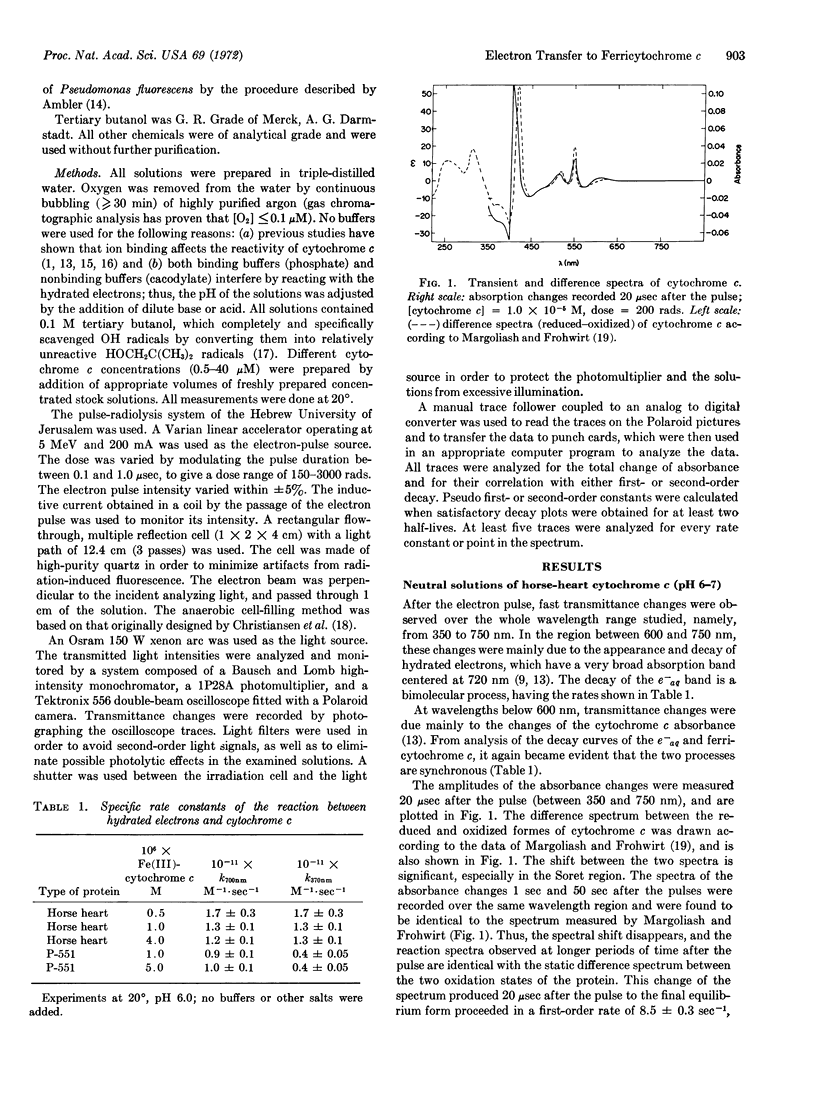
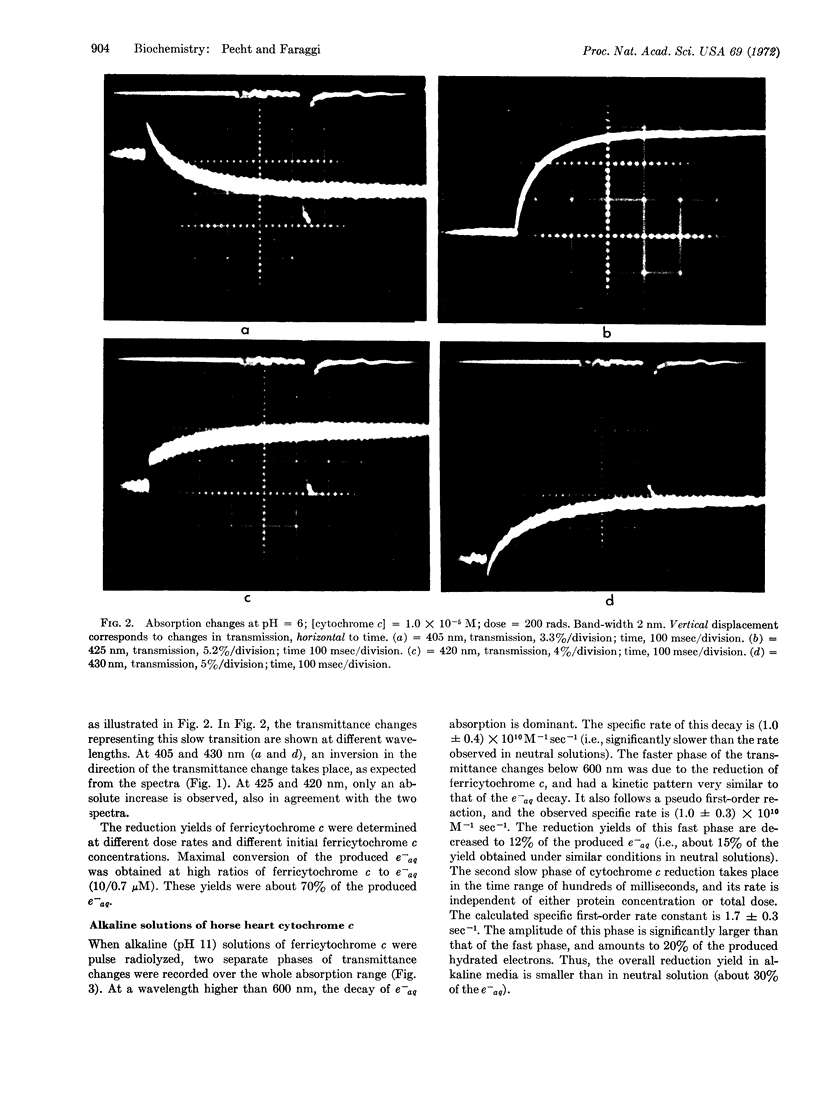
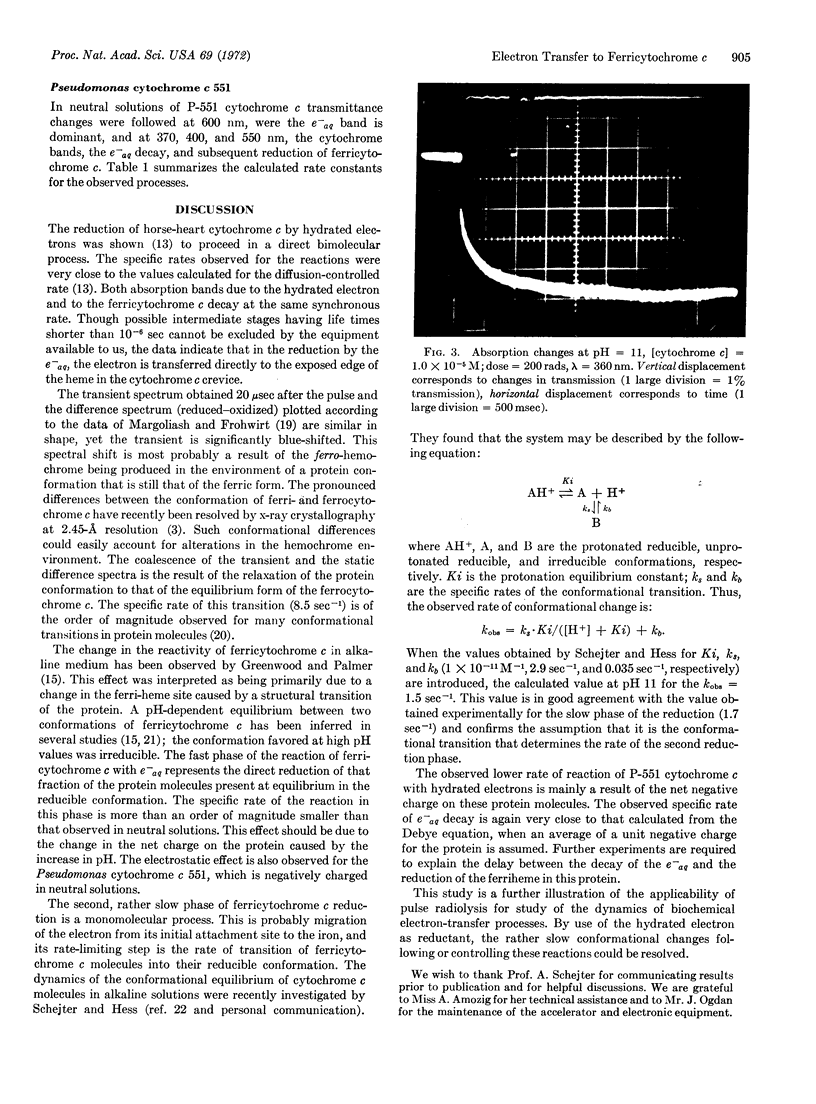

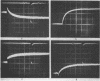
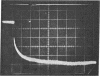
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K. G., Parks P. C., Czerlinski G. H., Hess G. P. On the elucidation of the pH dependence of the oxidation-reduction potential of cytochrome c at alkaline pH. J Biol Chem. 1966 Sep 25;241(18):4180–4185. [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- Greenwood C., Palmer G. Evidence for the existence of two functionally distinct forms cytochrome c manomer at alkaline pH. J Biol Chem. 1965 Sep;240(9):3660–3663. [PubMed] [Google Scholar]
- Hammes G. G. Relaxation spectrometry of biological systems. Adv Protein Chem. 1968;23:1–57. doi: 10.1016/s0065-3233(08)60399-x. [DOI] [PubMed] [Google Scholar]
- Land E. J., Swallow A. J. One-electron reactions in biochemical systems as studied by pulse radiolysis. 3. Ubiquinone. J Biol Chem. 1970 Apr 25;245(8):1890–1894. [PubMed] [Google Scholar]
- Land E. J., Swallow A. J. One-electron reactions in biochemical systems as studied by pulse radiolysis. I. Nicotinamide-adenine dinucleotide and related compounds. Biochim Biophys Acta. 1968 Oct 1;162(3):327–337. doi: 10.1016/0005-2728(68)90119-9. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash E., Schejter A. Cytochrome c. Adv Protein Chem. 1966;21:113–286. doi: 10.1016/s0065-3233(08)60128-x. [DOI] [PubMed] [Google Scholar]
- Morton R. A., Overnell J., Harbury H. A. Electron transfer between cytochromes c from horse and Pseudomonas. J Biol Chem. 1970 Sep 25;245(18):4653–4657. [PubMed] [Google Scholar]
- Pecht Israel, Faraggi Moshe. The reduction of cytochrome c by hydrated electrons. FEBS Lett. 1971 Mar 16;13(4):221–224. doi: 10.1016/0014-5793(71)80540-9. [DOI] [PubMed] [Google Scholar]
- Schejter A., Margalit R. The redox potential of cytochrome c: Ion binding and oxidation state as linked functions. FEBS Lett. 1970 Oct 5;10(3):179–181. doi: 10.1016/0014-5793(70)80447-1. [DOI] [PubMed] [Google Scholar]
- Winfield M. E. Electron transfer within and between haemoprotein molecules. J Mol Biol. 1965 Jul;12(3):600–611. doi: 10.1016/s0022-2836(65)80314-x. [DOI] [PubMed] [Google Scholar]