Abstract
Objectives. Although people with HIV experience significant oral health problems, many consistently identify oral health as an unmet health care need. We conducted a randomized controlled trial to evaluate the impact of a dental case management intervention on dental care use.
Methods. We evaluated the intervention according to self-reported dental care use at 6-, 12-, and 18-month follow-ups. Multivariable logistic models with generalized estimating equations were used to assess the effects of the intervention over time.
Results. The odds of having a dental care visit were about twice as high in the intervention group as in the standard care group at 6 months (adjusted odds ratio [OR] = 2.52; 95% confidence interval [CI] = 1.58, 4.08) and 12 months (adjusted OR = 1.98; 95% CI = 1.17, 3.35), but the odds were comparable in the 2 groups by 18 months (adjusted OR = 1.07; 95% CI = 0.62, 1.86). Factors significantly associated with having a dental care visit included frequent physician visits and dental care referrals.
Conclusions. We demonstrated that a dental case management intervention targeting people with HIV was efficacious but not sustainable over time. Barriers not addressed in the intervention must be considered to sustain its use over time.
In the era of antiretroviral therapy, people with HIV are living longer and the treatment of associated medical and oral manifestations of the disease has shifted to a chronic disease model.1 Previous studies have shown that a person living with HIV/AIDS is more likely than a person without the disease to experience oral health problems.2–5 Furthermore, the oral health problems of individuals with HIV can be more severe and difficult to treat than those of the general population and may also contribute to the onset of opportunistic infections.5
The oral health complications associated with HIV are well documented,2–6 and oral manifestations are increasingly being recognized as markers for monitoring treatment efficacy and predicting treatment failure.7 Oral manifestations, including Kaposi’s sarcoma, necrotizing ulcerative periodontitis, oral hairy leukoplakia, and candidiasis, may be present in up to 50% of people with HIV and 80% of people diagnosed with AIDS,5,6 and may predict low CD4 counts.8 In addition, individuals living with HIV/AIDS may experience difficulty in maintaining adequate salivary flow, which affects chewing, swallowing, and the ability to take medication.4 Chronic use of highly active antiretroviral therapy can also contribute to diminished salivary flow as well as an increased risk of oral candidiasis and oral hairy leukoplakia.9
Throughout the 1990s, a series of study findings highlighted the unmet needs for dental care among people with HIV infection.10–14 This gap in oral health care services was corroborated by findings from the oral health component of the HIV Cost and Services Utilization Study,15 which demonstrated that unmet dental needs were twice as common as unmet medical needs among HIV-positive adults16,17 and led to a national call to action to improve access to oral health care.18 That study also showed that approximately half of people living with HIV had dental insurance, and those without dental insurance had greater unmet needs for dental services.17,19,20
Recently published findings suggest that an unmet need still persists. One example is an initiative, funded by the Health Resources and Services Administration, that included 2469 people living with HIV who had not received dental care during the preceding year. Nearly half of these individuals (48%) reported an unmet dental need since their HIV diagnosis, 52% had not seen a dentist in more than 2 years, and 63% rated the health of their teeth and gums as fair or poor.21,22 An earlier investigation involving baseline data from the study presented here showed that oral health problems and symptoms were very prevalent among our study population, with 63% of participants having experienced an oral health impact very often or fairly often in the preceding 4 weeks.23
Barriers to dental care use among individuals living with HIV include fear of dental care, HIV-specific stigma, fear of disclosing their HIV status to health care providers, perceived cost barriers, and poor adherence to medical guidance.20,22,24–31 Compounding patient access barriers, dental care providers may be reluctant to treat patients with HIV owing to fears of HIV transmission and associated stigma.32–36
Previous research conducted in Florida revealed that more than one third of people with HIV do not discuss oral health with their primary care providers.37 Although clinical guidelines recommend that HIV care providers examine the oral cavity during initial and interim physical examinations of people living with HIV, this still may not be a regular clinical practice.37 To address underuse of oral health care services among individuals with HIV, we evaluated the efficacy of an intervention that linked individuals to dental care. The sample comprised a population of HIV-positive individuals in south Florida who had received HIV primary care but had not received oral health services in the preceding 12 months.
METHODS
The goal of this study, Project SMILE, was to evaluate the efficacy of a brief, client-centered dental case management intervention designed to increase access to and use of oral health services among low-income individuals with HIV. The intervention was based on the antiretroviral treatment and access to services (ARTAS) model, an effective individual-level, multisession, time-limited intervention designed to link HIV-positive individuals to medical care.38,39 The core components of ARTAS include strengths-based case management and the development of a barrier reduction plan.
The study sample in this 2-arm randomized clinical trial included 600 participants who had not received oral health services during the 12 months prior to their enrollment. Participants were enrolled and randomized between April 2005 and December 2007. Face-to-face interviews were conducted by the 2 designated field interviewers and the project director at baseline and at 6, 12, and 18 months after baseline; interviews focused on use of dental services in the preceding 6 months and predisposing, enabling, and need-related potential factors associated with dental care use.40,41 A small monetary incentive was offered for completion of the baseline interview ($20) and follow-up interviews and assessments ($25 at 6 months, $30 at 12 months, and $35 at 18 months).
Screening and Enrollment
Potential study participants were approached, screened, and enrolled from 5 HIV primary care clinics in 2 south Florida counties; 2 of the clinics had on-site dental facilities. For the purposes of our analyses, the 2 participating sites in one of the counties (both without on-site dental services) were combined because of the small sample size at one of the sites. To be included in the study, individuals were required to be HIV positive (documented through self-reports and validated with printed reports of most recent test results), to not have received oral health services during the preceding 12 months, to be 18 years of age or older, to currently be receiving HIV primary care, to be eligible for Ryan White grant program funding for dental services (this national program, based on financial eligibility, funds HIV-related services for those without access to care), to have plans to remain in south Florida for at least 24 months so that they could complete follow-up visits, and to be able to provide contact information for 2 individuals to assist with follow-up (in cases in which participants could not be located).
Flyers and active or passive referrals from clinic staff members were used in recruiting participants. Interested patients were referred to a research staff member who further described the study and obtained informed consent.
Intervention Groups
A gender- and site-stratified blocked randomization scheme generated via a computerized algorithm was used to randomize participants to study groups. Although randomization was not blinded, recruiters were not aware of group allocations until after participants had completed the baseline assessment. Information on group assignments was provided to the recruiters in sealed envelopes. Participants randomized to the standard care arm received care consistent with that provided to HIV-positive individuals who attended any of the study clinics and accessed services typically made available. Study participants randomized to receive the intervention were linked with a dental case manager trained to deliver the intervention model.
Participants randomized to the intervention arm received up to 3 sessions with the dental case manager. The goal of these sessions was to have the participant visit a dentist. The sessions focused on educating participants about the importance of oral health care and motivating them to seek care; identifying individual and structural barriers to obtaining dental care; identifying individual strengths, abilities, and skills that individuals can adapt to overcome such barriers; addressing structural barriers such as filling out paperwork to qualify for insurance; obtaining necessary documentation; making dental care appointments; arranging transportation to appointments; and linking participants to community case management services.
The number of sessions conducted with each participant was tailored to his or her readiness to visit a dentist as well as the number of perceived barriers identified. Sessions were conducted between baseline enrollment and 3 months postbaseline in any setting that was convenient for participants, such as in their home, in a local restaurant, in the study vehicle, or at the University of Miami. This 90-day period fit with the possibility of HIV-positive individuals having to wait 3 months for a dental care appointment. In some instances, the first intervention session occurred as early as directly after randomization and completion of the baseline appointment.
Primary Outcome
The primary outcome evaluated in the trial, self-reported use of dental care, was based on responses to the question “Have you visited a dentist in the last 6 months?” This question was asked at the 6-, 12-, and 18-month follow-ups. We used Andersen’s model of health service use to guide the selection of independent variables.40,41 This model suggests that individuals’ use of health services is a function of 3 components: predisposing factors, enabling factors, and need factors.
Study Variables
Predisposing characteristics included sociodemographic variables, oral health knowledge and attitudes, and general self-efficacy. Oral health knowledge was assessed by asking whether participants believed that they were more informed about how HIV infection affects oral health than other HIV-positive people (yes or no). Attitudes were assessed according to the extent to which the participant agreed with 3 statements concerning the importance of oral health for HIV-positive individuals. Finally, the 10-item General Self-Efficacy Scale was used to assess self-efficacy (e.g., “I can always manage to solve difficult problems if I try hard enough”); the 4-point response scale was dichotomized (exactly true vs less than exactly true).42
Enabling characteristics included variables related to dental and health care as well as social support, including whether a participant had received a referral to a dentist from any health care provider. General satisfaction with dental care was based on 5 items scored on a 4-point scale (strongly disagree to strongly agree); a mean score was computed. We used the Multidimensional Scale of Perceived Social Support to assess social support.43 An average score was computed, and the final score (ranging from 1 to 7) was dichotomized to denote a score of 6 or above (corresponding to strongly or very strongly agreeing that social support was available) versus a score below 6. Enabling characteristics also included variables related to participants’ socioeconomic status: annual household income and whether they had stable housing in the preceding 3 months. Unstable housing locations included someone else’s home, temporary housing (e.g., boarding house or shelter), prisons, and health care facilities.
Finally, need characteristics related to participants’ oral health as well as general health needs. The 49-item Oral Health Impact Profile44 was used to measure self-reported dysfunction, discomfort, and disability related to oral health. We asked how often in the preceding 4 weeks the respondent had experienced the oral health impact (defined as a dental symptom or problem) described in each of the items. We created a pair of binary summary measures from the scale’s 49 items, the first denoting that at least 1 impact occurred fairly often or very often and the second denoting that at least 1 impact occurred very often. We assessed oral health status according to whether participants had lost any permanent teeth as a result of decay or gum disease. Participants were also asked to rate their overall general and oral health on a 5-point scale (ranging from poor to excellent).
Data Analysis
In the preliminary statistical analysis, we examined bivariate associations between each independent variable and use of dental care in the preceding 6 months; in a logistic regression, we used the χ2 test to assess categorical variables and unadjusted odds ratio (OR) estimates to assess continuous predictors. Logistic models with generalized estimating equations and an autoregressive working correlation structure provided robust estimates of the variance in model parameters and accommodated correlations among repeated outcome measures. A stepwise multivariable analysis of the association between the dental case management intervention and use of dental care included an interaction term between intervention and time to allow assessment of potential differences in the intervention’s effects over time. In addition, site was included as a fixed effect so that sites with and without dental care services available could be compared. With the few number of sites and the many participants at each site, we were able to reliably estimate the impact of site as a fixed effect.45,46
We used a stepwise approach to assess all other potentially confounding factors. The factors initially considered were those having bivariate associations with dental care use at the 0.20 alpha level; factors were removed 1 at a time, beginning with those that had the highest P values, until only factors with P values of less than .05 remained. Throughout the process, however, we retained a set of specified variables regardless of their P values (age, gender, race/ethnicity, education, and recruitment site). At each step, the model was examined with respect to its goodness of fit, and covariates were assessed for any changes that might indicate confounding as a result of removed variables. There were no issues with collinearity among the predictor variables. We evaluated main effects and their corresponding interaction terms to determine whether there were any differences between the groups in the effects of the factors examined.
In addition, we conducted a sensitivity analysis to examine any potential bias caused by missing observations. We performed the generalized estimating equations analysis under 2 extreme scenarios in which missing outcomes were either replaced with a 0 (no dental care use) or a 1 (dental care use). We then assessed changes from the original analysis in estimates of treatment effects at the follow-up time points.
RESULTS
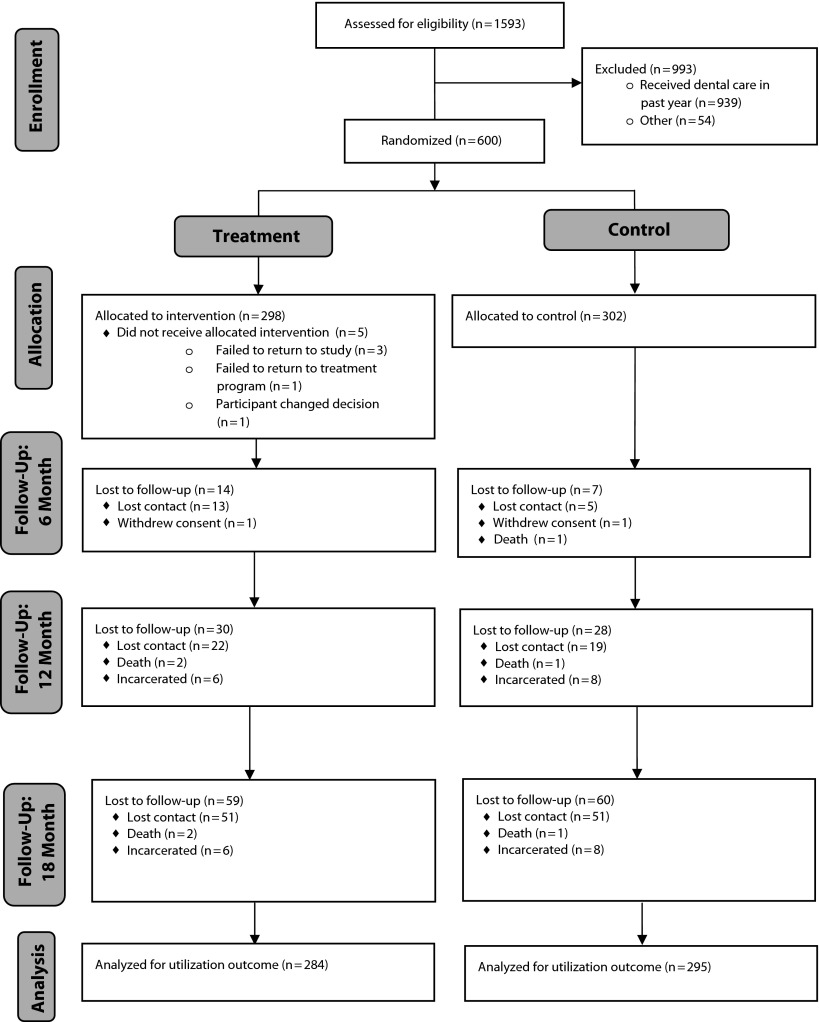
Study staff assessed 1593 individuals to determine whether they were eligible for the study. The CONSORT flow diagram in Figure 1 summarizes the study operations from initial enrollment to final follow-up assessment. Of those screened, 600 were randomized into the study and 993 were excluded as a result of not meeting the eligibility criteria, mostly because they had received dental care in the preceding year (n = 939). All 298 participants randomized to the intervention arm received services, with the exception of 5 participants who did not receive the intervention for the reasons documented in Figure 1.
FIGURE 1—
CONSORT flow diagram: Project SMILE.
Cross-sectional follow-up rates at 6, 12, and 18 months were 69%, 70%, and 67%, respectively. However, a notable number of participants who failed to follow-up at different visits throughout the 18-month study period were present for some of their study visits (the lost to follow-up data in Figure 1 indicate the numbers of participants who did not complete any subsequent visits and were lost to follow-up permanently). Participants who completed at least 1 of the 3 follow-up sessions were included in the outcome analysis (of note, only 3.5% of the sample was lost to follow-up after the baseline assessment).
There were no baseline differences in demographic characteristics or other study variables between participants in the intervention and standard care groups (Table 1). Overall, the majority of participants were male (70.9%), 35 to 54 years old (75.4%; median age = 44 years), and non-Hispanic Black (56.9%). Although 72.4% of the participants reported having a stable living environment, almost half (49.6%) reported an annual income of $5000 or less.
TABLE 1—
Selected Characteristics of Participants at Baseline, by Intervention Group: Project SMILE, Florida, 2005–2009
| Characteristic | Total (n = 594),a % | Control Group (n = 299), % | Intervention Group (n = 295), % |
| Age, y | |||
| 19–34 | 15.2 | 14.1 | 16.3 |
| 35–44 | 38.7 | 42.5 | 34.9 |
| 45–54 | 36.7 | 31.8 | 41.7 |
| ≥ 55 | 9.4 | 11.7 | 7.1 |
| Gender | |||
| Female | 29.1 | 29.1 | 29.2 |
| Male | 70.9 | 70.9 | 70.9 |
| Race/ethnicity | |||
| Hispanic | 28.8 | 31.8 | 25.8 |
| Non-Hispanic White | 13.3 | 13.7 | 12.9 |
| Non-Hispanic Black | 56.9 | 53.5 | 60.3 |
| Other | 1.0 | 1.0 | 1.0 |
| Education | |||
| < high school | 34.2 | 36.1 | 32.2 |
| High school | 38.2 | 35.8 | 40.7 |
| > high school | 27.6 | 28.1 | 27.1 |
| Stable housing | |||
| No | 27.6 | 26.8 | 28.5 |
| Yes | 72.4 | 73.2 | 71.5 |
| Annual income ≤ $5000b | |||
| No | 50.4 | 51.2 | 49.7 |
| Yes | 49.6 | 48.8 | 50.3 |
| Time since HIV diagnosis, y | |||
| < 5 | 25.4 | 27.4 | 23.4 |
| 5–9.99 | 29.0 | 30.4 | 27.5 |
| 10–14.99 | 26.6 | 28.4 | 24.8 |
| ≥ 15 | 19.0 | 13.7 | 24.4 |
| Better informed than other HIV-positive people | |||
| No | 26.3 | 26.8 | 25.8 |
| Yes | 73.7 | 73.2 | 74.2 |
| Knows the importance of oral health for HIV-positive people | |||
| No | 39.6 | 38.8 | 40.3 |
| Yes | 60.4 | 61.2 | 59.7 |
| Has lost teeth owing to decay/gum diseasec | |||
| No | 24.3 | 23.8 | 24.8 |
| Yes | 75.7 | 76.2 | 75.3 |
| Has experienced ≥ 1 oral health impacts fairly or very often | |||
| No | 37.4 | 37.1 | 37.6 |
| Yes | 62.6 | 62.9 | 62.4 |
| Has experienced ≥ 1 oral health impacts very often | |||
| No | 56.9 | 60.2 | 53.6 |
| Yes | 43.1 | 39.8 | 46.4 |
| Had ≥ 2 medical care visits in past 6 mo | |||
| No | 3.0 | 2.3 | 3.7 |
| Yes | 97.0 | 97.7 | 96.3 |
| Was referred to dentist by health care providerd | |||
| No | 76.5 | 77.9 | 75.1 |
| Yes | 23.5 | 22.2 | 24.9 |
| Has ≥ 1 health problem | |||
| No | 29.0 | 29.5 | 28.5 |
| Yes | 71.0 | 70.5 | 71.5 |
| Has high self-efficacy | |||
| No | 63.5 | 67.6 | 59.3 |
| Yes | 36.5 | 32.4 | 40.7 |
| Has a strong social support systeme | |||
| No | 53.6 | 53.0 | 54.1 |
| Yes | 46.4 | 47.0 | 45.9 |
| Time since most recent dental care visit, yf | |||
| 1–1.99 | 34.4 | 38.5 | 30.3 |
| 2–4.99 | 35.1 | 34.5 | 35.7 |
| ≥ 5 | 28.3 | 25.8 | 31.0 |
| Never visited a dentist | 2.2 | 1.3 | 3.1 |
Data missing for 6 participants at baseline.
Data missing for 5 participants (2 in control group, 3 in intervention group).
Data missing for 1 control group participant.
Data missing for 3 participants (1 in control group, 2 in intervention group).
Data missing for 62 participants (35 in control group, 27 in intervention group).
Data missing for 7 participants (3 in control group, 4 in intervention group).
Levels of self-reported oral health knowledge and perceptions of the importance of oral health for HIV-positive individuals were high overall: 73.7% of the participants believed that they were better informed regarding the dental implications of HIV infection than other HIV-positive individuals, and a majority (60.4%) emphasized the importance of oral health for those with HIV. When asked when their most recent dental visit had occurred, similar percentages of participants reported 1 to 2 years ago (34.4%) and 2 to 5 years ago (35.1%); 28.3% of the participants had not seen a dentist in more than 5 years, and only 2.2% had never seen one in their lifetime.
The presence of comorbidities was evident, with the majority of participants reporting that they had experienced at least 1 dental symptom or problem fairly often or very often (62.6%) and that they had experienced at least 1 general health problem (71%). Few had been referred to a dentist by a medical provider (23.5%), even though at baseline a majority reported having received medical care at least twice in the preceding year (97%). Fewer than half of the respondents (46.4%) claimed that they had a strong social support system, and only 36.5% had high perceived general self-efficacy.
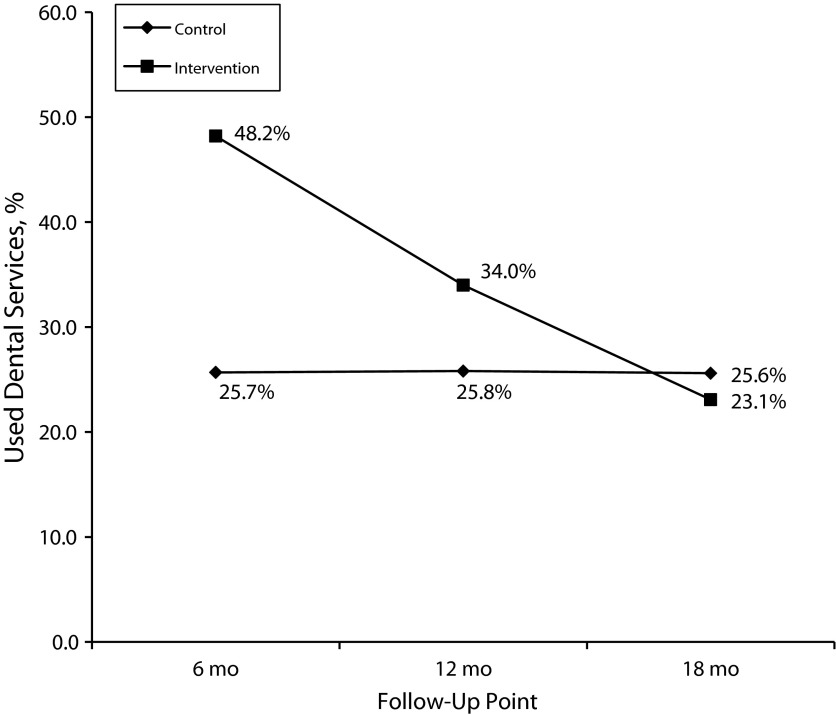
Rates of dental care use in the preceding 6 months were significantly higher in the intervention group than in the control group at the 6- and 12-month follow-up intervals (Figure 2 ). However, the difference in dental care use between the 2 groups was smaller at 12 months than at 6 months. Overall, the rate of dental care use in the intervention group declined over time, whereas the rate in the control group remained relatively constant. By the final (18-month) follow-up, the intervention and control groups did not exhibit any appreciable differences in use of dental care.
FIGURE 2—
Use of dental care in the preceding 6 months over time, by intervention group: Project SMILE, Florida, 2005–2009.
Results from the multivariable analysis indicate that, after control for other factors, the odds of having visited a dentist were more than twice as great in the intervention group than in the control group at 6 months (adjusted OR = 2.52; 95% confidence interval [CI] = 1.56, 4.08; P < .001; Table 2). At 12 months, the intervention group’s odds of having visited a dentist remained nearly twice those of the control group (adjusted OR = 1.98; 95% CI = 1.17, 3.35; P = .011). By 18 months, the odds of having visited a dentist were comparable between the 2 groups.
TABLE 2—
Results of Multivariable Model of Dental Care Use in the Preceding 6 Months: Project SMILE, Florida, 2005–2009
| Variable | Adjusted OR (95% CI) | P |
| Intervention effect: treatment vs control group | ||
| 6 mo | 2.52 (1.56, 4.08) | <.001 |
| 12 mo | 1.98 (1.17, 3.35) | .011 |
| 18 mo | 1.07 (0.62, 1.86) | .797 |
| Intervention effect over time | ||
| 12 mo vs 6 mo | 0.82 (0.52, 1.31) | .411 |
| 18 mo vs 12 mo | 0.59 (0.35, 1.01) | .053 |
| 18 mo vs 6 mo | 0.49 (0.29, 0.82) | .007 |
| Age, y | 1.01 (0.99, 1.03) | .168 |
| Gender | 0.95 (0.67, 1.35) | .776 |
| Race/ethnicity | ||
| Hispanic vs non-Hispanic White/other | 0.90 (0.51, 1.57) | .7 |
| Non-Hispanic Black vs non-Hispanic White/other | 0.64 (0.38, 1.09) | .101 |
| Education | ||
| < high school vs > high school | 0.93 (0.59, 1.44) | .731 |
| High school vs > high school | 0.75 (0.51, 1.10) | .14 |
| On-site dental care | 1.41 (0.95, 2.09) | .084 |
| ≥ 2 medical care visits in past 6 mo | 2.12 (1.40, 3.24) | <.001 |
| Referred to dentist by any health care provider | 6.80 (4.98, 9.30) | <.001 |
| Importance of oral health for people with HIV | 1.40 (1.02, 1.92) | .038 |
| Better informed than other HIV-positive people | 2.74 (1.54, 4.87) | <.001 |
| General satisfaction with dental care | 2.40 (1.51, 3.80) | <.001 |
| High self-efficacy | 1.44 (1.03, 2.03) | .035 |
Note. CI = confidence interval; OR = odds ratio. Data were derived from a generalized estimating equations analysis (n = 1200 observations).
Additional factors remained as predictors in the multivariable logistic regression analysis (Table 2). Participants who reported that they had visited their HIV primary care physician at least twice in the preceding 6 months were twice as likely as those who had not done so to have visited a dentist in the preceding 6 months (adjusted OR = 2.12; 95% CI = 1.40, 3.24; P < .001). Participants who had received a referral for dental care from either their HIV care provider or any other care provider in the preceding 6 months were more likely than those who did not receive a referral to visit a dentist (adjusted OR = 6.80; 95% CI = 4.98, 9.30; P < .001). Other factors positively and significantly associated with visiting a dentist were participants’ belief that oral health is important for those with HIV and their belief that they were better informed than other HIV-positive individuals regarding oral health, as well as higher levels of satisfaction with dental care and high general self-efficacy (Table 2). There was no interaction between treatment group and on-site dental services, indicating that the effect of the intervention was not dependent on whether sites had dental services available.
Under both extreme scenarios considered in the sensitivity analysis, the findings remained unchanged from the reported results. The estimates of the models fit to data with imputed outcomes representing extreme scenarios each indicated that the odds of receiving care at 6 and 12 months were significantly higher in the treatment group than in the standard care group, consistent with the findings from the data that were not imputed.
DISCUSSION
In April 2011, the Institute of Medicine issued a report, Advancing Oral Health in America, indicating that the most vulnerable populations in the United States continue to have difficulty in obtaining oral health care.47 One such vulnerable group is people living with HIV. Previous work has shown an association between case management services and use of oral health care among individuals living with HIV48–50; however, to our knowledge, our study is the first randomized controlled trial of an intervention addressing engagement in oral health care among HIV-positive individuals with low incomes.
Our findings are both encouraging and concerning. On the positive side, we demonstrated that a brief dental case management intervention based on the ARTAS model is efficacious in linking people with HIV to oral health care at 6 and 12 months after study randomization. It is promising that the ARTAS intervention can be adapted to focus on oral health care use among people living with HIV, and it might also be considered for linkage to other health care services.
Our results show that the effect of the intervention declined from 6 to 12 months and that it was not effective at 18 months after randomization. An issue of concern is that although participants in the intervention group increased their use of dental care, the overall percentage of intervention group participants who visited a dentist was low. This is surprising given that the participants should not have had concerns about dental care costs because they received services through the Ryan White grant program. This finding suggests that there are other barriers that may not have been addressed through this dental case management approach, such as fear of or anxiety regarding dental care, as well as other structural or cultural issues that may be related to the accessibility and acceptability of dental care.20,22,24–31 In addition, this diminishing effect of the intervention indicates that dental case management may need to be carried out on a periodic basis, not only after initial linkage to care, if it is to be effective.
We found that few of our participants had been encouraged by their HIV provider to seek dental care, which is also consistent with previous research.37 Yet, it is important to note that participants whose HIV primary care clinics or providers had given them referrals for dental treatment were more likely to have used dental care at the follow-up assessments. Although it has been found that the availability of on-site dental care in HIV care settings is associated with increased use of dental care, this was not the case in our study.28 Therefore, it is plausibly more imperative that HIV care providers be educated about the importance of dental health and providing active referrals as opposed to facilitating the provision of direct on-site dental care.
Limitations
The limitations of the study relate to the generalizability and validity of self-reported measures. First, our data were derived from a convenience sample of HIV-positive patients receiving services through the Ryan White grant program, an indication of low income, who were recruited from HIV primary care clinics in a single large urban area. Therefore, the generalizability of our findings to HIV-positive individuals in rural areas, other urban areas, or other countries may be limited. Nevertheless, although this randomized trial involved a convenience sample, our settings and patients are similar to those in other areas of the United States with high HIV prevalence rates.
Second, use of dental care was measured via self-report, which may have resulted in underreporting or overreporting of services received. The validity of self-reported service use has been shown to vary across different types of services, age groups, and medical conditions. Nevertheless, Gilbert et al. demonstrated good agreement between self-reported use of dental services and dental record information in the preceding 6 months in a study involving a diverse sample of Florida residents.51 Also, the original ARTAS intervention trial showed that there was a high degree of agreement between medical record reviews and self-reports of linkage to care at 6- and 12-month follow-up assessments. Specifically, medical record reviews showed that 93% and 86% of participants’ self-reported HIV care episodes were confirmed with medical record reviews conducted at 6-month and 12-month follow-up assessments, respectively.39 In addition, there is no reason to believe that the validity of self-reported rates of use were different in the 2 treatment groups.
Conclusions
Despite its limitations, this study offers a brief dental case management intervention approach that can be implemented in HIV care clinics to improve linkages to oral health care services among people living with HIV. In 2004, the American Dental Association advocated the use of “patient outreach efforts and care coordination” as a means of improving oral health accessibility in underserved populations.52 Some pilot studies have evaluated dental case management programs and whether they increase access to dental care in Medicaid-eligible, difficult-to-reach populations.50,53
Similarly, the importance of dental case management interventions has been recognized in the HIV field, and in some cases these interventions have been in use for many years (e.g., the Boston, MA, dental ombudsman program)48,49,54; however, there have been few (if any) rigorous efforts to evaluate these programs in randomized controlled or quasi-experimental investigations. Patient navigators in HIV care clinics also may help link HIV-positive patients to dental care. Unfortunately, it appears that little attention is given to oral health in HIV care settings. As the Affordable Care Act is fully implemented and the delivery of health care to people with HIV is continually reassessed, there are ongoing opportunities to improve the integration of oral health care and medical care.
Acknowledgments
Funding for this study was provided by the National Institute of Dental and Craniofacial Research (NIH grant number R01 DE015523).
Human Participant Protection
This study was approved by the institutional review boards of the University of Miami and Columbia University. Participants provided written informed consent before taking part in the study.
References
- 1.Younai F, Vincent-Jones C. Oral health and HIV infection: a chronic disease model. J Can Dent Assoc. 2009;37(11):811–819. [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services, Health Resources and Services Administration. Dental programs (part F) Available at: http://hab.hrsa.gov/abouthab/partfdental.html. Accessed March 15, 2014.
- 3.Patton L, McKaig R, Strauss R, Rogers D, Eron J. Changing prevalence of oral manifestations of human immunodeficiency virus in the era of protease inhibitor therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(3):299–304. doi: 10.1016/s1079-2104(00)70092-8. [DOI] [PubMed] [Google Scholar]
- 4.Weinert M, Grimes R, Lynch D. Oral manifestations of HIV infection. Ann Intern Med. 1996;125(6):485–496. doi: 10.7326/0003-4819-125-6-199609150-00010. [DOI] [PubMed] [Google Scholar]
- 5.Reznik DA. Oral manifestations of HIV disease. Top HIV Med. 2005;13(5):143–148. [PubMed] [Google Scholar]
- 6.Coogan M, Greenspan J, Challacombe S. Oral lesions in infection with human immunodeficiency virus. Bull World Health Organ. 2005;83(9):700–706. [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson N. The mouth in HIV/AIDS: markers of disease status and management challenges for the dental profession. Aust Dent J. 2010;55(suppl 1):85–102. doi: 10.1111/j.1834-7819.2010.01203.x. [DOI] [PubMed] [Google Scholar]
- 8.Bodhade AS, Ganvir S, Hazarey VK. Oral manifestations of HIV infection and their correlation with CD4 count. J Oral Sci. 2011;53(2):203–211. doi: 10.2334/josnusd.53.203. [DOI] [PubMed] [Google Scholar]
- 9.Nittayananta W, Talungchit S, Jaruratanasirikul S et al. Effects of long-term use of HAART on oral health status of HIV-infected subjects. J Oral Pathol Med. 2010;39(5):397–406. doi: 10.1111/j.1600-0714.2009.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capilouto E, Piette J, White BA, White B, Fleishman J. Perceived need for dental care among persons living with acquired immunodeficiency syndrome. Med Care. 1991;29(8):745–754. doi: 10.1097/00005650-199108000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Weissman JS, Makadon HJ, Seage GR, III et al. Changes in insurance status and access to care for persons with AIDS in the Boston Health Study. Am J Public Health. 1994;84(12):1997–2000. doi: 10.2105/ajph.84.12.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonuck K, Arno P, Green J et al. Self-perceived unmet health care needs of persons enrolled in HIV care. J Community Health. 1996;21(3):183–198. doi: 10.1007/BF01557998. [DOI] [PubMed] [Google Scholar]
- 13.Marx R, Katz MH, Park M, Gurley RJ. Meeting the service needs of HIV-infected persons: is the Ryan White CARE Act succeeding? J Acquir Immune Defic Syndr. 1997;14(1):44–55. doi: 10.1097/00042560-199701010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Shiboski C, Palacio H, Neuhaus JM, Greenblatt RM. Dental care access and use among HIV-infected women. Am J Public Health. 1999;89(6):834–839. doi: 10.2105/ajph.89.6.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson R, Bozette S, Shapiro M . Disparities in Care for HIV Patients: Results of the HCSUS Study. Santa Monica, CA: RAND; 2006. [Google Scholar]
- 16.Heslin K, Cunningham W, Marcus M et al. A comparison of unmet needs for dental and medical care among persons with HIV infection receiving care in the United States. J Public Health Dent. 2001;61(1):14–21. doi: 10.1111/j.1752-7325.2001.tb03350.x. [DOI] [PubMed] [Google Scholar]
- 17.Marcus M, Freed J, Coulter I et al. Perceived unmet need for oral treatment among a national population of HIV-positive medical patients: social and clinical correlates. Am J Public Health. 2000;90(7):1059–1063. doi: 10.2105/ajph.90.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Health Resources and Services Administration. Increasing access to dental care. Available at: http://hab.hrsa.gov/newspublications/careactionnewsletter/june2008.pdf. Accessed March 15, 2014.
- 19.Freed J, Marcus M, Freed B et al. Oral health findings for HIV-infected adult medical patients from the HIV Cost and Services Utilization Study. J Am Dent Assoc. 2005;136(10):1396–1405. doi: 10.14219/jada.archive.2005.0053. [DOI] [PubMed] [Google Scholar]
- 20.Patton LL, Strauss RP, McKaig RG, Porter DR, Eron JJ., Jr Perceived oral health status, unmet needs, and barriers to dental care among HIV/AIDS patients in a North Carolina cohort: impacts of race. J Public Health Dent. 2003;63(2):86–91. doi: 10.1111/j.1752-7325.2003.tb03480.x. [DOI] [PubMed] [Google Scholar]
- 21.Fox J, Tobias C, Bachman S, Reznik D, Rajabiun S, Verdecias N. Increasing access to oral health care for people living with HIV/AIDS in the US: baseline evaluation results of the Innovations in Oral Health Care Initiative. Public Health Rep. 2012;127(2):5–16. doi: 10.1177/00333549121270S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeanty Y, Cardenas G, Fox J et al. Correlates of unmet dental care need among HIV-positive people since being diagnosed with HIV. Public Health Rep. 2012;127(2):17–24. doi: 10.1177/00333549121270S204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomar S, Pereyra M, Metsch L. Oral health-related quality of life among low-income adults living with HIV. J Public Health Dent. 2011;71(3):241–247. [PubMed] [Google Scholar]
- 24.Singer R, Cardenas G, Xavier J et al. Dental anxiety and the use of oral health services among people attending two HIV primary care clinics in Miami. Public Health Rep. 2012;127(2):36–44. doi: 10.1177/00333549121270S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hastreiter RJ, Jiang P. Do regular dental visits affect the oral health care provided to people with HIV? J Am Dent Assoc. 2002;133(10):1343–1350. doi: 10.14219/jada.archive.2002.0049. [DOI] [PubMed] [Google Scholar]
- 26.Mascarenhas AK, Smith SR. Access and use of specific dental services in HIV disease. J Public Health Dent. 2000;60(3):172–181. doi: 10.1111/j.1752-7325.2000.tb03324.x. [DOI] [PubMed] [Google Scholar]
- 27.Shiboski CH, Cohen M, Weber K, Shansky A, Malvin K, Greenblatt RM. Factors associated with use of dental services among HIV-infected and high-risk uninfected women. J Am Dent Assoc. 2005;136(9):1242–1255. doi: 10.14219/jada.archive.2005.0340. [DOI] [PubMed] [Google Scholar]
- 28.Coulter I, Marcus M, Freed J et al. Use of dental care by HIV-infected medical patients. J Dent Res. 2000;79(6):1356–1361. doi: 10.1177/00220345000790060201. [DOI] [PubMed] [Google Scholar]
- 29.Kenagy G, Linsk N, Bruce D et al. Service utilization, service barriers, and gender among HIV-positive consumers in primary care. AIDS Patient Care STDS. 2003;17(5):235–244. doi: 10.1089/108729103321655881. [DOI] [PubMed] [Google Scholar]
- 30.Rajabiun S, Bachman S, Fox J, Tobias C, Bednarsh H. A typology of models for expanding access to oral health care for people living with HIV/AIDS. J Public Health Dent. 2011;71(3):212–219. [PubMed] [Google Scholar]
- 31.Rajabiun S, Fox J, McCluskey A et al. Patient perspectives on improving oral health-care practices among people living with HIV/AIDS. Public Health Rep. 2012;127(2):73–81. doi: 10.1177/00333549121270S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherry-Peppers G, Daniels CO, Meeks V, Sanders CF, Reznik D. Oral manifestations in the era of HAART. J Natl Med Assoc. 2003;95(2):21S–32S. [PMC free article] [PubMed] [Google Scholar]
- 33.Cleveland JL, Cardo DM. Occupational exposures to human immunodeficiency virus, hepatitis B virus, and hepatitis C virus: risk, prevention, and management. Dent Clin North Am. 2003;47(4):681–696. doi: 10.1016/s0011-8532(03)00041-7. [DOI] [PubMed] [Google Scholar]
- 34.Seacat J, Litt M, Daniels AS. Dental students treating patients living with HIV/AIDS: the influence of attitudes and HIV knowledge. J Dent Educ. 2009;73(4):437–444. [PubMed] [Google Scholar]
- 35.Giuliani M, Tumbarello M, Marino M et al. Dental hygienists behaviour towards HIV-positive patients in highly active antiretroviral therapy era: a pilot survey. Int J Dent Hyg. 2011;9(3):204–210. doi: 10.1111/j.1601-5037.2010.00472.x. [DOI] [PubMed] [Google Scholar]
- 36.Levett T, Slide C, Mallick F, Lau R. Access to dental care for HIV patients: does it matter and does discrimination exist? Int J STD AIDS. 2009;20(11):782–784. doi: 10.1258/ijsa.2009.009182. [DOI] [PubMed] [Google Scholar]
- 37.Pereyra M, Metsch L, Gooden L. HIV-positive patients’ discussion of oral health with their HIV primary care providers in Miami, Florida. AIDS Care. 2009;21(12):1578–1584. doi: 10.1080/09540120902923030. [DOI] [PubMed] [Google Scholar]
- 38.Craw JA, Gardner LI, Marks G et al. Brief strengths-based case management promotes entry into HIV medical care: results of the Antiretroviral Treatment Access Study-II. J Acquir Immune Defic Syndr. 2008;47(5):597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 39.Gardner LI, Metsch LR, Anderson-Mahoney P et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19(4):423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 40.Andersen R. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36(1):1–10. [PubMed] [Google Scholar]
- 41.Andersen R. A Behavioral Model of Families’ Use of Health Services. Chicago, IL: Center for Health Administration Studies, University of Chicago; 1968. [Google Scholar]
- 42.Schwarzer R, Jerusalem M. Generalized self-efficacy scale. In: Weinman J, Wright S, Johnston M, editors. Measures in Health Psychology: A User’s Portfolio. Windsor, England: NFER-Nelson; 1995. pp. 35–37. [Google Scholar]
- 43.Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support. J Pers Assess. 1988 5(1)2:30–41. [Google Scholar]
- 44.Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health. 1994;11(1):3–11. [PubMed] [Google Scholar]
- 45.Feaster D, Mikulich-Gilbertson S, Brincks A. Modeling site effects in the design and analysis of multi-site trials. Am J Drug Alcohol Abuse. 2011;37(5):383–391. doi: 10.3109/00952990.2011.600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peduzzi P, Henderson W, Hartigan P, Lavori P. Analysis of randomized controlled trials. Epidemiol Rev. 2002;24(1):26–38. doi: 10.1093/epirev/24.1.26. [DOI] [PubMed] [Google Scholar]
- 47.Institute of Medicine. Advancing Oral Health in America. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 48.Lemay C, Kretsedemas M, Graves JR. Satisfaction with dental case management among people living with HIV/AIDS. J Community Health. 2010;35(1):43–52. doi: 10.1007/s10900-009-9195-z. [DOI] [PubMed] [Google Scholar]
- 49.Lemay C, Cashman S, McDonald A, Graves J. A new approach to ensuring oral health care for people living with HIV/AIDS: the dental case manager. Prev Chronic Dis. 2012;9:E158. doi: 10.5888/pcd9.110297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lemay C, Tobias C, Umez-Eronini A et al. Dental case manager encounters: the association with retention in dental care and treatment plan completion. Spec Care Dentist. 2013;33(2):70–77. doi: 10.1111/j.1754-4505.2012.00293.x. [DOI] [PubMed] [Google Scholar]
- 51.Gilbert GH, Rose JS, Shelton BJ. A prospective study of the validity of data on self-reported dental visits. Community Dent Oral Epidemiol. 2002;30(5):352–362. doi: 10.1034/j.1600-0528.2002.00062.x. [DOI] [PubMed] [Google Scholar]
- 52.American Dental Association. Enhancing dental Medicaid outreach and care coordination. Available at: http://www.ada.org/sections/professionalResources/pdfs/medicaid_outreach.pdf. Accessed March 15, 2014.
- 53.Greenberg BJS, Kumar J, Stevenson H. Dental case management: increasing access to oral health care for families and children with low incomes. J Am Dent Assoc. 2008;139(8):1114–1121. doi: 10.14219/jada.archive.2008.0314. [DOI] [PubMed] [Google Scholar]
- 54.Boston Public Health Commission. HIV dental program and dental treatment fund. Available at: http://www.massresources.org/hiv-dental-program.html. Accessed March 15, 2014.