Abstract
Colonoscopy is the diagnostic modality of choice for investigation of symptoms suspected to be related to the colon and for the detection of polyps and colorectal cancer (CRC). Colonoscopy with removal of detected polyps has been shown to reduce the incidence and mortality of subsequent CRC. In many countries, population screening programs for CRC have been initiated, either by selection of patients for colonoscopy with fecal occult blood testing or by offering colonoscopy directly to average-risk individuals. Several endoscopy societies have formulated quality indicators for colonoscopy. These quality indicators are almost always incorporated as process indicators, rather than outcome measures. This review focuses on the quality indicators bowel preparation, cecal intubation rate, withdrawal time, adenoma detection rate, patient comfort, sedation and complication rate, and discusses the scientific evidence supporting them, as well as their potential shortcomings and issues that need to be addressed. For instance, there is still no clear and generally accepted definition of adequate bowel preparation, no robust scientific evidence is available supporting a cecal intubation rate ≥ 90% and the association between withdrawal time and occurrence of interval cancers has not been clarified. Adenoma detection rate is currently the only quality indicator that has been shown to be associated with interval colorectal cancer, but as an indicator it does not differentiate between subjects with one or more adenoma detected.
Keywords: Colonoscopy, Quality indicators, Bowel preparation, Cecal intubation, Withdrawal time, Adenoma detection rate, Screening, Complication, Interval colorectal cancer, Post-colonoscopy colorectal cancer
Core tip: Many endoscopy societies have formulated guidelines on quality indicators for colonoscopy, including bowel preparation, cecal intubation rate, withdrawal time and adenoma detection rate. These are mostly consensus-based process indicators, rather than outcome measures. The scientific evidence on which they are based is limited. Adenoma detection rate is currently the only quality indicator that has been shown to be directly associated with interval colorectal cancer, but also has its shortcomings.
INTRODUCTION
Colonoscopy is the diagnostic modality of choice for investigation of symptoms suspected to be related to the colon and for the detection of polyps and colorectal cancer (CRC). Colonoscopy with polypectomy has been shown to reduce both the incidence and mortality of subsequent CRC[1,2].
However, despite being the gold standard, colonoscopy is also known to be not a perfect test. From back-to-back colonoscopy studies, it is estimated that up to 25% of polyps are missed during colonoscopy[3,4]. Furthermore, the preventive effect of colonoscopy is most prominent for distal CRCs, whereas its role in preventing proximal CRCs is less evident[5,6]. Finally, up to 8% of CRCs occur within 3 years after a previous colonoscopy[7-12]. Despite technical advancements and increased professional awareness, this miss rate has not decreased over time[12]. Moreover, recent studies have shown that these so-called post-colonoscopy CRCs are most likely due to missed lesions, rather than being completely new lesions[13,14].
The incidence of CRC is steadily rising in many parts of the world[15]. Many countries have initiated population screening programs for CRC, either through selection of patients for colonoscopy with fecal occult blood testing (FOBT) or by offering colonoscopy directly to average-risk individuals[16,17]. This has resulted in an increase in the number of colonoscopies performed. For these mass screening programs to be successful, it is of utmost importance that colonoscopies are of high quality and performed according to the latest state of knowledge.
In an effort to optimize general performance of colonoscopy and to decrease inter-individual variation between physicians performing colonoscopy, several quality indicators have been suggested in recent years[18]. These quality indicators however all are process indicators rather than indicators of outcome. Ideally, the quality of colonoscopy should be measured by clinical outcome measures. The goal of colonoscopy in most cases is the detection of neoplastic lesions. After removal of premalignant neoplastic lesions, patients enter a surveillance program. The rate of the occurrence of interval cancers or post-colonoscopy CRCs, defined as CRCs diagnosed in the period between the last colonoscopy and the scheduled surveillance colonoscopy, is a more direct and probably better reflection of the quality of the colonoscopy performed than the main current quality indicators proposed in guidelines.
In this review, we will discuss the main current quality indicators for colonoscopy, the scientific evidence supporting them, as well as their potential shortcomings and issues that still need to be addressed.
BOWEL PREPARATION
A quality indicator issued by several international guidelines is that the endoscopist should report the quality of the bowel preparation for each colonoscopy[18,19]. Several guidelines state that ≥ 90% of patients undergoing colonoscopy should have had a bowel preparation rated as excellent or at least adequate[19,20]. The quality of bowel cleansing has been shown to impact the ability and time needed to reach the cecum and the detection of polyps, both small and large (≥ 10 mm)[21,22].
There are several bowel preparation medications available and regimens used for bowel preparation before colonoscopy. These vary from polyethylene glycol (PEG) based solutions, osmotic laxatives (sodium phosphate, magnesium citrate, sodium sulphate) or stimulant laxatives (senna, bisacodyl, sodium picosulphate), either alone or in combination.
In a meta-analysis of randomized controlled trials, split dose bowel preparation before colonoscopy has been demonstrated to significantly improve the number of satisfactory bowel preparations, and is associated with increased patient compliance and decreased nausea compared with full-dose PEG[23]. In a systematic review and meta-analysis, Enestvedt et al[24] concluded that bowel preparation with 4 liter of split dose PEG-solution is superior than other bowel preparation methods. Several endoscopy societies now recommend 4 liter split dose PEG-solution as the first choice bowel preparation[25], although 2 liter PEG-solution with ascorbate may be an alternative in the non-constipated patient. Routine use of sodium phosphate preparations is not recommended because of safety concerns, especially in patients with renal insufficiency[25]. In patients using PEG-solutions, the interval between the last ingested dose of PEG-solution and the colonoscopy should be 3-5 h, as this has been shown to result in significantly better bowel preparation[26,27].
In the literature, several risk factors for inadequate bowel preparation have been identified. Increasing age[28-31] and male gender[29-32] have repeatedly been reported. A medical history of colorectal surgery[28,29], diabetes[28,29] and cirrhosis[29,32], as well as inpatient status[30,32] have also been identified as risk factors for inadequate bowel preparation in several studies. Other risk factors that have been suggested in the literature are a procedural indication of constipation, a reported failure to successfully complete the bowel lavage, the use of tricyclic antidepressants, a history of stroke or dementia[32], a history of Parkinson’s disease, being overweight, having had a positive FOBT[29], a history of hysterectomy[28] and being of African-American descent[31]. A history of previous polypectomy was a negative predictive factor for inadequate bowel preparation in the study by Ness et al[32]. Furthermore, a later colonoscopy starting time during the day[30-32] was associated with inadequate bowel preparation in several studies. Most of these studies however were conducted before the wide application of a split-dose bowel preparation regimen. Whether this association currently still is valid remains to be elucidated.
Several scales have been developed to standardize the reporting of bowel preparation quality. Aronchick et al[33] were the first to propose a validated bowel preparation scale. This is a 5 point categorical scale, rating bowel preparation as excellent (small volume of clear liquid; > 95% of surface seen), good (large volume of clear liquid covering 5%-25% of surface; > 90% of surface see), fair (some semi-solid stool suctioned or washed away; > 90% of surface seen), poor (semi-solid stool that could not be suctioned or washed away; < 90% of surface seen) or inadequate (repeat bowel preparation necessary). Unfortunately, the reliability of this scale for the distal colon is rather poor.
Rostom and Jolicoeur developed and prospectively validated another bowel preparation scale, the Ottawa scale[34]. In this scale, the colon is divided into three segments: right colon (cecum and ascending colon), mid colon (transverse and descending colon) and rectosigmoid. For each segment, bowel preparation is qualified using a 4 point scale (0: perfectly clear to 4: solid stools and lots of fluid) for each colon segment individually and a 0 to 2 fluid quantity rating as a global value for the entire colon. The scale thus has a range from 0 (perfect bowel preparation) to 14 (completely unprepared).
Finally, in 2009 Lai et al[35] introduced the Boston Bowel Preparation Scale (BBPS). In this validated bowel preparation scale, the colon is divided into the right colon (cecum and ascending colon), transverse colon (including both the hepatic and splenic flexure) and the left colon (descending colon and rectosigmoid). The BBPS is a ten point scale (0-9) with 0-3 points allocated to each colon segment, i.e., 0 (unprepared colon segment that cannot be cleared), 1 (portion of mucosa of the colon segment seen, but other areas of the colon segment not well seen due to staining, residual stool and/or opaque liquid), 2 (minor residual staining, small fragments of stool and/or opaque liquid, but mucosa of colon segment seen well) 3 (entire mucosa of colon segment seen well with no residual staining, small fragments of stool or opaque liquid). In the validation study, a score of ≥ 5 was considered adequate.The BBPS differs from other preparation scales in that the score is applied after the endoscopist has performed cleansing maneuvers, like suctioning and washing.
All these scales have mainly been used in studies comparing new formulas or different schemes for bowel preparation[33,36-40], rather than being used to assist in clinical decision making. In a recent retrospective study, Calderwood et al[41] reported that the BBPS correlated with endoscopist behavior with regard to the advice for follow-up intervals for colonoscopy. A total BBPS score of ≥ 6 and/or all segment scores ≥ 2 provided a standardized definition of an “adequate” bowel preparation, whereas in 96% of examinations with a total score of ≤ 2 a repeat examination within 1 year was recommended. For scores 3 to 5 however, recommended surveillance intervals varied widely between endoscopists. Future studies should focus on prospectively evaluating these cut-offs for surveillance interval recommendations and ideally associating them with relevant clinical outcome measures.
The widely adopted quality indicator for bowel preparation has several shortcomings. First of all, there is still no clear and generally accepted definition of adequate bowel preparation. Furthermore, the mere reporting of the quality of bowel preparation in itself is unlikely to significantly affect the quality of the colonoscopies performed, unless it becomes more clear what bowel preparation quality is the absolute minimum to detect relevant findings and to prevent interval cancers. There is also no clear policy on how to proceed when a patient’s bowel is inadequately cleansed; the only relevant published studies on this topic had either small patient numbers[42] or a retrospective design[43].
The rule that ≥ 90% of patients undergoing colonoscopy should have an excellent or adequate bowel preparation is consensus based and has found its way into several guidelines[19,20]. However, there is no scientific evidence to support this cut-off at 90%. Although inadequate bowel preparation has been shown to negatively affect the rate of detected polyps, this does not appear to be the case for CRCs[21]. It is conceivable that, through the negative effect on the detection of adenomas, an inadequate bowel preparation is associated with a higher rate of interval cancers, but to date, there is no direct evidence to support this.
CECAL INTUBATION RATE
In order to visualize the entire colonic mucosa, intubation of the endoscope to the cecum is mandatory. Cecal intubation is defined as introduction of tip of the colonoscope into the cecal pole, proximal of the ileocecal valve in order to have the entire cecum visualized. Although this sometimes may be challenging, there is consensus that each endoscopist should have a cecal intubation rate of ≥ 90% of all cases[18-20,44,45]. When not taking into account obstructing CRCs, inadequate bowel preparation or severe colitis, this adjusted cecal intubation rate should be ≥ 95%[18]. Also, in ≥ 95% of all screening colonoscopies the cecum should be intubated[18,19]. Furthermore, cecal intubation should be documented by naming and photographing the landmarks of the cecum, i.e., the appendiceal orifice, the ileocecal valve and/or the terminal ileum.
In the literature, several factors have been associated with a higher risk of incomplete colonoscopy or more difficult intubation, with female gender being the most frequently reported predictive factor[46-50]. In addition, patients with advanced age[46,49,50] or a low body mass index[48-50], or in women with a history of hysterectomy[47] or diverticular disease[50], colonoscopy is reported to be more difficult and more often incomplete. Finally, poor bowel preparation and lower endoscopist annual case volume have been reported to be associated with a higher risk of incomplete colonoscopy[49].
Completeness of the colonoscopy is associated with a reduction in mortality from CRC[6]. In a study by Neerincx et al[51], a secondary colonoscopy after previous incomplete colonoscopy yielded initially missed advanced neoplasia (CRC or advanced adenoma) in 4.3% of patients. In a study on the yield of CT-colonography after incomplete colonoscopy in 136 patients, in 13.9% of patients one or more additional colonic neoplastic lesions (polyp(s) and/or CRC) were found[52].
These findings suggest that in cases of incomplete colonoscopy the clinician should always perform additional imaging to visualize the remaining colon. Following incomplete colonoscopy, the cecum can usually be intubated in the majority of patients during a repeat colonoscopy with readily available endoscopic instruments, suggesting that a repeat colonoscopy should always be considered[47,53]. CT-colonography might be a useful alternative in these cases, with the additional benefit of detecting potentially relevant extra-colonic findings[52].
It is important to keep in mind that there is no robust scientific evidence for a cecal intubation rate of ≥ 90%. Although it is obvious that an endoscopist is not able to adequately inspect colon segments that were not intubated, the accepted minimal cecal intubation rate is based on consensus rather than on a scientific basis.
WITHDRAWAL TIME
In 2006, Barclay et al[54] were the first to report that colonoscopists with a mean withdrawal time of 6 minutes or more had higher detection rates of any neoplasia and advanced neoplasia. Since then, a recommended mean withdrawal time of at least 6 min has been formulated as a quality indicator in several colonoscopy guidelines[18-20].
However, colonoscopic withdrawal time as a quality indicator is not undisputed. Since the initial publication by Barclay et al[54], several observational studies have reported on the association between colonoscopic withdrawal time and the number of detected polyps[55-59]. Other large studies could however not confirm these findings[60-62]. Furthermore, interventions directed at optimizing withdrawal time, in an attempt to improve polyp detection, have yielded conflicting results. Although Barclay et al[63] did report higher rates of overall and advanced neoplasia detection during screening colonoscopy after implementing a time-dependent colonoscopic withdrawal protocol, other authors were not able to find a difference in overall polyp detection rate after formally implementing such a policy[64,65].
Gellad et al[66] were the first to study the association between withdrawal time during an initial, negative colonoscopy and the risk of developing neoplasia in the next five years. They did not detect any significant association. However, mean baseline withdrawal time in the 13 participating centers was rather long (greater than 12 min), possibly explaining the non-confirmatory results. It is possible that withdrawal time no longer is an adequate quality measure for screening colonoscopy above a certain threshold.
The use of the indicator withdrawal time is based on the assumption that endoscopists who take longer to withdraw the colonoscope also use specific techniques to improve visualization of the entire colonic mucosa. A study of two endoscopists with different rates of missed adenomas indeed showed that a better quality colonoscopic withdrawal technique was associated with a longer withdrawal time[67]. Lee et al[62] reported that the number of detected adenomas was found to be associated with the quality of withdrawal technique, but not necessarily related to withdrawal time. Withdrawal technique may therefore be a more important indicator for colonoscopy quality than withdrawal time. At present, there is however no generally accepted way to quantify an optimal withdrawal technique.
It is conceivable that the derived quality indicator withdrawal time in the future will be replaced by a measure of the proportion of the colonic mucosa that is adequately visualized during colonoscopy. Interestingly, Hong et al[68] recently reported on a fully automated three-dimensional reconstruction technique from individual colonoscopy images. Such a technique might eventually give real time feedback to the endoscopist on areas of the colonic wall that are not adequately inspected, thus enabling revisiting these areas during the same procedure. The percentage of the colon surface that is visualized by the endoscopist may potentially serve as a new quality indicator for colonoscopy. Furthermore, information on inspected and uninspected areas of the colonic wall may help in training endoscopists, giving insight in possible “blind spots” during scope withdrawal.
As mentioned above, the association between the quality indicator withdrawal time and the occurrence of interval cancers has not yet been elucidated.
ADENOMA DETECTION RATE
The adenoma detection rate (ADR) is defined as the proportion of screened subjects in whom at least one adenomatous lesion is identified[18,19,69]. In an asymptomatic screening population, an ADR of ≥ 25% in men and of ≥ 15% in women over 50 years old has been proposed in the American screening guidelines[18], whereas the British Quality Assurance Guidelines for Colonoscopy has set the standard ADR, based on their own pilot data, at ≥ 35% of all screening colonoscopies in patients who had a positive FOBT[19].
Repeatedly, considerable variations between endoscopists in the rate of detected polyps and adenomas have been shown[70-74]. The ADR is the only current quality indicator that has been demonstrated to be directly associated with interval colorectal cancer. In the landmark study by Kaminski et al[69], an ADR ≥ 20% was associated with a reduction in interval colorectal cancers. A recent study by Corley et al[75] showed that the ADR was inversely associated with the risk of interval CRC, but also with advanced-stage interval cancers and fatal interval cancers.
In line with these findings, many recent studies have focused on ways to optimize adenoma detection, ranging from inexpensive and easy to implement interventions in daily clinical practice, to minor adaptations of currently used colonoscopy equipment to completely new colonoscopy platforms.
Position changes during colonoscope withdrawal have been reported to increase luminal distension and may reduce the rate of missed lesions[76]. Two small randomized studies have indeed suggested that dynamic patient position changes may improve polyp detection[77,78], but there was no difference in polyp or adenoma detection rates in another, larger randomized study[79].
Endoscopy nurse participation as a second observer during colonoscopy has been reported to significantly increase the overall number of detected polyps and adenomas found during colonoscopy[80], and appears an easy to implement intervention to increase polyp detection rate (PDR) and ADR[81].
Furthermore, the time of performing the colonoscopy may have an effect on the ADR. Testing the hypothesis that fatigue of the endoscopist, which increases as the day progresses, might affect ADR, Sanaka et al[82] were the first to report that the ADR of endoscopists was significantly higher in morning colonoscopies than in afternoon colonoscopies. The time of the colonoscopy during the day was an independent predictor for adenoma detection. These findings have been confirmed by almost all other studies on this subject[83-86]. Gurudu et al[83] proposed that colonoscopies should best be performed in half-day blocks by different physicians. They found no significant difference in ADR between morning and afternoon colonoscopies when endoscopists only perform colonoscopies in half-day blocks.
The use of high definition colonoscopy as compared to standard video colonoscopy has been reported to have only a marginal beneficial effect on the detection of colonic polyps and adenomas in a recent meta-analysis[87]. Due to heterogeneity of the included studies and the fact that no randomized trials were available, these results should be interpreted with some caution.
Virtual chromoendoscopy consists of multiple techniques that use a narrow spectrum of wavelengths with a decreased penetration depth to enhance visualization. Light of short wavelengths increases vascular contrast of the mucosa, potentially improving visualization and the identification of neoplastic lesions. Although there are some conflicting data, most studies and meta-analyses have not been able to demonstrate a substantial increase in ADRs with pan-colonic virtual chromoendoscopy[88-90].
Cap-assisted colonoscopy is performed by attaching a transparant cap to the tip of the colonoscope. These caps were originally designed to be used during endoscopic mucosa resection, but they might also aid in depressing colonic folds to improve visualization of the entire colonic mucosa. However, in a meta-analysis of 16 randomized controlled trials including 8991 subjects, Ng et al[91] concluded that cap-assisted colonoscopy only had a limited effect on ADR, although a higher proportion of patients with polyp(s) were detected when a cap was attached (relative risk 1.08; 95%CI: 1.00-1.17).
It has been reported that retroflexion of the colonoscope might aid in the removal of polyps that are difficult to access endoscopically[92,93]. Conceivably, inspection with a retroflexed colonoscope may also help in increasing visualization of the proximal aspects of colonic folds, especially in the right colon, and thereby increasing ADR. However, although this technique appears safe in experienced hands, both a randomized study and a large prospective observational study failed to demonstrate a relevant increase in the number of detected polyps[94,95].
In recent years, several new devices have been developed to improve visualization of the proximal sides of colonic folds and inner curvatures. First, the Third-Eye Retroscope® (Avantis Medical Systems, Inc) is a through-the-scope catheter with a camera and light source at the tip. After advancement through the working channel of the colonoscope, the catheter is retroflexed 180° (Figure 1). It then provides a 135° retrograde view of the colon. In a randomized, multicenter back-to-back study, the Third-Eye Retroscope yielded a net additional detection rate of 29.8% for polyps and 23.2% for adenomas compared to standard colonoscopy[96]. An advantage of this device is that it can be used with standard colonoscopy equipment. However, use of this device in clinical practice may be hampered by the fact that the Third-Eye Retroscope needs to be removed from the working channel in case a polypectomy snare or biopsy forceps is used. Furthermore, when the device is in place, the colonoscope has reduced suctioning capacity. These factors may increase procedural time and may be experienced as bothersome by the endoscopist.
Figure 1.

Third-Eye retroscope.
Recently, Gralnek et al[97] reported the results of the first international, multicenter, randomized, back-to-back study with the new Full Spectrum Endoscopy™ platform (FUSE; EndoChoice®, Alpharetta, Georgia, United States). The full spectrum colonoscope allows a high resolution 330° view of the colonic lumen, as compared to the 140°-170° of standard colonoscopes (Figure 2). In their study including 185 subjects, the adenoma miss rate was significantly lower in patients in whom colonoscopy was performed with the full-spectrum endoscope first: in the latter group five (7%) of 67 adenomas were missed vs 20 (41%) of 49 adenomas in the group that underwent standard colonoscopy first (P < 0.0001). Although these results seem promising, further studies are required to determine the potential role for this system in non-expert centers. The obvious disadvantage in the implementation of this new device in daily clinical practice, is that new colonoscopes and main control units are required.
Figure 2.
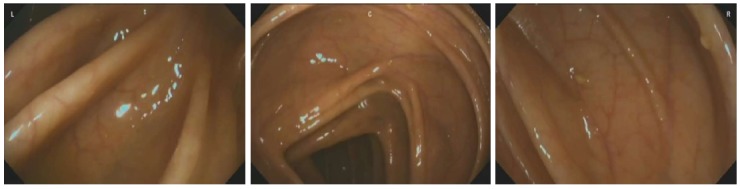
Endoscopic view using the Full Spectrum Endoscopy™ platform.
A potential downside of the current definition of ADR is that it does not discriminate between subjects in whom the endoscopist detects one vs more than one adenoma. It has been shown that physicians are more likely to miss additional adenomas during colonoscopy, when they have already detected two or more[4].
Wang et al[98] concluded that, despite comparable and adequate ADRs, there can be considerable variability between endoscopists with regard to the total number of adenomas detected per colonoscopy. They introduced a metric called the ADR-plus, the mean number of incremental adenomas after the first, and by coupling this to the ADR the authors were better able to distinguish high- from low-performing endoscopists. Lee et al[99] introduced two new measures in addition to the ADR that also may provide additional information on the inter-individual variation in the quality of performing colonoscopy: mean adenomas per procedure (MAP) and mean adenomas per positive procedure (MAP+). However, how these new metrics translate to the occurrence of interval cancers is currently not known.
PATIENT COMFORT AND SEDATION
Several guidelines recommend that sedation dosages as well as patient comfort scores should routinely be reported and monitored[19,20]. In their position statement on quality in screening colonoscopy, the European Society of Gastrointestinal Endoscopy proposed that no more than 1% of patients should have a saturation below 85% for more than 30 s or should require administration of a reversal agent[20].
Patient comfort in the screening setting is important, as patients who consider screening colonoscopy as being too uncomfortable, are less likely to participate[100]. It may obviously impact the effect of population screening when a significant proportion of the target population does not participate. Recently, Rostom et al[101] have prospectively validated a nurse-assisted patient comfort score in a multicenter, international setting, allowing for a uniform registration of patient comfort and comparison of colonoscopy practices. The various endoscopic societies have not yet adopted this validated comfort score. Which scores are considered acceptable and how to avoid drop-outs from the screening program has yet to be determined. Measuring comfort has the obvious caveat that endoscopists, nurses and patients may have different opinions about the level of (dis)comfort during the procedure.
Discomfort during colonoscopy can be reduced by the administration of sedatives. There is worldwide a large variation in the use of sedation for colonoscopy[102-105]. In some countries the majority of patients undergo colonoscopy unsedated, while elsewhere sedation with benzodiazepines combined with opiates is the standard of care. Entonox (nitrous oxide and oxygen) is frequently used in some countries, while elsewhere propofol and general anesthesia are increasingly being used in daily practice. Severe sedation-related complications have been reported to be rare: Behrens et al[106] reported a rate of 0.01% in their study of 388404 endoscopies. However, sedation-related adverse events need to be prevented, especially in an otherwise healthy screening population. There is however no validated score to record the level of sedation during colonoscopy, nor is there an accepted gold standard regarding sedation for colonoscopy.
Interestingly, a recent study from the United Kingdom screening program shows that, although there are wide variations in the use of sedation, colonoscopists’ individual medication practice does not appear to be related to the occurrence of significant discomfort[102]. Instead, it is suggested that the best endoscopists cause less patient discomfort while using less sedation[103].
COMPLICATION RATE
Colonoscopy is an invasive procedure that inadvertently will lead to complications in a small subset of patients. The rate of complications obviously is not necessarily associated with the interval CRCs. However, for a population screening program to have an overall beneficial effect, it is crucial that complication rates are low.
Perforation is the most serious complication of colonoscopy. It is defined as the presence of air, luminal contents or instrumentation outside the gastrointestinal tract[19]. It may result from mechanical trauma to the bowel wall, overinsufflation of the colon, or as a result of a therapeutic procedure. In the literature, reported overall rates of perforation range from 0.1%-0.6%[107-109]. The perforation rate for diagnostic colonoscopies is lower than that of therapeutic interventions. The British guidelines for screening colonoscopy state a standard of < 1:1000 risk of perforation in all colonoscopies[19,20], and a < 1:500 risk of perforation in colonoscopies in which polypectomy is performed[19]. This is largely consistent with the American guidelines[18], although it is important to keep in mind that there may be a significant variation in perforation risk between a screening population in which each participant undergoes a colonoscopy and a screening population that is pre-selected by means of fecal occult blood testing. Proportionally, it can be expected that more polypectomies will be performed in the latter. Each country should set its own standards according to the local screening strategy.
Historically, surgical closure or resection of the perforated colon segment was the only therapeutic option in case of iatrogenic colonic perforation. Several case series have reported on successful endoscopic closure of small iatrogenic bowel wall defects using metallic endoclips, either with endoclips alone or using a combined technique of endoclips and endoloops[110,111]. In recent years, the over-the-scope clip (Ovesco Endoscopy GmbH, Tuebingen, Germany) has become available, with high rates of successful perforation closure in the first reported case series[112,113].
Bleeding is the most common complication after polypectomy. Based on the literature, several guidelines set a standard of post-polypectomy bleeding in < 1:100 colonoscopies with polypectomy[18,19]. It is known that the risk of bleeding increases with size of the lesion and a more proximal location in the colon[114]. Several endoscopic techniques can be used to prevent bleeding. Cold snaring of small, non-pedunculated polyps may prevent delayed bleeding[115], even in anticoagulated patients[116]. Submucosal injection with saline and epinephrin prevents immediate bleeding but probably not delayed bleeding[117]. Furthermore, prophylactic placement of a detachable snare around the stalk of a pedunculated polyp may prevent bleeding[118,119], as well as prophylactic closure of the polypectomy site with metallic clips after removal of large (> 2 cm) sessile or flat lesions[120].
Post-polypectomy coagulation syndrome (PPCS), or transmural burn syndrome, is a known complication of colonoscopic polypectomy. It is defined by the development of abdominal pain, fever, leukocytosis and peritoneal inflammation in the absence of frank perforation that occurs after polypectomy with electrocoagulation[121]. To our knowledge, there is only one study that specifically focused on PPCS. In this large retrospective study, its incidence is reported to be 0.07% of all colonoscopies with polypectomy. Hypertension, a lesion size ≥ 10 mm and non-polypoid configuration of the lesion were independently associated with PPCS[121]. Correct identification of this entity is important, as this may avoid unnecessary explorative laparotomy. PPCS can usually be treated medically without a need for surgical intervention and without mortality. PPCS is not yet included in the current guidelines.
CONCLUSION
In summary, the main quality indicators for colonoscopy all have their shortcomings (Table 1). Most of these have been formulated based on consensus. Following the guideline Quality Indicators for Colonoscopy from the American Society of Gastrointestinal Endoscopy from 2006[18], many other countries have adopted these same quality indicators. The scientific evidence on which they are based is however limited. Potential measures to improve performance on individual quality indicators are summarized in Table 2.
Table 1.
Quality indicators and their shortcomings
| Quality indicator | Proposed standard | Unresolved issues |
| Bowel preparation | Each endoscopy report should state the quality of the bowel preparation[18,19] ≥ 90% of patients undergoing colonoscopy should have had a bowel preparation rated as excellent or at least adequate[19,20] | No evidence to support a cut-off of ≥ 90% No clear and generally accepted definition of adequate bowel preparation Unclear what bowel preparation quality is the absolute minimum to detect relevant findings and prevent interval cancers No clear policy on how to proceed in case of inadequate bowel preparation |
| Cecal intubation rate | Overall cecal intubation rate of ≥ 90%[18-20] Adjusted cecal intubation rate of ≥ 95%[18,19] Cecal intubation rate of ≥ 95% in all screening colonoscopies[18,19] | No robust scientific evidence to support a cut-off of ≥ 90% No evidence supporting an association between cecal intubation rate and the occurrence of interval CRC |
| Withdrawal time | ≥ 6 min on withdrawal from cecal pole to anus[18-20] | Conflicting reports on the association between withdrawal time and the number of detected polyps Interventions directed at optimizing withdrawal time have yielded conflicting results No evidence supporting an association between withdrawal time and the occurrence of interval CRC Better endoscopic withdrawal technique is not necessarily associated with withdrawal time An indirect measure to quantify the proportion of the colonic mucosa that is adequately visualized |
| Adenoma detection rate | ≥ 25% in men and ≥ 15% in women over 50 yr[18] ≥ 35% of all screening colonoscopies in patients with a positive fecal occult blood testing[19] | The only quality indicator that has been shown to be directly associated with interval CRC Does not discriminate between subjects in whom the endoscopist detects one vs more than one adenoma Does not optimally differentiate between high- and low-performing endoscopists |
| Patient comfort and sedation | Routinely reporting and monitoring of patient comfort scores and sedation dosages[19,20] | Until recently no validated patient comfort score was available Not yet clear what patient comfort scores are considered acceptable The endoscopist, the nurse and the patient may have different opinions about the level of comfort during the procedure No gold standard regarding sedation during colonoscopy No validated score to assess the level of sedation during colonoscopy |
| Complication rate | Perforation in < 1:1000 colonoscopies[18-20] Post-polypectomy bleeding in < 1:100 colonoscopies with polypectomy[18,19] | Consensus based Complication rate is mainly dependent on the number of therapeutic colonoscopies, which may vary between screening strategies (colonoscopic screening of the entire population vs selection of high-risk individuals through fecal occult blood testing) |
CRC: Colorectal cancer.
Table 2.
Potential measures to improve performance per quality indicator
| Quality indicator | Potential intervention to improve performance | Strength of scientific evidence |
| Bowel preparation | Split dose bowel preparation Last ingested dose of PEG-solution 3-5 h before colonoscopy | Meta-analysis of randomized controlled trials Observational, prospective studies |
| Cecal intubation rate | Additional training and use of auxiliary endoscopic instruments (e.g., pediatric colonoscope) | Expert opinion |
| Adenoma detection rate | Endoscopy nurse participation as a second observer Perform colonoscopy in the morning or in half-day blocks High definition colonoscopy (compared to standard video colonoscopy, marginal effect) Cap-assisted colonoscopy (marginal effect) Third-Eye Retroscope Full Spectrum Endoscopy | Randomized, multicenter studies Retrospective studies Meta-analysis Meta-analysis of randomized controlled trials Randomized, multicenter study Randomized, multicenter study |
| Complication rate | Cold snaring of small, non-pedunculated polyps may prevent bleeding | Prospective, multicenter, observational study and small single center randomized controlled study |
| Submucosal injection with saline and epinephrin prevents immediate bleeding | Randomized study | |
| Prophylactic placement of a detachable snare around the stalk of a pedunculated polyp prevents bleeding | Randomized studies | |
| Prophylactic closure of the polypectomy site with metallic clips after removal of large (> 2 cm) sessile or flat lesions may prevent bleeding | Retrospective study |
What is not yet clear is how to proceed when a fellow or senior endoscopist does not meet the required standards. Individualized additional training or a binding negative advice to continue the fellowship could be an option for endoscopists in training. However, this could be difficult for senior endoscopists that have practiced for years, especially when the scientific basis for these quality indicators is still not well established. What further needs to be addressed, is how to check that endoscopists indeed perform colonoscopy according to the standard of care set by their peers or national guidelines.
ADR currently is the only quality indicator that has been shown to be directly associated with the outcome measure interval colorectal cancer. As such, it seems reasonable to let this indicator prevail in discussions with endoscopists who fail to meet the set standards.
Ideally, endoscopists should only be evaluated and compared by the most relevant outcome measure in the context of screening colonoscopies, i.e. the occurrence of interval CRCs. Since the incidence of interval CRCs is fortunately rather low, and the duration between colonoscopy and interval CRC is rather long, this may prove to be too slow and rigid a quality indicator in daily practice to timely intervene in case of substandard colonoscopy performance.
Until we find a better measure to approximate the risk of interval CRCs, the current set of quality indicators will have to suffice. However, they need to be interpreted with caution and continuously adjusted as more information becomes available. For instance, both withdrawal time and ADR are a derivative of the quality with which the entire colonic mucosa is visualized during colonoscopy and in time may be replaced with a more direct measure for the proportion of the colonic mucosa that is inspected.
Footnotes
P- Reviewer: Herszenyi L, Jonaitis L, Yao CL S- Editor: Tian YL L- Editor: A E- Editor: Zhang DN
References
- 1.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 2.Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–350. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 4.Leufkens AM, van Oijen MG, Vleggaar FP, Siersema PD. Factors influencing the miss rate of polyps in a back-to-back colonoscopy study. Endoscopy. 2012;44:470–475. doi: 10.1055/s-0031-1291666. [DOI] [PubMed] [Google Scholar]
- 5.Lakoff J, Paszat LF, Saskin R, Rabeneck L. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin Gastroenterol Hepatol. 2008;6:1117–1121; quiz 1064. doi: 10.1016/j.cgh.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK, Rahmani EY, Haseman JH, Lemmel GT, Kaster S, Buckley JS. Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology. 1997;112:17–23. doi: 10.1016/s0016-5085(97)70213-0. [DOI] [PubMed] [Google Scholar]
- 8.Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96–102. doi: 10.1053/j.gastro.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Bressler B, Paszat LF, Vinden C, Li C, He J, Rabeneck L. Colonoscopic miss rates for right-sided colon cancer: a population-based analysis. Gastroenterology. 2004;127:452–456. doi: 10.1053/j.gastro.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 10.Hosokawa O, Shirasaki S, Kaizaki Y, Hayashi H, Douden K, Hattori M. Invasive colorectal cancer detected up to 3 years after a colonoscopy negative for cancer. Endoscopy. 2003;35:506–510. doi: 10.1055/s-2003-39665. [DOI] [PubMed] [Google Scholar]
- 11.Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010;105:2588–2596. doi: 10.1038/ajg.2010.390. [DOI] [PubMed] [Google Scholar]
- 12.Pullens HJ, Leenders M2, Schipper ME3, van Oijen MG2, Siersema PD. No Decrease in the Rate of Early or Missed Colorectal Cancers After Colonoscopy With Polypectomy Over a 10-Year Period: A Population-Based Analysis. Clin Gastroenterol Hepatol. 2014:Epub ahead of print. doi: 10.1016/j.cgh.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 13.le Clercq CM, Bouwens MW, Rondagh EJ, Bakker CM, Keulen ET, de Ridder RJ, Winkens B, Masclee AA, Sanduleanu S. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014;63:957–963. doi: 10.1136/gutjnl-2013-304880. [DOI] [PubMed] [Google Scholar]
- 14.Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8:858–864. doi: 10.1016/j.cgh.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18:1688–1694. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- 16.Davila RE, Rajan E, Baron TH, Adler DG, Egan JV, Faigel DO, Gan SI, Hirota WK, Leighton JA, Lichtenstein D, et al. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc. 2006;63:546–557. doi: 10.1016/j.gie.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Rees CJ, Bevan R. The National Health Service Bowel Cancer Screening Program: the early years. Expert Rev Gastroenterol Hepatol. 2013;7:421–437. doi: 10.1586/17474124.2013.811045. [DOI] [PubMed] [Google Scholar]
- 18.Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2006;63:S16–S28. doi: 10.1016/j.gie.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Chilton A, Rutter M, editors . Quality Assurance Guidelines for Colonoscopy. Sheffield: NHS Cancer Screening Programmes; 2011. [Google Scholar]
- 20.Rembacken B, Hassan C, Riemann JF, Chilton A, Rutter M, Dumonceau JM, Omar M, Ponchon T. Quality in screening colonoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) Endoscopy. 2012;44:957–968. doi: 10.1055/s-0032-1325686. [DOI] [PubMed] [Google Scholar]
- 21.Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 22.Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75:1197–1203. doi: 10.1016/j.gie.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Kilgore TW, Abdinoor AA, Szary NM, Schowengerdt SW, Yust JB, Choudhary A, Matteson ML, Puli SR, Marshall JB, Bechtold ML. Bowel preparation with split-dose polyethylene glycol before colonoscopy: a meta-analysis of randomized controlled trials. Gastrointest Endosc. 2011;73:1240–1245. doi: 10.1016/j.gie.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Enestvedt BK, Tofani C, Laine LA, Tierney A, Fennerty MB. 4-Liter split-dose polyethylene glycol is superior to other bowel preparations, based on systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2012;10:1225–1231. doi: 10.1016/j.cgh.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Hassan C, Bretthauer M, Kaminski MF, Polkowski M, Rembacken B, Saunders B, Benamouzig R, Holme O, Green S, Kuiper T, et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2013;45:142–150. doi: 10.1055/s-0032-1326186. [DOI] [PubMed] [Google Scholar]
- 26.Seo EH, Kim TO, Park MJ, Joo HR, Heo NY, Park J, Park SH, Yang SY, Moon YS. Optimal preparation-to-colonoscopy interval in split-dose PEG bowel preparation determines satisfactory bowel preparation quality: an observational prospective study. Gastrointest Endosc. 2012;75:583–590. doi: 10.1016/j.gie.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Eun CS, Han DS, Hyun YS, Bae JH, Park HS, Kim TY, Jeon YC, Sohn JH. The timing of bowel preparation is more important than the timing of colonoscopy in determining the quality of bowel cleansing. Dig Dis Sci. 2011;56:539–544. doi: 10.1007/s10620-010-1457-1. [DOI] [PubMed] [Google Scholar]
- 28.Chung YW, Han DS, Park KH, Kim KO, Park CH, Hahn T, Yoo KS, Park SH, Kim JH, Park CK. Patient factors predictive of inadequate bowel preparation using polyethylene glycol: a prospective study in Korea. J Clin Gastroenterol. 2009;43:448–452. doi: 10.1097/MCG.0b013e3181662442. [DOI] [PubMed] [Google Scholar]
- 29.Hassan C, Fuccio L, Bruno M, Pagano N, Spada C, Carrara S, Giordanino C, Rondonotti E, Curcio G, Dulbecco P, et al. A predictive model identifies patients most likely to have inadequate bowel preparation for colonoscopy. Clin Gastroenterol Hepatol. 2012;10:501–506. doi: 10.1016/j.cgh.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55:2014–2020. doi: 10.1007/s10620-009-1079-7. [DOI] [PubMed] [Google Scholar]
- 31.Appannagari A, Mangla S, Liao C, Reddy KG, Kupfer SS. Risk factors for inadequate colonoscopy bowel preparations in African Americans and whites at an urban medical center. South Med J. 2014;107:220–224. doi: 10.1097/SMJ.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ness RM, Manam R, Hoen H, Chalasani N. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96:1797–1802. doi: 10.1111/j.1572-0241.2001.03874.x. [DOI] [PubMed] [Google Scholar]
- 33.Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52:346–352. doi: 10.1067/mge.2000.108480. [DOI] [PubMed] [Google Scholar]
- 34.Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482–486. doi: 10.1016/s0016-5107(03)02875-x. [DOI] [PubMed] [Google Scholar]
- 35.Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620–625. doi: 10.1016/j.gie.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gentile M, De Rosa M, Cestaro G, Forestieri P. 2 L PEG plus ascorbic acid versus 4 L PEG plus simethicon for colonoscopy preparation: a randomized single-blind clinical trial. Surg Laparosc Endosc Percutan Tech. 2013;23:276–280. doi: 10.1097/SLE.0b013e31828e389d. [DOI] [PubMed] [Google Scholar]
- 37.Brahmania M, Ou G, Bressler B, Ko HK, Lam E, Telford J, Enns R. 2 L versus 4 L of PEG3350 + electrolytes for outpatient colonic preparation: a randomized, controlled trial. Gastrointest Endosc. 2014;79:408–416.e4. doi: 10.1016/j.gie.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 38.Samarasena JB, Muthusamy VR, Jamal MM. Split-dosed MiraLAX/Gatorade is an effective, safe, and tolerable option for bowel preparation in low-risk patients: a randomized controlled study. Am J Gastroenterol. 2012;107:1036–1042. doi: 10.1038/ajg.2012.115. [DOI] [PubMed] [Google Scholar]
- 39.Hjelkrem M, Stengel J, Liu M, Jones DP, Harrison SA. MiraLAX is not as effective as GoLytely in bowel cleansing before screening colonoscopies. Clin Gastroenterol Hepatol. 2011;9:326–332.e1. doi: 10.1016/j.cgh.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Gerard DP, Holden JL, Foster DB, Raiser MW. Randomized Trial of Gatorade/Polyethylene Glycol With or Without Bisacodyl and NuLYTELY for Colonoscopy Preparation. Clin Transl Gastroenterol. 2012;3:e16. doi: 10.1038/ctg.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calderwood AH, Schroy PC, Lieberman DA, Logan JR, Zurfluh M, Jacobson BC. Boston Bowel Preparation Scale scores provide a standardized definition of adequate for describing bowel cleanliness. Gastrointest Endosc. 2014;80:269–276. doi: 10.1016/j.gie.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibáñez M, Parra-Blanco A, Zaballa P, Jiménez A, Fernández-Velázquez R, Fernández-Sordo JO, González-Bernardo O, Rodrigo L. Usefulness of an intensive bowel cleansing strategy for repeat colonoscopy after preparation failure. Dis Colon Rectum. 2011;54:1578–1584. doi: 10.1097/DCR.0b013e31823434c8. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Horin S, Bar-Meir S, Avidan B. The outcome of a second preparation for colonoscopy after preparation failure in the first procedure. Gastrointest Endosc. 2009;69:626–630. doi: 10.1016/j.gie.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 44.Marshall JB, Barthel JS. The frequency of total colonoscopy and terminal ileal intubation in the 1990s. Gastrointest Endosc. 1993;39:518–520. doi: 10.1016/s0016-5107(93)70162-5. [DOI] [PubMed] [Google Scholar]
- 45.Valori R, Rey JF, Atkin WS, Bretthauer M, Senore C, Hoff G, Kuipers EJ, Altenhofen L, Lambert R, Minoli G. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Quality assurance in endoscopy in colorectal cancer screening and diagnosis. Endoscopy. 2012;44 Suppl 3:SE88–S105. doi: 10.1055/s-0032-1309795. [DOI] [PubMed] [Google Scholar]
- 46.Gupta M, Holub JL, Eisen G. Do indication and demographics for colonoscopy affect completion? A large national database evaluation. Eur J Gastroenterol Hepatol. 2010;22:620–627. doi: 10.1097/MEG.0b013e3283352cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cirocco WC, Rusin LC. Factors that predict incomplete colonoscopy. Dis Colon Rectum. 1995;38:964–968. doi: 10.1007/BF02049733. [DOI] [PubMed] [Google Scholar]
- 48.Anderson JC, Gonzalez JD, Messina CR, Pollack BJ. Factors that predict incomplete colonoscopy: thinner is not always better. Am J Gastroenterol. 2000;95:2784–2787. doi: 10.1111/j.1572-0241.2000.03186.x. [DOI] [PubMed] [Google Scholar]
- 49.Bernstein C, Thorn M, Monsees K, Spell R, O’Connor JB. A prospective study of factors that determine cecal intubation time at colonoscopy. Gastrointest Endosc. 2005;61:72–75. doi: 10.1016/s0016-5107(04)02461-7. [DOI] [PubMed] [Google Scholar]
- 50.Anderson JC, Messina CR, Cohn W, Gottfried E, Ingber S, Bernstein G, Coman E, Polito J. Factors predictive of difficult colonoscopy. Gastrointest Endosc. 2001;54:558–562. doi: 10.1067/mge.2001.118950. [DOI] [PubMed] [Google Scholar]
- 51.Neerincx M, Terhaar sive Droste JS, Mulder CJ, Räkers M, Bartelsman JF, Loffeld RJ, Tuynman HA, Brohet RM, van der Hulst RW. Colonic work-up after incomplete colonoscopy: significant new findings during follow-up. Endoscopy. 2010;42:730–735. doi: 10.1055/s-0030-1255523. [DOI] [PubMed] [Google Scholar]
- 52.Pullens HJ, van Leeuwen MS, Laheij RJ, Vleggaar FP, Siersema PD. CT-colonography after incomplete colonoscopy: what is the diagnostic yield? Dis Colon Rectum. 2013;56:593–599. doi: 10.1097/DCR.0b013e3182781668. [DOI] [PubMed] [Google Scholar]
- 53.Brahmania M, Park J, Svarta S, Tong J, Kwok R, Enns R. Incomplete colonoscopy: maximizing completion rates of gastroenterologists. Can J Gastroenterol. 2012;26:589–592. doi: 10.1155/2012/353457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 55.Simmons DT, Harewood GC, Baron TH, Petersen BT, Wang KK, Boyd-Enders F, Ott BJ. Impact of endoscopist withdrawal speed on polyp yield: implications for optimal colonoscopy withdrawal time. Aliment Pharmacol Ther. 2006;24:965–971. doi: 10.1111/j.1365-2036.2006.03080.x. [DOI] [PubMed] [Google Scholar]
- 56.Overholt BF, Brooks-Belli L, Grace M, Rankin K, Harrell R, Turyk M, Rosenberg FB, Barish RW, Gilinsky NH. Withdrawal times and associated factors in colonoscopy: a quality assurance multicenter assessment. J Clin Gastroenterol. 2010;44:e80–e86. doi: 10.1097/MCG.0b013e3181bf9b02. [DOI] [PubMed] [Google Scholar]
- 57.Lee TJ, Blanks RG, Rees CJ, Wright KC, Nickerson C, Moss SM, Chilton A, Goddard AF, Patnick J, McNally RJ, et al. Longer mean colonoscopy withdrawal time is associated with increased adenoma detection: evidence from the Bowel Cancer Screening Programme in England. Endoscopy. 2013;45:20–26. doi: 10.1055/s-0032-1325803. [DOI] [PubMed] [Google Scholar]
- 58.Butterly L, Robinson CM, Anderson JC, Weiss JE, Goodrich M, Onega TL, Amos CI, Beach ML. Serrated and adenomatous polyp detection increases with longer withdrawal time: results from the New Hampshire Colonoscopy Registry. Am J Gastroenterol. 2014;109:417–426. doi: 10.1038/ajg.2013.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benson ME, Reichelderfer M, Said A, Gaumnitz EA, Pfau PR. Variation in colonoscopic technique and adenoma detection rates at an academic gastroenterology unit. Dig Dis Sci. 2010;55:166–171. doi: 10.1007/s10620-008-0703-2. [DOI] [PubMed] [Google Scholar]
- 60.Moritz V, Bretthauer M, Ruud HK, Glomsaker T, de Lange T, Sandvei P, Huppertz-Hauss G, Kjellevold Ø, Hoff G. Withdrawal time as a quality indicator for colonoscopy - a nationwide analysis. Endoscopy. 2012;44:476–481. doi: 10.1055/s-0032-1306898. [DOI] [PubMed] [Google Scholar]
- 61.Adler A, Wegscheider K, Lieberman D, Aminalai A, Aschenbeck J, Drossel R, Mayr M, Mroß M, Scheel M, Schröder A, et al. Factors determining the quality of screening colonoscopy: a prospective study on adenoma detection rates, from 12,134 examinations (Berlin colonoscopy project 3, BECOP-3) Gut. 2013;62:236–241. doi: 10.1136/gutjnl-2011-300167. [DOI] [PubMed] [Google Scholar]
- 62.Lee RH, Tang RS, Muthusamy VR, Ho SB, Shah NK, Wetzel L, Bain AS, Mackintosh EE, Paek AM, Crissien AM, et al. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos) Gastrointest Endosc. 2011;74:128–134. doi: 10.1016/j.gie.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6:1091–1098. doi: 10.1016/j.cgh.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 64.Sawhney MS, Cury MS, Neeman N, Ngo LH, Lewis JM, Chuttani R, Pleskow DK, Aronson MD. Effect of institution-wide policy of colonoscopy withdrawal time > or = 7 minutes on polyp detection. Gastroenterology. 2008;135:1892–1898. doi: 10.1053/j.gastro.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 65.Velásquez J, Espinoza-Ríos J, Huerta-Mercado J, Pinto J, De los Ríos R, Piscoya A, OR C, Zegarra A, Bussalleu A. [Impact assessment of increasing the time of withdrawal of colonoscopy in the detection rate of polyps in our midst] Rev Gastroenterol Peru. 2009;29:321–325. [PubMed] [Google Scholar]
- 66.Gellad ZF, Weiss DG, Ahnen DJ, Lieberman DA, Jackson GL, Provenzale D. Colonoscopy withdrawal time and risk of neoplasia at 5 years: results from VA Cooperative Studies Program 380. Am J Gastroenterol. 2010;105:1746–1752. doi: 10.1038/ajg.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rex DK. Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest Endosc. 2000;51:33–36. doi: 10.1016/s0016-5107(00)70383-x. [DOI] [PubMed] [Google Scholar]
- 68.Hong D, Tavanapong W, Wong J, Oh J, de Groen PC. 3D Reconstruction of virtual colon structures from colonoscopy images. Comput Med Imaging Graph. 2014;38:22–33. doi: 10.1016/j.compmedimag.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 69.Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 70.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102:856–861. doi: 10.1111/j.1572-0241.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 71.Imperiale TF, Glowinski EA, Juliar BE, Azzouz F, Ransohoff DF. Variation in polyp detection rates at screening colonoscopy. Gastrointest Endosc. 2009;69:1288–1295. doi: 10.1016/j.gie.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 72.Bretagne JF, Hamonic S, Piette C, Manfredi S, Leray E, Durand G, Riou F. Variations between endoscopists in rates of detection of colorectal neoplasia and their impact on a regional screening program based on colonoscopy after fecal occult blood testing. Gastrointest Endosc. 2010;71:335–341. doi: 10.1016/j.gie.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 73.van Lelyveld N, van Oijen MG, Schwartz MP. [Quality indicators for colonoscopy: differences in polyp detection between endoscopists at one hospital] Ned Tijdschr Geneeskd. 2012;156:A4219. [PubMed] [Google Scholar]
- 74.Ricci E, Hassan C, Petruzziello L, Bazzoli F, Repici A, Di Giulio E. Inter-centre variability of the adenoma detection rate: a prospective, multicentre study. Dig Liver Dis. 2013;45:1022–1027. doi: 10.1016/j.dld.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 75.Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298–1306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.East JE, Suzuki N, Arebi N, Bassett P, Saunders BP. Position changes improve visibility during colonoscope withdrawal: a randomized, blinded, crossover trial. Gastrointest Endosc. 2007;65:263–269. doi: 10.1016/j.gie.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 77.East JE, Bassett P, Arebi N, Thomas-Gibson S, Guenther T, Saunders BP. Dynamic patient position changes during colonoscope withdrawal increase adenoma detection: a randomized, crossover trial. Gastrointest Endosc. 2011;73:456–463. doi: 10.1016/j.gie.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 78.Köksal AŞ, Kalkan IH, Torun S, Taşkıran I, Öztaş E, Kayaçetin E, Şaşmaz N. A simple method to improve adenoma detection rate during colonoscopy: altering patient position. Can J Gastroenterol. 2013;27:509–512. doi: 10.1155/2013/276043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ou G, Kim E, Lakzadeh P, Tong J, Enns R, Ramji A, Whittaker S, Ko HH, Bressler B, Halparin L, et al. A randomized controlled trial assessing the effect of prescribed patient position changes during colonoscope withdrawal on adenoma detection. Gastrointest Endosc. 2014;80:277–283. doi: 10.1016/j.gie.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 80.Aslanian HR, Shieh FK, Chan FW, Ciarleglio MM, Deng Y, Rogart JN, Jamidar PA, Siddiqui UD. Nurse observation during colonoscopy increases polyp detection: a randomized prospective study. Am J Gastroenterol. 2013;108:166–172. doi: 10.1038/ajg.2012.237. [DOI] [PubMed] [Google Scholar]
- 81.Lee CK, Park DI, Lee SH, Hwangbo Y, Eun CS, Han DS, Cha JM, Lee BI, Shin JE. Participation by experienced endoscopy nurses increases the detection rate of colon polyps during a screening colonoscopy: a multicenter, prospective, randomized study. Gastrointest Endosc. 2011;74:1094–1102. doi: 10.1016/j.gie.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 82.Sanaka MR, Deepinder F, Thota PN, Lopez R, Burke CA. Adenomas are detected more often in morning than in afternoon colonoscopy. Am J Gastroenterol. 2009;104:1659–1664; quiz 1665. doi: 10.1038/ajg.2009.249. [DOI] [PubMed] [Google Scholar]
- 83.Gurudu SR, Ratuapli SK, Leighton JA, Heigh RI, Crowell MD. Adenoma detection rate is not influenced by the timing of colonoscopy when performed in half-day blocks. Am J Gastroenterol. 2011;106:1466–1471. doi: 10.1038/ajg.2011.125. [DOI] [PubMed] [Google Scholar]
- 84.Lurix E, Hernandez AV, Thoma M, Castro F. Adenoma detection rate is not influenced by full-day blocks, time, or modified queue position. Gastrointest Endosc. 2012;75:827–834. doi: 10.1016/j.gie.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 85.Paeck KH, Heo WJ, Park DI, Kim YH, Lee SH, Lee CK, Eun CS, Han DS. Colonoscopy scheduling influences adenoma and polyp detection rates. Hepatogastroenterology. 2013;60:1647–1652. [PubMed] [Google Scholar]
- 86.Lee A, Iskander JM, Gupta N, Borg BB, Zuckerman G, Banerjee B, Gyawali CP. Queue position in the endoscopic schedule impacts effectiveness of colonoscopy. Am J Gastroenterol. 2011;106:1457–1465. doi: 10.1038/ajg.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Subramanian V, Mannath J, Hawkey CJ, Ragunath K. High definition colonoscopy vs. standard video endoscopy for the detection of colonic polyps: a meta-analysis. Endoscopy. 2011;43:499–505. doi: 10.1055/s-0030-1256207. [DOI] [PubMed] [Google Scholar]
- 88.Pasha SF, Leighton JA, Das A, Harrison ME, Gurudu SR, Ramirez FC, Fleischer DE, Sharma VK. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol. 2012;107:363–370; quiz 371. doi: 10.1038/ajg.2011.436. [DOI] [PubMed] [Google Scholar]
- 89.Chung SJ, Kim D, Song JH, Kang HY, Chung GE, Choi J, Kim YS, Park MJ, Kim JS. Comparison of detection and miss rates of narrow band imaging, flexible spectral imaging chromoendoscopy and white light at screening colonoscopy: a randomised controlled back-to-back study. Gut. 2014;63:785–791. doi: 10.1136/gutjnl-2013-304578. [DOI] [PubMed] [Google Scholar]
- 90.Chung SJ, Kim D, Song JH, Park MJ, Kim YS, Kim JS, Jung HC, Song IS. Efficacy of computed virtual chromoendoscopy on colorectal cancer screening: a prospective, randomized, back-to-back trial of Fuji Intelligent Color Enhancement versus conventional colonoscopy to compare adenoma miss rates. Gastrointest Endosc. 2010;72:136–142. doi: 10.1016/j.gie.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 91.Ng SC, Tsoi KK, Hirai HW, Lee YT, Wu JC, Sung JJ, Chan FK, Lau JY. The efficacy of cap-assisted colonoscopy in polyp detection and cecal intubation: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2012;107:1165–1173. doi: 10.1038/ajg.2012.135. [DOI] [PubMed] [Google Scholar]
- 92.Rex DK, Khashab M. Colonoscopic polypectomy in retroflexion. Gastrointest Endosc. 2006;63:144–148. doi: 10.1016/j.gie.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 93.Pishvaian AC, Al-Kawas FH. Retroflexion in the colon: a useful and safe technique in the evaluation and resection of sessile polyps during colonoscopy. Am J Gastroenterol. 2006;101:1479–1483. doi: 10.1111/j.1572-0241.2006.00606.x. [DOI] [PubMed] [Google Scholar]
- 94.Harrison M, Singh N, Rex DK. Impact of proximal colon retroflexion on adenoma miss rates. Am J Gastroenterol. 2004;99:519–522. doi: 10.1111/j.1572-0241.2004.04070.x. [DOI] [PubMed] [Google Scholar]
- 95.Hewett DG, Rex DK. Miss rate of right-sided colon examination during colonoscopy defined by retroflexion: an observational study. Gastrointest Endosc. 2011;74:246–252. doi: 10.1016/j.gie.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 96.Leufkens AM, DeMarco DC, Rastogi A, Akerman PA, Azzouzi K, Rothstein RI, Vleggaar FP, Repici A, Rando G, Okolo PI, et al. Effect of a retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE study. Gastrointest Endosc. 2011;73:480–489. doi: 10.1016/j.gie.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 97.Gralnek IM, Siersema PD, Halpern Z, Segol O, Melhem A, Suissa A, Santo E, Sloyer A, Fenster J, Moons LM, et al. Standard forward-viewing colonoscopy versus full-spectrum endoscopy: an international, multicentre, randomised, tandem colonoscopy trial. Lancet Oncol. 2014;15:353–360. doi: 10.1016/S1470-2045(14)70020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang HS, Pisegna J, Modi R, Liang LJ, Atia M, Nguyen M, Cohen H, Ohning G, van Oijen M, Spiegel BM. Adenoma detection rate is necessary but insufficient for distinguishing high versus low endoscopist performance. Gastrointest Endosc. 2013;77:71–78. doi: 10.1016/j.gie.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 99.Lee TJ, Rutter MD, Blanks RG, Moss SM, Goddard AF, Chilton A, Nickerson C, McNally RJ, Patnick J, Rees CJ. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut. 2012;61:1050–1057. doi: 10.1136/gutjnl-2011-300651. [DOI] [PubMed] [Google Scholar]
- 100.de Wijkerslooth TR, de Haan MC, Stoop EM, Bossuyt PM, Thomeer M, van Leerdam ME, Essink-Bot ML, Fockens P, Kuipers EJ, Stoker J, et al. Reasons for participation and nonparticipation in colorectal cancer screening: a randomized trial of colonoscopy and CT colonography. Am J Gastroenterol. 2012;107:1777–1783. doi: 10.1038/ajg.2012.140. [DOI] [PubMed] [Google Scholar]
- 101.Rostom A, Ross ED, Dubé C, Rutter MD, Lee T, Valori R, Bridges RJ, Pontifex D, Webbink V, Rees C, et al. Development and validation of a nurse-assessed patient comfort score for colonoscopy. Gastrointest Endosc. 2013;77:255–261. doi: 10.1016/j.gie.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 102.Ball A, Riley S. PWE-028 Patient Comfort And Sedation And Analgesic Practices During Colonoscopy In The English Bowel Cancer Screening Programme. Gut. 2014;63 Suppl 1:A134. [Google Scholar]
- 103.Ekkelenkamp VE, Dowler K, Valori RM, Dunckley P. Patient comfort and quality in colonoscopy. World J Gastroenterol. 2013;19:2355–2361. doi: 10.3748/wjg.v19.i15.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ristikankare MK, Julkunen RJ. Premedication for gastrointestinal endoscopy is a rare practice in Finland: a nationwide survey. Gastrointest Endosc. 1998;47:204–207. [PubMed] [Google Scholar]
- 105.Liu H, Waxman DA, Main R, Mattke S. Utilization of anesthesia services during outpatient endoscopies and colonoscopies and associated spending in 2003-2009. JAMA. 2012;307:1178–1184. doi: 10.1001/jama.2012.270. [DOI] [PubMed] [Google Scholar]
- 106.Behrens A, Labenz J, Schuler A, Schröder W, Rünzi M, Steinmann RU, de Mas CR, Kreuzmayr A, Barth K, Bahr MJ, et al. [How safe is sedation in gastrointestinal endoscopy? A multicentre analysis of 388,404 endoscopies and analysis of data from prospective registries of complications managed by members of the Working Group of Leading Hospital Gastroenterologists (ALGK)] Z Gastroenterol. 2013;51:432–436. doi: 10.1055/s-0032-1325524. [DOI] [PubMed] [Google Scholar]
- 107.Silvis SE, Nebel O, Rogers G, Sugawa C, Mandelstam P. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy Survey. JAMA. 1976;235:928–930. doi: 10.1001/jama.235.9.928. [DOI] [PubMed] [Google Scholar]
- 108.Rabeneck L, Paszat LF, Hilsden RJ, Saskin R, Leddin D, Grunfeld E, Wai E, Goldwasser M, Sutradhar R, Stukel TA. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–1906, 1906.e1. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 109.Lüning TH, Keemers-Gels ME, Barendregt WB, Tan AC, Rosman C. Colonoscopic perforations: a review of 30,366 patients. Surg Endosc. 2007;21:994–997. doi: 10.1007/s00464-007-9251-7. [DOI] [PubMed] [Google Scholar]
- 110.Cho SB, Lee WS, Joo YE, Kim HR, Park SW, Park CH, Kim HS, Choi SK, Rew JS. Therapeutic options for iatrogenic colon perforation: feasibility of endoscopic clip closure and predictors of the need for early surgery. Surg Endosc. 2012;26:473–479. doi: 10.1007/s00464-011-1903-y. [DOI] [PubMed] [Google Scholar]
- 111.Ladas SD, Kamberoglou D, Vlachogiannakos J, Tomos P. Combined use of metallic endoclips and endoloops using a single-channel scope in closing iatrogenic perforations and fistulas: two case reports and a literature review. Eur J Gastroenterol Hepatol. 2014;26:119–122. doi: 10.1097/MEG.0b013e328365a464. [DOI] [PubMed] [Google Scholar]
- 112.Gubler C, Bauerfeind P. Endoscopic closure of iatrogenic gastrointestinal tract perforations with the over-the-scope clip. Digestion. 2012;85:302–307. doi: 10.1159/000336509. [DOI] [PubMed] [Google Scholar]
- 113.Nishiyama N, Mori H, Kobara H, Rafiq K, Fujihara S, Kobayashi M, Oryu M, Masaki T. Efficacy and safety of over-the-scope clip: including complications after endoscopic submucosal dissection. World J Gastroenterol. 2013;19:2752–2760. doi: 10.3748/wjg.v19.i18.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sorbi D, Norton I, Conio M, Balm R, Zinsmeister A, Gostout CJ. Postpolypectomy lower GI bleeding: descriptive analysis. Gastrointest Endosc. 2000;51:690–696. doi: 10.1067/mge.2000.105773. [DOI] [PubMed] [Google Scholar]
- 115.Repici A, Hassan C, Vitetta E, Ferrara E, Manes G, Gullotti G, Princiotta A, Dulbecco P, Gaffuri N, Bettoni E, et al. Safety of cold polypectomy for & lt; 10mm polyps at colonoscopy: a prospective multicenter study. Endoscopy. 2012;44:27–31. doi: 10.1055/s-0031-1291387. [DOI] [PubMed] [Google Scholar]
- 116.Horiuchi A, Nakayama Y, Kajiyama M, Tanaka N, Sano K, Graham DY. Removal of small colorectal polyps in anticoagulated patients: a prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc. 2014;79:417–423. doi: 10.1016/j.gie.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 117.Hsieh YH, Lin HJ, Tseng GY, Perng CL, Li AF, Chang FY, Lee SD. Is submucosal epinephrine injection necessary before polypectomy? A prospective, comparative study. Hepatogastroenterology. 2001;48:1379–1382. [PubMed] [Google Scholar]
- 118.Iishi H, Tatsuta M, Narahara H, Iseki K, Sakai N. Endoscopic resection of large pedunculated colorectal polyps using a detachable snare. Gastrointest Endosc. 1996;44:594–597. doi: 10.1016/s0016-5107(96)70015-9. [DOI] [PubMed] [Google Scholar]
- 119.Di Giorgio P, De Luca L, Calcagno G, Rivellini G, Mandato M, De Luca B. Detachable snare versus epinephrine injection in the prevention of postpolypectomy bleeding: a randomized and controlled study. Endoscopy. 2004;36:860–863. doi: 10.1055/s-2004-825801. [DOI] [PubMed] [Google Scholar]
- 120.Liaquat H, Rohn E, Rex DK. Prophylactic clip closure reduced the risk of delayed postpolypectomy hemorrhage: experience in 277 clipped large sessile or flat colorectal lesions and 247 control lesions. Gastrointest Endosc. 2013;77:401–407. doi: 10.1016/j.gie.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 121.Cha JM, Lim KS, Lee SH, Joo YE, Hong SP, Kim TI, Kim HG, Park DI, Kim SE, Yang DH, et al. Clinical outcomes and risk factors of post-polypectomy coagulation syndrome: a multicenter, retrospective, case-control study. Endoscopy. 2013;45:202–207. doi: 10.1055/s-0032-1326104. [DOI] [PubMed] [Google Scholar]


